Abstract
Over the last several decades, the cardiac intensive care unit (CICU) has seen a substantial evolution in the patient population, comorbidities, and diagnoses. However, generation of high-quality evidence to manage these complex and critically ill patients has been slow. Given the scarcity of clinical trials focused on critical care cardiology (CCC), CICU clinicians are often left to extrapolate from studies conducted which either exclude or poorly represented the patient population admitted to CICUs. The lack of high-quality evidence and limited guidance from society guidelines has led to significant variation in practice patterns for many of the most common CICU diagnoses. Several barriers, both common to critical care research and unique to CCC, have impeded progress. In this multinational perspective, we describe key areas of priority for CCC research, current challenges for investigation in the CICU, and essential elements of a path forward for the field.
Keywords: Cardiac intensive care unit, Shock, Mechanical circulatory support, Research methods
Condensed Abstract
The cardiac intensive care unit (CICU) has seen an evolution in the patient population, comorbidities, and diagnoses. However, generation of high-quality evidence to manage these complex and critically ill patients has been slow. The lack of high-quality evidence and limited guidance from society guidelines has led to significant variation in practice patterns for many of the most common CICU diagnoses. Progress has been impeded by significant barriers to CCC-related research. In this multinational perspective, we describe key areas of priority for critical care cardiology research, current challenges for investigation in the CICU, and suggestions for a path forward.
Introduction
The changing demographics, case-mix, and acuity in the contemporary cardiac intensive care unit (CICU) are well described.1,2 This evolution has stimulated development of new training and staffing paradigms in critical care cardiology (CCC) and highlighted major gaps in the evidence guiding CCC.3–5 Given the scarcity of clinical trials focused on CCC, CICU clinicians are often left to extrapolate from studies conducted in general medical and surgical intensive care units, and which frequently either excluded or poorly represented the patient population admitted to CICUs. Wide variation in practice patterns for management of common CICU disorders reflect the absence of evidence-based professional society guidelines for many of the most common and morbid syndromes cared for in CICUs.6,7 Progress has been impeded by significant barriers to CCC-related research. To address these challenges, it is important to first delineate research priorities specific to CCC. In this multinational perspective, we describe key areas of CICU-specific CCC research, current challenges for clinical and translational investigation in the CICU, and essential elements of a path forward for the field. While numerous CICU-specific research questions remain (Table 1), we focus on four broad themes that we view as priorities in CCC research, including defining practice variation, evaluating and refining CICU monitoring, advancing device management, and improvement in patient phenotyping.
Table 1.
Broad areas and specific topics in critical care cardiology research
| Broad Area | Specific Topics |
|---|---|
| Hemodynamic Monitoring | |
| PAC use in cardiogenic shock | |
| Non-invasive hemodynamics devices | |
| Evidenced based use of POCUS | |
| Respiratory Management | |
| Strategies to avoid invasive mechanical ventilation | |
| Efficacy and safety of alternative ventilatory modes and settings | |
| Heart-lung interactions with positive pressure ventilation | |
| Optimal extubation strategies | |
| Sedation and analgesia for mechanical ventilation | |
| Arterial oxygen and carbon dioxide targets for cardiac patients | |
| Acute Arrhythmias | |
| Ventricular tachycardia storm management | |
| Acute treatment of rapid atrial fibrillation | |
| Medical management of unstable bradyarrhythmias | |
| Prognostication/Advanced Phenotyping | |
| Appropriateness criteria for triage to the CICU | |
| Risk prediction across various diagnoses, (e.g., acute heart failure, cardiogenic shock, cardiac arrest) | |
| Neuroprognostication after cardiac arrest | |
| Deep phenotyping to tailor therapies (e.g., cardiogenic shock type to tailored medical or mechanical therapy) | |
| Application of biomarkers and proteomics for prognostication | |
| Identifying futility to better counsel patients/families | |
| Quantifying the impact of frailty on outcomes and response to therapies | |
| Selection of patients for transfer to advanced care centers | |
| Timing of palliative care involvement | |
| Cardiogenic Shock | |
| Improving medical management paradigms | |
| Identifying evidence-based approaches to and selection of MCS | |
| Optimal hemodynamic targets (e.g., mean arterial pressure) | |
| Management of acute and chronic right ventricular failure | |
| Strategies for identifying and managing mixed shock | |
| Mitigation of device complications | |
| Venting strategies for ECMO | |
| Cardiac Arrest | |
| Post-cardiac arrest management (e.g., temperature targets) | |
| Strategies to optimize ECMO for cardiopulmonary resuscitation | |
| CCC Education/Staffing | |
| Delineation of the skills and training for CICU practitioners | |
| Appropriate staffing models stratified by CICU level | |
| Renal Management | |
| Identification and treatment of cardiorenal syndrome | |
| Timing and modes of renal replacement therapy in cardiac patients | |
| Research Methods | |
| Methods and strategies for implementation of structured severity staging within registries and clinical trials | |
| Applications of artificial intelligence in the data rich CICU | |
| Ethical and efficient informed consent in CCC research | |
| Implementation of novel and pragmatic trial designs, including Bayesian adaptive designs, registry and EHR-based studies | |
| Post-Cardiotomy Critical Care | |
PAC = Pulmonary artery catheter; POCUS = Point-of-care ultrasound; CICU = Cardiac intensive care unit; MCS = Mechanical circulatory support; ECMO = Extracorporeal membrane oxygenation; CCC = Critical care cardiology; EHR = Electronic heath record
Key Priority Areas for Research in Critical Care Cardiology
Defining epidemiology and practice variation in the CICU
Where there is variability in practice, there is often clinical uncertainty as well as the opportunity to improve. Understanding the epidemiology of the population, defining usual care in the CICU, and assessing variability in practice between centers, regions, and nations is a foundational step to identify and quantify unmet needs, and develop testable hypotheses for CCC comparative effectiveness research.8 Over the last decade, reports from North America and Europe have detailed profound inconsistency in structures and patterns of care in CICUs.6,9,10 For example, in North American tertiary care CICUs, use of mechanical circulatory support (MCS) varied between 17% to 50% of patients with cardiogenic or mixed shock. Moreover, among patients receiving MCS, between-center use of intra-aortic balloon pumps (IABP) ranged from 40 to 100% independently of severity of illness.7 MCS use in Europe is similarly varied with reports of IABP use ranging from 1% in Germany to 25% in England.11,12 Such heterogeneity in practice is not unique to MCS, as variation exists for numerous other domains in CCC, including acute myocardial infarction, respiratory insufficiency, renal replacement therapy, and pulmonary artery catheter (PAC) use.6,9,13,14
Critical evaluation of available and emerging CICU monitoring
The balance of risk and potential benefit of invasive hemodynamic monitoring in the CICU remains uncertain. There is an emerging consensus among experts that PAC monitoring is needed for many patients in the CICU, especially those with MCS.15 This expert perspective is supported by non-randomized observational studies demonstrating a favorable association between use of a PAC with mortality among patients with shock.14,16,17 However, the lack of randomized data for this population leaves a significant evidence gap relevant to practice in the CICU.
Two ongoing lines of investigation are important to address the unmet need for critical evaluation of the risks and benefits of hemodynamic monitoring in the CICU. First, randomized, controlled trials (RCTs) are aimed at providing rigorous evidence to guide PAC practice. For example, the currently enrolling PAC in Cardiogenic Shock trial (PACCS; NCT05445376) is studying early routine PAC placement in patients with heart failure-related cardiogenic shock. Second, as the efficacy and safety of PACs is being reexamined, minimally invasive and non-invasive technologies for periodic and continuous bedside hemodynamic monitoring are emerging and need to be similarly evaluated. Several devices use proprietary algorithms to assess the arterial pressure waveform from an arterial catheter to estimate stroke volume and cardiac output. To date, small trials have evaluated these technologies and reported a reduction in hypotensive episodes. However, these studies poorly represent patients commonly seen in the CICU and have not captured major clinical outcomes. As well, the accuracy of some may be insufficient, as exemplified in one study showing a ~60% discrepancy in the cardiac index when compared with 3-dimensional echocardiography.18 If validated in the CICU patient population, such devices not only offer the advantage of being non-invasive, but also the potential for remote monitoring. Non-invasive devices could facilitate “hub-and-spoke” integrated care models by allowing providers at referral centers to provide real-time guidance and improve triage. Given increasing mobility due to miniaturization, point-of-care ultrasonography itself has become nearly ubiquitous and indispensable in the modern ICU.19 Quantitative methods can be used to assess cardiac output and filling pressures; although the applicability to critically ill patients is less clear.20 While promising, non-invasive devices and strategies for hemodynamic monitoring require further study and validation in patients with cardiovascular dysfunction before any should enter widespread use in the CICU.
Improved phenotyping of patients with cardiac critical illness
Both the availability of more granular epidemiological data from ongoing registries as well as sobering neutral results from clinical trials in CCC have brought into sharper focus the profound heterogeneity of the major acute syndromes presenting to the CICU, including cardiogenic shock and cardiac arrest. These conditions carry unacceptably high mortality, with little improvement in outcomes over the last several decades.21 One possible reason for the lack of progress is our incomplete understanding of these complex disease phenotypes, including patient-specific factors such as frailty which may influence response to certain therapies. Improved disease phenotyping and “sub-phenotyping” present a hope to evolve toward a precision medicine approach in CCC. Given the vast amount of clinical information generated and collected for each patient, the CICU is particularly well positioned to develop such strategies integrating multiple streams of data. However, there is a need for improved interoperability among electronic medical records (EMR) and incorporation of device generated data directly into the EMR to spur this forward. Although in a nascent phase, advanced machine learning approaches may also accelerate such efforts.22 Application of artificial intelligence (AI) and machine learning algorithms to the data rich CICU holds intriguing promise. AI offers the potential to incorporate the enormous amount of complex clinical, non-invasive, and invasive data to predict decompensation, aid in diagnosis, and improve in decision making.23 As an example, using machine learning techniques from two international cohorts of patients with cardiogenic shock, a recent study identified 3 distinct phenotypes, including non-congested, cardiorenal, and cardiometabolic clusters, based on routine information at the time of admission.24 Although previous results have been mixed, recent advances in proteomic analyses may also improve risk prediction in patients with cardiogenic shock beyond commonly used risk scores. Applying a comprehensive quantitative proteomics approach, a recent study found that 4 circulating proteins measured during the first twenty-four hours significantly improved the performance of previously validated cardiogenic shock risk scores.25 Such approaches may identify unique clinical and laboratory characteristics that could lead to more granular phenotyping with the prospect of improving treatment paradigms.
Advancing medical and device management
Acute cardiovascular critical care consumes a substantial proportion of healthcare resources and often does so disproportionately at the end-stages of disease. However, many of the therapies used have not been adequately studied in well-controlled clinical trials. Despite the use of advanced, and often invasive, CICU monitoring and therapies, outcomes for many conditions remain poor. Moreover, with the increased medical complexity of the CICU population, investigation of the medical and device management of non-cardiac disease (e.g., mechanical ventilation and renal replacement therapy) in critically ill cardiac patients has taken on a new importance (Table 1).
In particular, the use of MCS devices for cardiogenic shock lacks high quality evidence demonstrating clinical benefit.26 A single adequately sized RCT demonstrated no benefit of IABP vs. medical therapy in patients with acute myocardial infarction-cardiogenic shock.27 The largest trial (n=48) comparing IABPs to percutaneous left ventricular assist devices was underpowered and enrolled a population at high risk for futility, with >90% of participants having had a cardiac arrest.28 A recent RCT (n=122) of extracorporeal membrane oxygenation for refractory (SCAI stage D or E) shock showed no difference in survival but was designed with an optimistic estimate of possible effect size.29 Several clinical trials in acute myocardial infarction-cardiogenic shock evaluating extracorporeal life support (ECLS-SHOCK, NCT03637205) or other percutaneous ventricular assist devices (DanGerShock, NCT01633502) will provide important data. While a single trial is actively enrolling (Altshock-2, NCT04369573), no completed RCTs have evaluated MCS for cardiogenic shock due to decompensated heart failure, despite growing evidence that it is a distinct entity which may respond differently to certain therapies (e.g., “super-responders” to IABP therapy).30
These important trials draw focus to 4 major aspects of the need for research to advance medical and device management of patients in the CICU. First, glaring gaps in evidence remain for the most fundamental therapies used in CCC. Second, there is a need for commitment of adequate public, philanthropic, and industry resources to support the conduct of clinical trials sized to confidently detect effect sizes that are clinically relevant. Third, the identification of appropriate populations for clinical trials is complicated and will likely rely on more careful phenotyping than in the past to enroll populations that are generalizable to most patients encountered in usual clinical CICU practice. Fourth, delivery of therapies in the CICU are often part of overarching strategies that may involve algorithms for escalation, titration, de-escalation and monitoring for complications and that may influence the efficacy and safety of specific devices and interventions. Such strategies also require prospective comparative effectiveness research.
Barriers to Critical Care Cardiology Research
Critical care research is challenging, facing questions around equipoise, issues regarding informed consent, heterogeneity of many of the clinical syndromes, sometimes scarce patient populations, narrow inclusion criteria, difficulties with recruitment, and often limited resources.31–34 These barriers are particularly relevant to clinical research in the CICU (Central Illustration).
Central Illustration. Barriers and potential solutions to research in critical care cardiology.
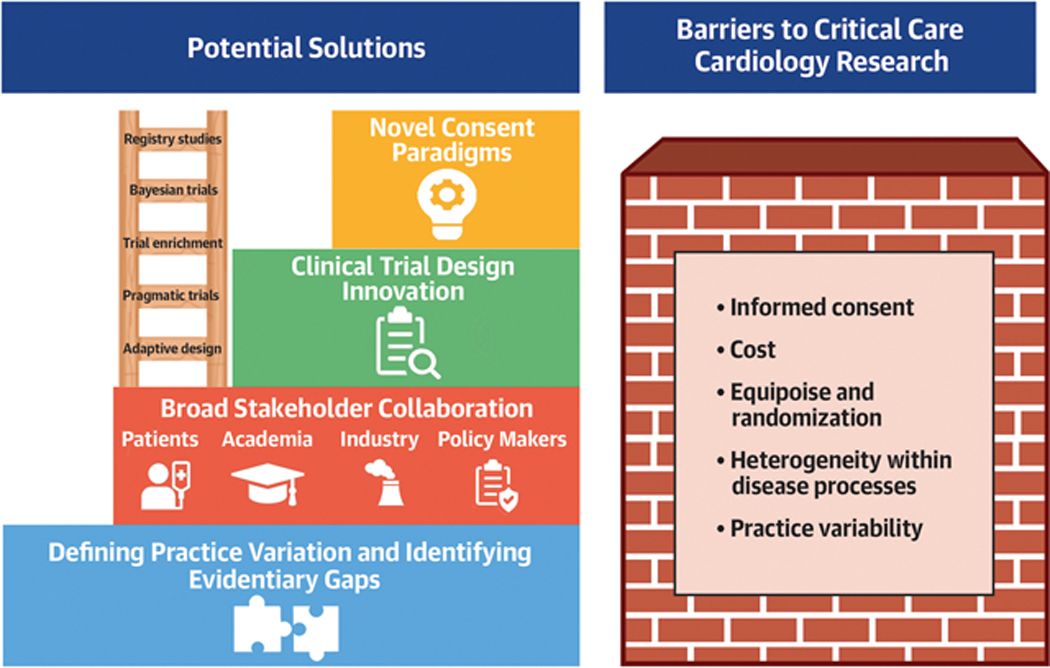
Barriers to critical care cardiology research are listed on the right and potential solutions on the left. Defining variation both within disease processes and practice variation is a key first step on which to build with broad stakeholder collaboration and trial design innovation.
The substantial variability in usual care for the most common CICU diagnoses is partly driven by differences in organizational structure and delivery of care across health systems, by region, and country.10,35 Such variability complicates the design of comparative effectiveness studies. Up to one-third of critical care comparative effectiveness trials in widely read medical journals do not include a specified control arm or therapies typical of contemporary practices.33 Practice patterns have developed largely based on expert opinion and experiential observations, making questions around equipoise and randomization for providers very difficult, especially for critically ill patients for whom potentially life-saving interventions are viewed as essential. Differences in MCS device selection and differing societal guidelines are an example.36,37
Enormous heterogeneity exists in the etiology and severity of many acute cardiovascular disease processes. Cardiogenic shock can be precipitated by numerous causes (e.g., ischemia, myocarditis, heart failure, etc.) and includes a wide spectrum of end-organ consequences,38 thus, making it challenging to both identify patients who may benefit most from a therapy as well as individuals for whom the same therapy would be futile. Furthermore, because CCC diagnoses often require emergent interventions, time consuming processes for informed consent of the critically ill patient can be a significant barrier to recruitment. The informed consent process also differs substantially between countries. Patients presenting with acute cardiovascular disease often lack capacity and require surrogate consent, which may delay treatment and is associated with significant stress for families. Even when informed consent is obtained from a fully oriented ICU patient, studies have shown that the majority are unable to recall the purpose of the study, its risks, or benefits.39 In stark comparison to previous research for highly prevalent cardiac diseases, such as acute myocardial infarction, the lower number of eligible participants for trials addressing the most pressing questions in CCC leads to slow enrollment, limits industry-supported research and thus hampers the development of new therapeutic tools. Lastly, clinical trial costs have become prohibitive in many cases, and <5% of government funded ICU studies include cardiac-specific research.5
A Path Forward
As detailed, numerous challenges and unanswered questions remain for the field of CCC. Some of these challenges are inherently related to difficulties of prospective research in critically ill patients, while others may be specific to CCC (Central Illustration). To date, much of the clinical research in CCC has been descriptive or predictive, and while RCTs remain the gold standard of clinical research and must be pursued in CCC, RCTs with sufficient recruitment to provide definitive answers may not be possible for every remaining research priority. Real-world evidence, using sophisticated statistical methods to mitigate confounding, will be required to evaluate broad implementation of therapies emerging from RCTs. High-quality data from multicenter registries, leveraging efficient data collection from EMRs, provide opportunities to apply advanced statistical methods to improve casual inference when RCTs are not feasible.
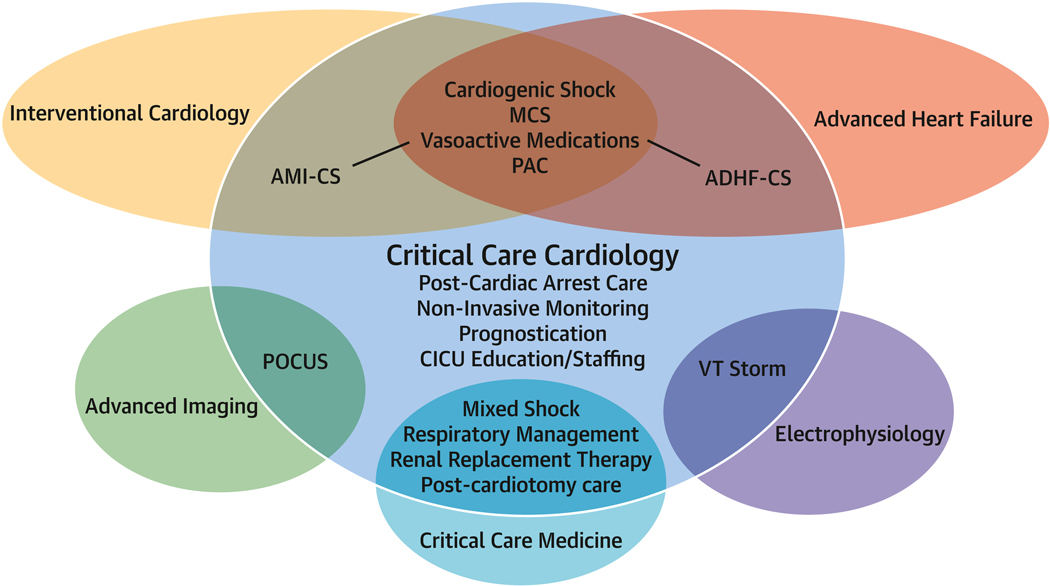
However, RCTs will be required to change practice and inform guidelines. Four important strategies will be necessary to conduct RCTs in CCC. First, broad partnership between medical professional societies, the CCC community, governmental and policy agencies, non-profit groups, and industry will be necessary to generate funding for such trials. Collaborations with industry have driven many of the major successes in modern cardiovascular medicine and are likely to be important in CCC with multistakeholder engagement to support rigorous and balanced assessment of safety and efficacy. Importantly, there is significant overlap of CCC research priorities with other fields, both within and outside of cardiology, and collaboration with these specialties will be essential to advance the field (Figure 1).
Figure 1. Key areas of research collaboration across specialties.
Venn diagraph showing the overlap between acute cardiovascular topics, various specialties, and critical care cardiology.
MCS = Mechanical circulatory support; AMI-CS = Acute myocardial infarction-cardiogenic shock; ADHF = Acute decompensated heart failure; POCUS = Point-of-care ultrasound; VT = Ventricular tachycardia
Second, cooperation that is international and across disciplines will be essential. Multicenter CCC registries are important examples of collaborations that are building the infrastructure for future clinical trials. Registry-based RCTs offer pragmatic approaches with the potential to lower cost, improve generalizability, and accelerate patient recruitment. However, care must be taken to ensure data quality, adherence to the protocolized comparative interventions, and reliability of event ascertainment. Lessons learned from the COVID-19 pandemic also highlight the importance of emerging methodologies for efficient and timely evidence generation. As an example, Randomised Evaluation of COVID-19 Therapy (RECOVERY) was a pragmatic trial that utilized an adaptive platform design, which allowed for multiple interventions to be studied on a single, shared infrastructure, with Bayesian sequential analysis for efficient conduct until efficacy or futility was reached.40
Third, a critical evaluation of informed consent in the setting of emergency research, with processes that have been more successful in Europe than the United States (US), is warranted.38 Implementation would be aided by promoting consistency in the interpretation and application of existing regulations. As well, CCC research may be aided by broad stakeholder involvement, including patients and regulators, to develop ethically sound, forward-thinking approaches to informed consent in the setting of emergency research.
Finally, the identification of appropriate populations for clinical trials is complicated and will likely rely on more careful phenotyping than in the past to enroll populations that are generalizable to most patients encountered in usual clinical CICU practice. Given the significant heterogeneity within many acute cardiovascular disease processes, advanced phenotyping through biomarkers, proteomics, and AI offers the potential for prognostic and predictive trial enrichment, which has been successfully applied within cardiology as well as acute care research such as sepsis.41 These methods also facilitate the possibility of moving beyond “syndromes” to identify shared pathophysiologic features within disease processes.42 The generalizability of the results of clinical trials must be artfully balanced with the inclusion of a population with potential for benefit from the intervention(s) under investigation accounting for the likelihood of futility for some patients. Use of objective criteria to identify patients with such high probability of medical futility, e.g. using SCAI shock stage E or comatose state after cardiac arrest as exclusion criteria for some shock trials, is a relevant concept in the design of future trials.29
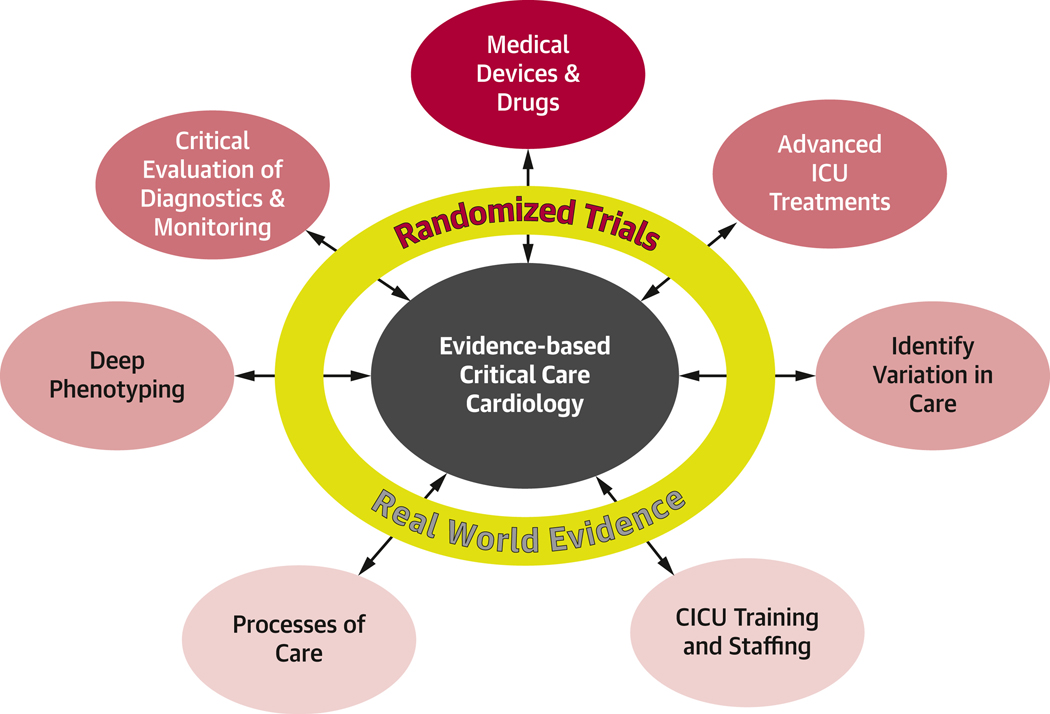
To accomplish these goals, the CCC community should be creative, nimble, and collaborative across disciplines to improve the evidence base needed to care for our patients of our sickest patients. (Figure 2). Understanding the epidemiology of CCC and variations in practice is foundational to identifying key areas of research. More comprehensive phenotyping will allow for more tailored patient selection for clinical trials and better targeting of therapies. Technological advances continue to expand monitoring and treatment options in CCC but require critical evaluation and thoughtful application. Engagement of all involved stakeholders is essential to advance the care of our sickest patients.
Figure 2. Evidence generation for critical care cardiology.
At the center, evidence based critical care cardiology will require both randomized and real-world data to accomplish each outer goal. Arrows represent the need for constant refinement and reevaluation as new data arises.
Highlights.
Evidence generation in the modern cardiac intensive care unit has not matched the evolution of the patient population.
Beyond difficulties of studying critically ill patients, there are unique barriers to research in critical care cardiology.
Overcoming these barriers will require novel research designs and collaboration among multiple stakeholders.
Disclosures:
Drs. Bohula, and Morrow are members of the TIMI Study Group, which has received institutional research grant support through Brigham and Women’s Hospital from Abbott Laboratories, Abiomed, Amgen, Anthos Therapeutics, Arca Biopharma, AstraZeneca, Daiichi-Sankyo, Intarcia, Janssen, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Regeneron, Roche, Siemens, and Zora Biosciences. Dr. Morrow has received consulting fees from Abbott Laboratories, Arca Biopharma, InCarda, Inflammatix, Merck, Novartis, and Roche Diagnostics. Dr. Solomon receives research support from the National Institutes of Health Clinical Center intramural research funds. Dr. Krychtiuk receives speaker fees from Zoll Medical, Sanofi and Daiichi-Sankyo. Dr.Kristensen is National Coordinator of the SOS-AMI study (Idorsia departmental grant). Dr. Pöss receives institutional research fees from German Cardiac Society, German Heart Research Foundation, Dr. Rolf M. Schwiete Foundation and Getinge.
Abbreviations
- CICU
Cardiac intensive care unit
- CCC
Critical care cardiology
- MCS
Mechanical circulatory support
- IABP
Intra-aortic balloon pumps
- PAC
Pulmonary artery catheter
- RCT
Randomized controlled trial
- AI
Artificial intelligence
- EMR
Electronic medical record
Footnotes
The remaining authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sinha SS, Sjoding MW, Sukul D et al. Changes in Primary Noncardiac Diagnoses Over Time Among Elderly Cardiac Intensive Care Unit Patients in the United States. Circ Cardiovasc Qual Outcomes 2017;10:e003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casella G, Cassin M, Chiarella F et al. Epidemiology and patterns of care of patients admitted to Italian Intensive Cardiac Care units: the BLITZ-3 registry. J Cardiovasc Med (Hagerstown) 2010;11:450–61. [DOI] [PubMed] [Google Scholar]
- 3.Miller PE, Kenigsberg BB, Wiley BM. Cardiac Critical Care: Training Pathways and Transition to Early Career. J Am Coll Cardiol 2019;73:1726–1730. [DOI] [PubMed] [Google Scholar]
- 4.Czerwinska-Jelonkiewicz K, Montero S, Baneras J et al. Current status and needs for changes in critical care training: the voice of the young cardiologists. Eur Heart J Acute Cardiovasc Care 2021;10:94–101. [DOI] [PubMed] [Google Scholar]
- 5.van Diepen S, Granger CB, Jacka M, Gilchrist IC, Morrow DA, Katz JN. The unmet need for addressing cardiac issues in intensive care research. Crit Care Med 2015;43:128–34. [DOI] [PubMed] [Google Scholar]
- 6.Metkus TS, Miller PE, Alviar CL et al. Advanced respiratory support in the contemporary cardiac ICU. Crit Care Explor 2020;2:e0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg DD, Barnett CF, Kenigsberg BB et al. Clinical Practice Patterns in Temporary Mechanical Circulatory Support for Shock in the Critical Care Cardiology Trials Network (CCCTN) Registry. Circ Heart Fail 2019;12:e006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schenck C, Banna S, Heck C, Ali T, Miller PE. Rocuronium Versus Succinylcholine in Patients With Acute Myocardial Infarction Requiring Mechanical Ventilation. J Am Heart Assoc 2023;12:e8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagundes A Jr, Berg DD, Park JG et al. Patients With Acute Coronary Syndromes Admitted to Contemporary Cardiac Intensive Care Units: Insights From the CCCTN Registry. Circ Cardiovasc Qual Outcomes 2022;15:e008652. [DOI] [PubMed] [Google Scholar]
- 10.Quien M, Thomas A, Ludmir J, Miller PE. Staffing models in the cardiac intensive care unit. Curr Opin Crit Care 2022;28:453–459. [DOI] [PubMed] [Google Scholar]
- 11.Backhaus T, Fach A, Schmucker J et al. Management and predictors of outcome in unselected patients with cardiogenic shock complicating acute ST-segment elevation myocardial infarction: results from the Bremen STEMI Registry. Clin Res Cardiol 2018;107:371–379. [DOI] [PubMed] [Google Scholar]
- 12.Rathod KS, Koganti S, Iqbal MB et al. Contemporary trends in cardiogenic shock: Incidence, intra-aortic balloon pump utilisation and outcomes from the London Heart Attack Group. Eur Heart J Acute Cardiovasc Care 2018;7:16–27. [DOI] [PubMed] [Google Scholar]
- 13.van Diepen S, Tymchak W, Bohula EA et al. Incidence, underlying conditions, and outcomes of patients receiving acute renal replacement therapies in tertiary cardiac intensive care units: An analysis from the Critical Care Cardiology Trials Network Registry. Am Heart J 2020;222:8–14. [DOI] [PubMed] [Google Scholar]
- 14.Kadosh BS, Berg DD, Bohula EA et al. Pulmonary Artery Catheter Use and Mortality in the Cardiac Intensive Care Unit. JACC Heart Fail 2023. [Google Scholar]
- 15.Saxena A, Garan AR, Kapur NK et al. Value of Hemodynamic Monitoring in Patients With Cardiogenic Shock Undergoing Mechanical Circulatory Support. Circulation 2020;141:1184–1197. [DOI] [PubMed] [Google Scholar]
- 16.Garan AR, Kanwar M, Thayer KL et al. Complete Hemodynamic Profiling With Pulmonary Artery Catheters in Cardiogenic Shock Is Associated With Lower In-Hospital Mortality. JACC Heart Fail 2020;8:903–913. [DOI] [PubMed] [Google Scholar]
- 17.Kanwar MK, Blumer V, Zhang Y et al. Pulmonary Artery Catheter Use and Risk of In-hospital Death in Heart Failure Cardiogenic Shock. J Card Fail 2023. [DOI] [PubMed] [Google Scholar]
- 18.Hattori K, Maeda T, Masubuchi T et al. Accuracy and Trending Ability of the Fourth-Generation FloTrac/Vigileo System in Patients With Low Cardiac Index. J Cardiothorac Vasc Anesth 2017;31:99–104. [DOI] [PubMed] [Google Scholar]
- 19.Chamsi-Pasha MA, Sengupta PP, Zoghbi WA. Handheld Echocardiography: Current State and Future Perspectives. Circulation 2017;136:2178–2188. [DOI] [PubMed] [Google Scholar]
- 20.Tavazzi G, Spiegel R, Rola P, Price S, Corradi F, Hockstein M. Multi-organ Evaluation of Perfusion and Congestion Using Ultrasound in Patients with Shock. Eur Heart J Acute Cardiovasc Care 2023. [DOI] [PubMed] [Google Scholar]
- 21.van Diepen S, Katz JN, Albert NM et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2017;136:e232–e268. [DOI] [PubMed] [Google Scholar]
- 22.Jentzer J, Rayfield C, Soussi S et al. Machine learning approaches for phenotyping in cardiogenic shock and critical illness. JACC: Adv 2022;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon JH, Pinsky MR, Clermont G. Artificial Intelligence in Critical Care Medicine. Crit Care 2022;26:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zweck E, Thayer KL, Helgestad OKL et al. Phenotyping Cardiogenic Shock. J Am Heart Assoc 2021;10:e020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rueda F, Borras E, Garcia-Garcia C et al. Protein-based cardiogenic shock patient classifier. Eur Heart J 2019;40:2684–2694. [DOI] [PubMed] [Google Scholar]
- 26.Miller PE, Bromfield SG, Ma Q et al. Clinical Outcomes and Cost Associated With an Intravascular Microaxial Left Ventricular Assist Device vs Intra-aortic Balloon Pump in Patients Presenting With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA Intern Med 2022;182:926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiele H, Zeymer U, Neumann FJ et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–96. [DOI] [PubMed] [Google Scholar]
- 28.Ouweneel DM, Eriksen E, Sjauw KD et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol 2017;69:278–287. [DOI] [PubMed] [Google Scholar]
- 29.Ostadal P, Rokyta R, Karasek J et al. Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock: Results of the ECMO-CS Randomized Clinical Trial. Circulation 2023;147:454–464. [DOI] [PubMed] [Google Scholar]
- 30.Fried JA, Nair A, Takeda K et al. Clinical and hemodynamic effects of intra-aortic balloon pump therapy in chronic heart failure patients with cardiogenic shock. J Heart Lung Transplant 2018;37:1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pattison N, Arulkumaran N, Humphreys S, Walsh T. Exploring obstacles to critical care trials in the UK: A qualitative investigation. J Intensive Care Soc 2017;18:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Granholm A, Alhazzani W, Derde LPG et al. Randomised clinical trials in critical care: past, present and future. Intensive Care Med 2022;48:164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Applefeld WN, Wang J, Klein HG, Danner RL, Eichacker PQ, Natanson C. Comparative effectiveness research in critically ill patients: risks associated with mischaracterising usual care. Crit Care Resusc 2020;22:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller PE, van Diepen S, Ahmad T . Acute Decompensated Heart Failure Complicated by Respiratory Failure. Circ Heart Fail 2019;12:e006013. [DOI] [PubMed] [Google Scholar]
- 35.Claeys MJ, Roubille F, Casella G et al. Organization of intensive cardiac care units in Europe: Results of a multinational survey. Eur Heart J Acute Cardiovasc Care 2020;9:993–1001. [DOI] [PubMed] [Google Scholar]
- 36.Ibanez B, James S, Agewall S et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 37.O’Gara PT, Kushner FG, Ascheim DD et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:e78–e140. [DOI] [PubMed] [Google Scholar]
- 38.Samsky MD, Krucoff MW, Morrow DA et al. Cardiac safety research consortium “shock II” think tank report: Advancing practical approaches to generating evidence for the treatment of cardiogenic shock. Am Heart J 2020;230:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chenaud C, Merlani P, Luyasu S, Ricou B. Informed consent for research obtained during the intensive care unit stay. Crit Care 2006;10:R170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casey JD, Beskow LM, Brown J et al. Use of pragmatic and explanatory trial designs in acute care research: lessons from COVID-19. Lancet Respir Med 2022;10:700–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanski NL, Wong HR. Prognostic and predictive enrichment in sepsis. Nat Rev Nephrol 2020;16:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maslove DM, Tang B, Shankar-Hari M et al. Redefining critical illness. Nat Med 2022;28:1141–1148. [DOI] [PubMed] [Google Scholar]