Abstract
BACKGROUND:
Among patients treated with statin therapy to guideline-recommended cholesterol levels, residual inflammatory risk assessed by high-sensitivity C-reactive protein (hsCRP) is at least as strong a predictor of future cardiovascular events as is residual risk assessed by low-density lipoprotein cholesterol (LDLC). Whether these relationships are present among statin-intolerant patients with higher LDLC levels is uncertain but has implications for the choice of preventive therapies, including bempedoic acid, an agent that reduces both LDLC and hsCRP.
METHODS:
The multinational CLEAR-Outcomes trial (Cholesterol Lowering via Bempedoic Acid, an ACL-Inhibiting Regimen Outcomes Trial) randomly allocated 13 970 statin-intolerant patients to 180 mg of oral bempedoic acid daily or matching placebo and followed them for a 4-component composite of incident myocardial infarction, stroke, coronary revascularization, or cardiovascular death, and for all-cause mortality. Quartiles of increasing baseline hsCRP and LDLC were assessed as predictors of future adverse events after adjustment for traditional risk factors and randomized treatment assignment.
RESULTS:
Compared with placebo, bempedoic acid reduced median hsCRP by 21.6% and mean LDLC levels by 21.1% at 6 months. Baseline hsCRP was significantly associated with the primary composite end point of major cardiovascular events (highest versus lowest hsCRP quartile; hazard ratio [HR], 1.43 [95% CI, 1.24–1.65]), cardiovascular mortality (HR, 2.00 [95% CI, 1.53–2.61]), and all-cause mortality (HR, 2.21 [95% CI, 1.79–2.73]). By contrast, the relationship of baseline LDLC quartile (highest versus lowest) to future events was smaller in magnitude for the primary composite cardiovascular end point (HR, 1.19 [95% CI, 1.04–1.37]) and neutral for cardiovascular mortality (HR, 0.90 [95% CI, 0.70–1.17]) and all-cause mortality (HR, 0.95 [95% CI, 0.78–1.16]). Risks were high for those with elevated hsCRP irrespective of LDLC level. Bempedoic acid demonstrated similar efficacy in reducing cardiovascular events across all levels of hsCRP and LDLC.
CONCLUSIONS:
Among contemporary statin-intolerant patients, inflammation assessed by hsCRP predicted risk for future cardiovascular events and death more strongly than hyperlipidemia assessed by LDLC. Compared with placebo, bempedoic acid had similar efficacy for reducing cardiovascular risk across hsCRP and LDLC strata.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02993406.
Keywords: atherosclerosis, cholesterol, clinical trials as topic, C-reactive protein, inflammation
Clinical Perspective.
What Is New?
Among 13 970 contemporary statin-intolerant patients with or at high risk of atherosclerosis, inflammation detected by high-sensitivity C-reactive protein was at least as strong a predictor of cardiovascular events, cardiovascular death, and all-cause mortality as was hyperlipidemia detected by either low-density lipoprotein cholesterol or non–high-density lipoprotein cholesterol.
What Are the Clinical Implications?
Vascular inflammation is a major determinant of atherosclerotic risk among statin-intolerant patients in a manner identical to that observed in statin-tolerant patients. These data suggest that targeting low-density lipoprotein cholesterol alone is unlikely to completely reduce atherosclerotic risk and that inflammatory pathways have yet to be fully exploited for patient benefit.
In primary prevention studies initiated 30 years ago, inflammation (as detected by high-sensitivity C-reactive protein [hsCRP]) and hyperlipidemia (as detected by low-density lipoprotein cholesterol [LDLC]) predicted future cardiovascular events with similar magnitude.1,2 Those initial epidemiological data, crucial to the development of the inflammation hypothesis of atherothrombosis, eventually led to clinical trials demonstrating that anti-inflammatory agents such as canakinumab3 and low-dose colchicine4,5 can substantially reduce cardiovascular event rates without changing LDLC.
With increasingly effective therapies for cholesterol reduction in wide use, inflammation has emerged as an important source of residual cardiovascular risk. In a recent analysis of 31 245 contemporary patients with atherosclerosis receiving guideline-directed medical care, including statin therapy, hsCRP was a stronger predictor of future vascular risk than LDLC, particularly for cardiovascular death.6 These contemporary data have potential implications for the selection of adjunctive therapies to lower cardiovascular risk because they suggest that anti-inflammatory treatments have yet to be fully exploited for clinical care and that focusing on LDLC reduction alone will unlikely eliminate all vascular risk. This issue has taken on greater relevance because low-dose colchicine has become the first anti-inflammatory therapy to be approved by the US Food and Drug Administration for use as an adjunctive therapy to reduce the risk of recurrent myocardial infarction, stroke, coronary revascularization, and cardiovascular death.7,8
Statins lower both hsCRP and LDLC. Thus, to address the relative effect of inflammation and hyperlipidemia as predictors of risk while avoiding the potential for therapeutic confounding, data are required among contemporary patients not taking statins. Completion of the recent CLEAR-Outcomes trial (Cholesterol Lowering via Bempedoic Acid, an ACL-Inhibiting Regimen Outcomes Trial)9 comparing bempedoic acid with placebo among statin-intolerant patients affords an opportunity to address this issue.
METHODS
Study Population
In the multinational CLEAR-Outcomes trial, 13 970 statin-intolerant patients with LDLC >100 mg/dL randomly received 180 mg of oral bempedoic acid or matching placebo between December 2016 and August 2019. The trial protocol was approved by an institutional review committee and all participants provided written informed consent. Consistent with policies set in the primary CLEAR-Outcomes report,9 the data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results. Participants were followed for incident myocardial infarction, stroke, coronary revascularization, cardiovascular death, and all-cause mortality during a median period of 40.6 months (maximal follow-up 5 years). The CLEAR-Outcomes trial was conducted at 1250 sites in 32 countries. As reported in detail elsewhere,9 eligible participants either had a previous cardiovascular event (secondary prevention cohort, n=9764) or were at high risk for cardiovascular events (primary prevention cohort, n=4206) and reported being unable or unwilling to take statin therapy owing to an adverse effect that had started or increased during statin therapy and resolved or improved after statin discontinuation. The trial used a 4-week single-blind placebo run-in to eliminate individuals intolerant of placebo and to increase long-term compliance with trial medications.
Statistical Analysis
To provide an unbiased comparison, the baseline distributions of hsCRP and LDLC among statin-intolerant patients were initially divided into quartiles. Then, for increasing quartiles of each biomarker, we calculated hazard ratios, 95% confidence intervals, and P values for the occurrence of the primary 4-component cardiovascular end point, and for the end points of cardiovascular mortality, and all-cause mortality, as well. Proportional hazard models were used to estimate the adjusted relative hazard for each end point among subjects in each of the 3 higher quartiles, relative to those in the lowest quartile. Following the prespecified CLEAR-Outcomes statistical analysis plan, adjustment was made on an a priori basis for age, sex, ethnicity, region, diabetes, body mass index, estimated glomerular filtration rate, blood pressure, alcohol use, smoking status, known atherosclerotic disease, and randomized treatment assignment. Proportional hazard assumptions were tested.
This analysis was repeated across 4 predefined risk groups, dividing the cohort on an a priori basis into 4 groups on the basis of median baseline hsCRP and LDLC values: those with hsCRP <2.3 mg/L and LDLC <135 mg/dL (the referent group); those with hsCRP <2.3 mg/L and LDLC ≥13 5 mg/dL; those with hsCRP ≥2.3 mg/L and LDLC <135 mg/dL; and those with hsCRP ≥2.3 mg/L and LDLC ≥135 mg/dL. For ease of clinical interpretation and to address generalizability, these joint-effects analyses were repeated using the clinical thresholds of < or ≥2.0 mg/L for baseline hsCRP and < or ≥130 mg/dL for baseline LDLC, values close to the trial medians.
The relative benefit of bempedoic acid compared with placebo for the primary 4-component cardiovascular outcome was assessed in subgroups of baseline hsCRP (< or ≥2 mg/L), baseline LDLC (< or ≥130 mg/dL), and the 4 risk groups defined above.
In sensitivity analyses, we used non–high-density lipoprotein cholesterol as an alternative to LDLC, and repeated all analyses, removing patients who were allowed to be enrolled in the CLEAR-Outcomes trial while receiving a very low average daily statin dose (lower than the lowest approved dose). On a post hoc basis, additional analyses were performed that censored trial participants 30 days after receiving any nontrial lipid-lowering therapy.
All primary analyses were performed in the intent-to-treat analysis set, including all randomly assigned patients. All P values are 2-sided with significance and confidence intervals computed at the 0.05 level. Multiplicity was not adjusted. The first and last author had full access to and vouch for the validity of the trial data and the statistical analyses.
Role of the Funding Sources
The CLEAR-Outcomes trial was conducted by Esperion Therapeutics in collaboration with the Cleveland Clinic Coordinating Center for Clinical Research (C5Research) and an academic executive committee. The first and last author designed the analysis plan for this article, which was then conducted by the CLEAR-Outcomes trial biostatistician (LL), who is an employee of Esperion, Inc. The analyses were then independently verified by statisticians at C5Reseach. The manuscript was written by the first author, reviewed by all authors, and the decision to publish was made by the first and last authors independent of the trial sponsor.
RESULTS
Baseline clinical characteristics of the 13 970 statin-intolerant participants in CLEAR-Outcomes have been previously reported in detail.9 In brief, the mean age was 65.5 years, 48.2% had diabetes, 69.9% had a previous cardiovascular event, and the mean body mass index was 29.9 kg/m2. At trial entry, median hsCRP was 2.30 mg/L (interquartile range, 1.15–4.47), and median LDLC was 134.5 mg/dL (interquartile range, 115.0–158.5).
Over the median follow-up period of 40.6 months, 1746 primary 4-component cardiovascular end points accrued. Of 854 deaths that occurred during trial follow-up, 526 were adjudicated as cardiovascular deaths.
Relationships of Inflammation and Cholesterol to Future Cardiovascular Events and Mortality Among Statin-Intolerant Patients
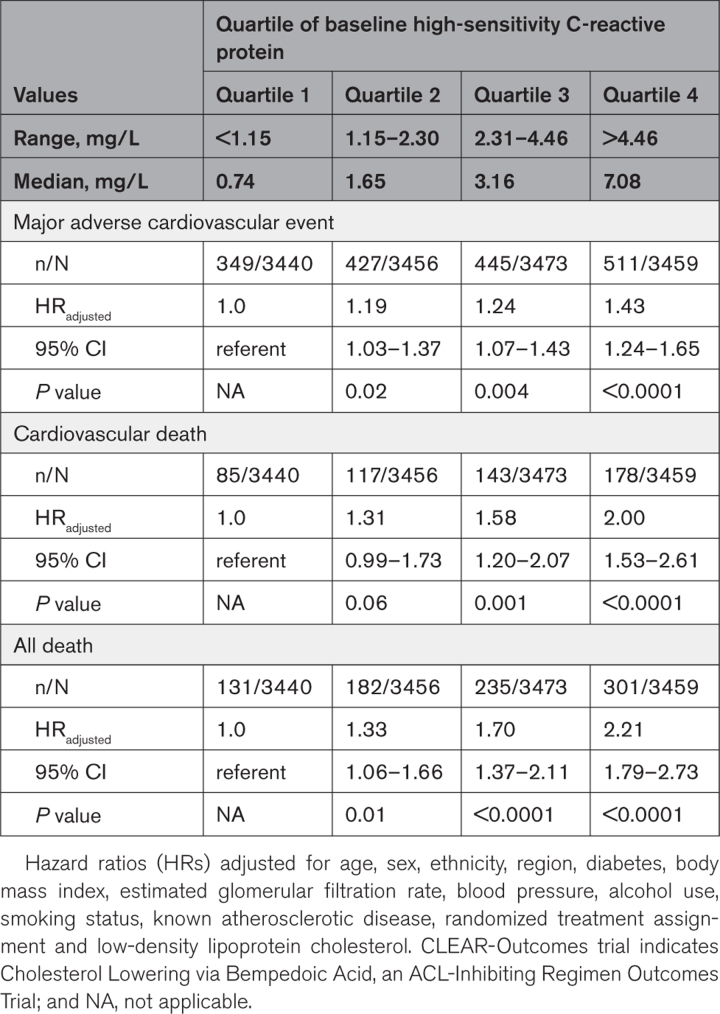
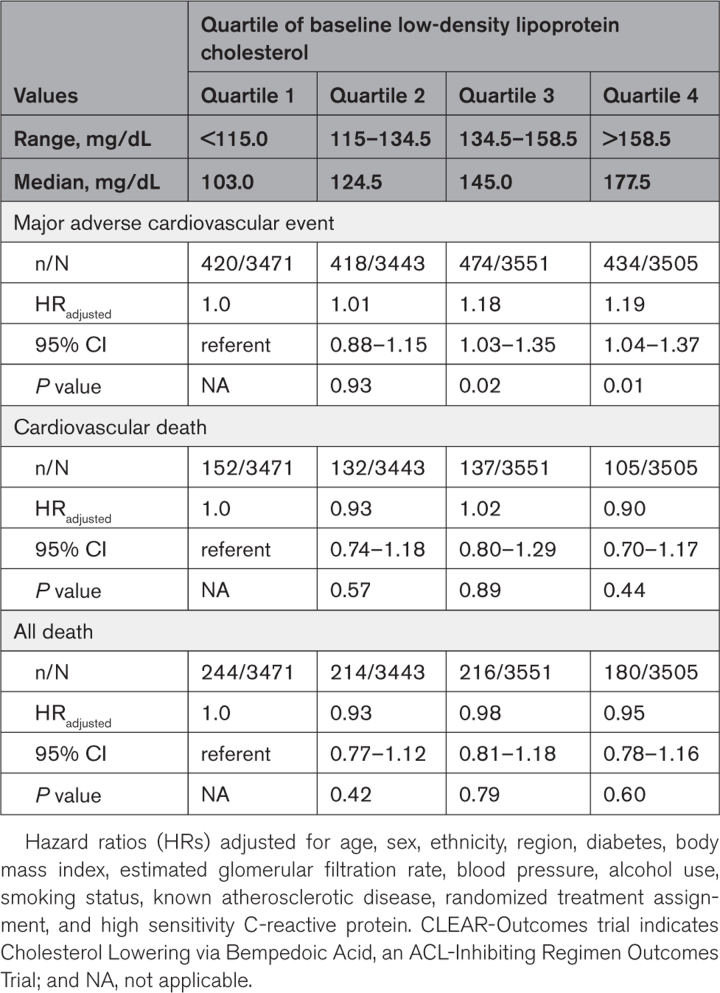
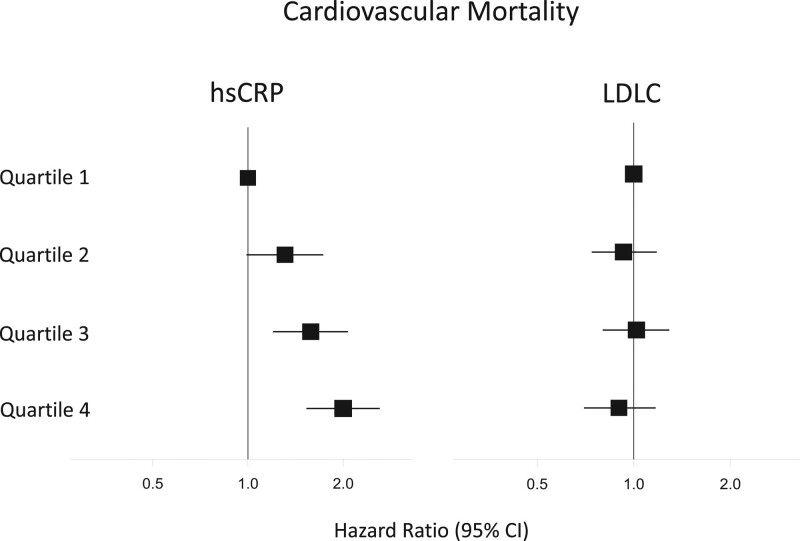
Baseline hsCRP was significantly associated with the primary composite end point of future myocardial infarction, stroke, coronary revascularization, and cardiovascular death (highest versus lowest hsCRP quartile, adjusted hazard ratio [HR], 1.43 [95% CI, 1.24–1.65]; P<0.0001), and the end points of cardiovascular mortality (adjusted HR, 2.00 [95% CI, 1.53–2.61]; P<0.0001) and all-cause mortality (adjusted HR, 2.21 [95% CI, 1.79–2.73]; P<0.0001; Table 1). By contrast, the relationship of baseline LDLC quartile (highest versus lowest) to future cardiovascular events was of smaller magnitude for the primary composite end point (adjusted HR, 1.19 [95% CI, 1.04–1.37]; P=0.01) and neutral for cardiovascular mortality (adjusted HR, 0.90 [95% CI, 0.70–1.17]; P=0.44) and all-cause mortality (adjusted HR, 0.95 [95% CI, 0.78–1.16]; P=0.60; Table 2). Figure 1 presents a comparison of increasing quartiles of hsCRP (left) to increasing quartiles of LDLC (right) for the end point of cardiovascular mortality.
Table 1.
Predictive Value of Baseline High-Sensitivity C-Reactive Protein for Incident Major Adverse Cardiovascular Events, Cardiovascular Death, and All-Cause Mortality in the CLEAR-Outcomes Trial
Table 2.
Predictive Value of Baseline, Low-Density Lipoprotein Cholesterol for Incident Major Adverse Cardiovascular Events, Cardiovascular Death, and All-Cause Mortality in the CLEAR-Outcomes Trial
Figure 1.
Relative impact of inflammation and cholesterol as independent determinants of risk for cardiovascular death. Increasing quartiles of inflammatory risk (as assessed by hsCRP; left) and increasing quartiles of cholesterol risk (as assessed by LDLC; right) as predictors of cardiovascular death among 13 970 statin-intolerant patients. Hazard ratios and 95% CIs adjusted for age, sex, ethnicity, region, diabetes, body mass index, estimated glomerular filtration rate, blood pressure, alcohol use, smoking status, known atherosclerotic disease, and randomized treatment assignment. hsCRP indicates high-sensitivity C-reactive protein; and LDLC, low-density lipoprotein cholesterol.
Joint Effects of Inflammation and Cholesterol on Risk
As anticipated, risks for the primary composite end point, cardiovascular mortality, and all-cause mortality were all significantly higher for individuals with hsCRP and LDLC values above the trial median baseline levels compared with individuals with hsCRP and LDLC below the trial baseline median levels (all P values <0.001; Table S1). However, risks for the primary composite end point, cardiovascular mortality, and all-cause mortality were all significantly higher for those with above-median compared with below-median hsCRP, irrespective of LDLC strata (all P values ≤0.001). These effects were particularly evident for cardiovascular death and all-cause mortality where individuals with below median hsCRP had no evidence of increased risk when LDLC was above median, yet risk was almost as high for those with above median hsCRP and below median LDLC as among those with elevated levels of both biomarkers.
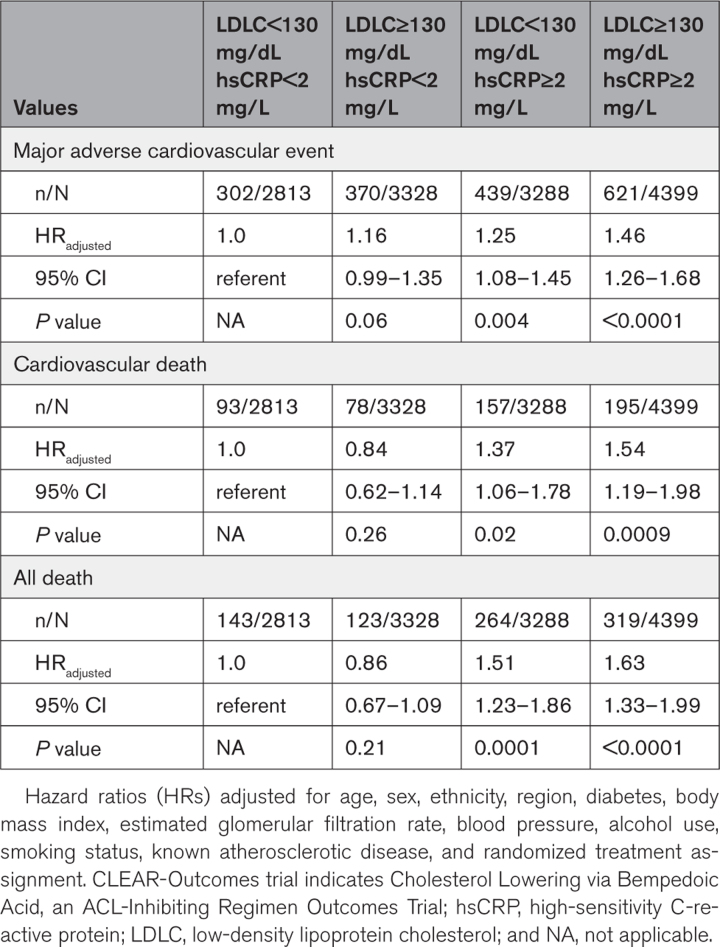
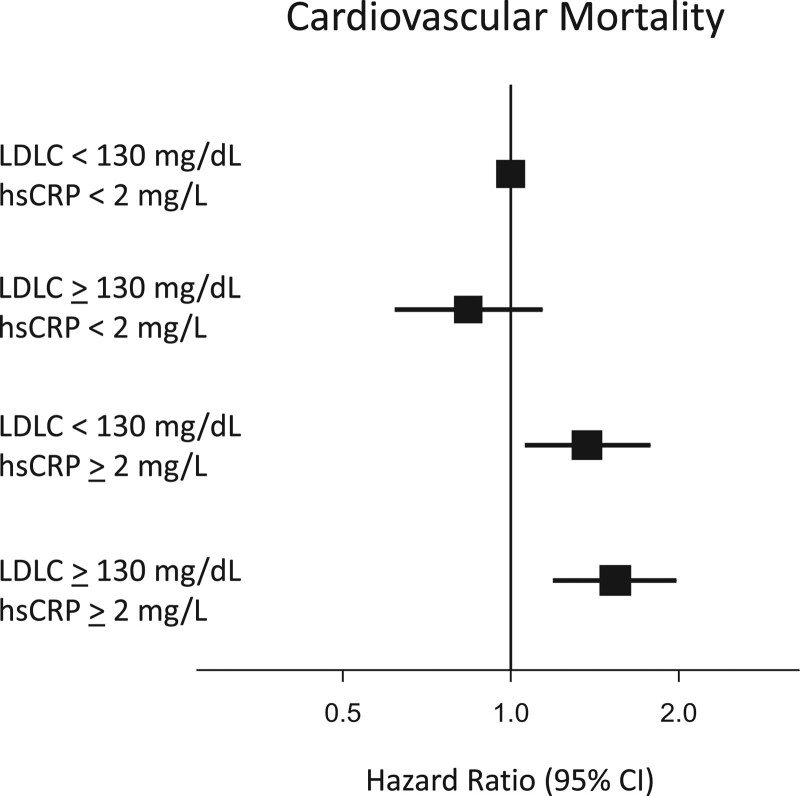
For ease of clinical interpretation, Table 3 presents comparable data using the clinical cut points of < or ≥2 mg/L for hsCRP and < or ≥130 mg/dL for LDLC and Figure 2 shows comparable data for the end point of cardiovascular mortality.
Table 3.
Joint Analyses of Baseline hsCRP (< or ≥2 mg/L) and Baseline LDLC (< or ≥130 mg/dL) as Predictors for Incident Major Adverse Cardiovascular Events, Cardiovascular Death, and All-Cause Mortality in the CLEAR-Outcomes Trial
Figure 2.
Inflammation determines risk of cardiovascular death at both high and low levels of LDLC. Joint analysis of hsCRP (≥ or <2 mg/L) and LDLC (≥ or <130 mg/dL) as predictors of cardiovascular death among 13 970 statin-intolerant patients. Hazard ratios and 95% CIs adjusted for age, sex, ethnicity, region, diabetes, body mass index, estimated glomerular filtration rate, blood pressure, alcohol use, smoking status, known atherosclerotic disease, and randomized treatment assignment. hsCRP indicates high-sensitivity C-reactive protein; and LDLC, low-density lipoprotein cholesterol.
Effects of Bempedoic Acid Compared With Placebo Across hsCRP and LDLC Strata
Compared with placebo among these statin-intolerant patients, bempedoic acid reduced median hsCRP by 21.6% and mean LDLC levels by 21.1% at 6 months.
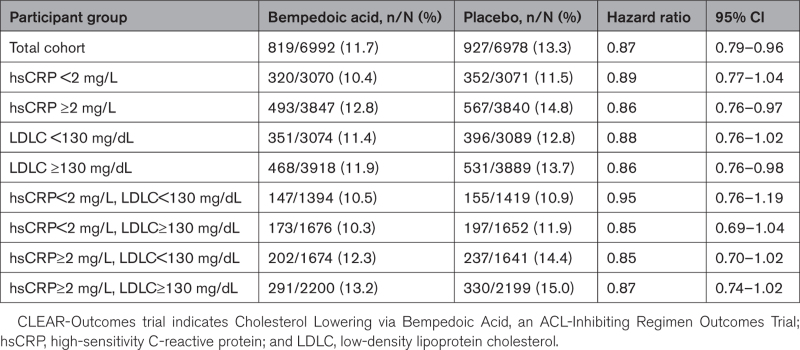
In the overall CLEAR-Outcomes trial, bempedoic acid compared with placebo reduced the primary trial composite end point by 13% (HR, 0.87 [95% CI, 0.79–0.96]). As shown in Table 4, the relative efficacy of bempedoic acid compared with placebo was of similar magnitude across all baseline subgroups defined by either hsCRP or LDLC.
Table 4.
Efficacy of Bempedoic Acid Compared With Placebo for the Primary Major Adverse Cardiovascular Events End Point of the CLEAR-Outcomes Trial for the Total Trial Cohort and Stratified by Baseline hsCRP and LDLC Levels
Sensitivity Analyses
Within the CLEAR-Outcomes trial, 22.3% of participants were receiving a very low average daily statin dose (lower than the lowest approved dose). None of the above analysis changed in any substantive manner when these individuals were excluded from analysis. Likewise, no substantive differences in outcomes were observed in sensitivity analyses on the basis of non–high-density lipoprotein cholesterol rather than LDLC. An additional sensitivity analysis that censored patient data 30 days after receiving any nontrial lipid-lowering therapy revealed a nearly identical primary trial composite end point (HR, 0.86 [95% CI, 0.77-0.94]) suggesting that any differential drop-in to adjunctive lipid-lowering therapy had little to no effect on trial outcomes. This is likely related to the fact that the great majority of primary composite end points (92.9% and 90.9% of events in the bempedoic acid and placebo groups, respectively) occurred before any nontrial adjunctive therapy was given.
DISCUSSION
Lowering LDLC with statin therapy is the most important pharmacological intervention to reduce the risk of atherosclerotic disease.10 Yet not all patients tolerate statin therapy, and data from the CLEAR-Outcomes trial of bempedoic acid provide an alternative treatment for such individuals. Bempedoic acid, a drug that lowers LDLC and hsCRP,11,12 appears to have similar efficacy for reducing cardiovascular event rates across baseline levels for both biomarkers.
We believe the finding that hsCRP predicts cardiovascular events and death at least as strongly as LDLC among contemporary statin-intolerant patients has clinical importance. First, the current data extend recent work in the PROMINENT (Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes), REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl - Intervention Trial), and STRENGTH (Long-term Outcomes Study to Assess Statin Residual Risk with Epanova in High Cardiovascular Risk Patents with Hypertriglyceridemia) trials, in which nearly identical findings were seen among 31 245 contemporary individuals taking statin therapy.6 In that previous analysis, LDLC levels were relatively low (median baseline LDLC=76 mg/dL), as all participants were receiving statin therapy. By contrast, in the current contemporary data among statin-intolerant patients, LDLC levels are higher (median LDLC=135 mg/dL). These data thus confirm that low-grade inflammation assessed by hsCRP accurately reflects high risk across a full range of LDLC levels and among those taking and not taking statins. That the differential magnitude of effect for hsCRP compared with LDLC is most evident for cardiovascular death also confirms, as previously reported, that inflammation heralds potentially catastrophic events.
Second, the median hsCRP level of 2.3 mg/L in the current analysis of statin-intolerant individuals is similar to the median value of 2.2 mg/L observed in the previous trials of individuals taking statins. These data confirm that hsCRP levels are concordant across different populations and indicate that current lipid-lowering therapies have relatively modest effects on vascular inflammation. Completed randomized trials of canakinumab3 and low-dose colchicine4,5 demonstrate that targeted anti-inflammatory therapy given on a background of statin therapy can reduce residual cardiovascular risk at least as much as adjunctive lipid-lowering agents.8
Third, the current data corroborate evidence from epidemiological studies published 30 years ago in apparently healthy men and women where the predictive value of hsCRP and LDLC was similar in magnitude among individuals not taking lipid-lowering therapy.1,2 That hsCRP today remains a powerful predictor of risk likely reflects adverse secular trends over time in the prevalence of obesity, insulin resistance, and metabolic syndrome, factors that promote low-grade vascular inflammation.13,14
Fourth, the current data reinforce the clinical concept that both LDLC and hsCRP should be measured to quantify the risk of incident cardiovascular events and potentially inform selection of the most appropriate targeted therapies. Likewise, these data add evidence that combination therapies that aggressively lower both LDLC and hsCRP may optimally address prevention of cardiovascular events. After trials with interleukin-1 blockade3 and low-dose colchicine,4,5,8 ongoing trials of interleukin-6 blockade are now underway to address whether vascular risk can be reduced with targeted anticytokine therapy in diverse high-risk settings, including chronic kidney disease, heart failure with preserved ejection fraction, and acute coronary ischemia.15
Finally, these data have implications for the primary prevention of atherosclerotic events. Among the 4206 participants in the CLEAR-Outcomes trial who did not have a history of cardiovascular disease, random allocation to bempedoic acid compared with placebo resulted in a 30% reduction in the risk of first major adverse cardiovascular events (HR, 0.70 [95% CI, 0.54–0.89]; P=0.002).16 This magnitude of risk reduction is nearly as large as that observed in the primary prevention JUPITER trial (Crestor 20 mg Versus Placebo in Prevention of Cardiovascular Events) of rosuvastatin compared with placebo in individuals with moderate levels of LDLC and high levels of hsCRP (HR, 0.56 [95% CI, 0.46–0.69]; P<0.0001).17 The current data confirm that individuals with LDLC <130 mg/dL but hsCRP ≥2 mg/L are at high risk, and that bempedoic acid offers a potential choice for primary and secondary prevention to patients with inflammatory risk who do not tolerate statin therapy.
A potential limitation of our analysis is that CLEAR-Outcomes participants were selected for LDLC levels >100 mg/dL. Although we cannot eliminate bias on this basis, we think it unlikely that having moderately higher LDLC levels than in the general population would substantively affect our results, in particular, because these data are consistent with previous work that included participants with lower ranges of LDLC.6 In addition, although this analysis was conducted to address controversies in our previous work comparing residual inflammatory risk and residual cholesterol risk among patients taking statins, the analyses presented here among statin-intolerant patients were not prespecified in the CLEAR-Outcomes protocol.
In conclusion, the current data among statin-intolerant patients confirm and extend the recent observation in statin-tolerant patients that inflammation assessed by hsCRP predicts future cardiovascular risk at least as strongly as LDLC. As we previously noted among those taking statins,6 these data must not be construed to diminish the proven role of lipid-lowering therapies for primary and secondary prevention patients with hypercholesterolemia. Yet, as also previously noted, accumulating data do suggest that targeting LDLC alone is unlikely to completely reduce atherosclerotic risk and that inflammatory pathways have yet to be fully exploited for patient benefit.
ARTICLE INFORMATION
Acknowledgments
Dr Ridker designed the a priori analysis plan for this manuscript, which was conducted by Drs Lei and Louie. Dr Ridker wrote the first draft of the manuscript, which was reviewed, edited, and approved by all authors. Drs Ridker, Lei, Louie, and Nicholls had full access to the data. All authors had final responsibility for the decision to submit for publication.
Sources of Funding
This work was supported by Esperion Therapeutics, Ann Arbor, MI.
Disclosures
Dr Ridker has received institutional research grant support from Kowa, Novartis, Amarin, Pfizer, Esperion, NovoNordisk, and the National Heart, Lung, and Blood Institute; has served as a consultant to Novartis, Flame, Agepha, Ardelyx, AstraZeneca, Janssen, Civi Biopharm, Glaxo Smith Kline, SOCAR, Novo Nordisk, Health Outlook, Montai Health, Eli Lilly, New Amsterdam, Boehringer-Ingelheim, RTI; Zomagen, Cytokinetics, Horizon Therapeutics, and Cardio Therapeutics; has minority shareholder equity positions in Uppton, Bitteroot Bio, and Angiowave; and receives compensation for service on the Peter Munk Advisory Board (University of Toronto), the Leducq Foundation, Paris FR, and the Baim Institute (Boston, MA). Drs Lei and Louie are employees of Esperion Therapeutics. Dr Haddad reports no conflicts. Dr Nicholls has received research support from AstraZeneca, New Amsterdam Pharma, Amgen, Anthera, Eli Lilly, Esperion, Novartis, Cerenis, The Medicines Company, Resverlogix, InfraReDx, Roche, Sanofi-Regeneron, Fauna Bio and LipoScience and is a consultant for AstraZeneca, Amarin, Akcea, Eli Lilly, Anthera, Omthera, Merck, Takeda, Resverlogix, Sanofi-Regeneron, CSL Behring, Esperion, Boehringer Ingelheim, Vaxxinity, Sequiris, Silence Therapeutics. Dr Lincoff has received institutional research grant support from AbbVie, AstraZeneca, CSL Behring, Eli Lilly, Esperion, Novartis and has served as a consultant to Akebia, Ardelyx, Becton-Dickson, Eli Lilly, Endologix, Fibrogen, GlaxoSmithKline, Medtronic, Neovasc, Novo Nordisk, Provention Bio, and ReCor. Dr Libby is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Moderna, Novo Nordisk, Novartis, Pfizer, and Sanofi-Regeneron. He is a member of the scientific advisory board for Amgen, Caristo Diagnostics, Cartesian Therapeutics, CSL Behring, DalCor Pharmaceuticals, Eulicid Bioimaging, Kancera, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, PlaqueTec, TenSixteen Bio, Soley Thereapeutics, and XBiotech, Inc. Dr Libby’s laboratory has received research funding the past 2 years from Novartis, Novo Nordisk, and Genentech and receives current funding support from National Heart, Lung, and Blood Institute (1R01HL134892 and 1R01HL163099-01), the RRM Charitable Fund and the Simard Fund. He is on the Board of Directors of XBiotech, Inc. Dr Libby has a financial interest in Xbiotech, a company developing therapeutic human antibodies, in TenSixteen Bio, a company targeting somatic mosaicism and clonal hematopoiesis of indeterminate potential (CHIP) to discover and develop novel therapeutics to treat age-related diseases, and in Soley Therapeutics, a biotechnology company that is combining artificial intelligence with molecular and cellular response detection for discovering and developing new drugs, currently focusing on cancer therapeutics. Dr Libby’s interests were reviewed and are managed by Brigham and Women’s Hospital and Mass General Brigham in accordance with their conflict-of-interest policies. Dr Nissen reports that the Cleveland Clinic Center for Clinical Research has received funding to perform clinical trials from Abbvie, AstraZeneca, Amgen, Bristol Myers Squibb, Eli Lilly, Esperion, Medtronic, MyoKardia, New Amsterdam Pharmaceuticals, Novartis, Pfizer, and Silence Therapeutics. Dr Nissen is involved in these clinical trials but receives no personal remuneration for his participation.
Supplemental Material
Table S1
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CLEAR
- Cholesterol Lowering via Bempedoic Acid, an ACL-Inhibiting Regimen Outcomes Trial
- HR
- hazard ratio
- hsCRP
- high-sensitivity C-reactive protein
- LDLC
- low-density lipoprotein cholesterol
- PROMINENT
- Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes
- REDUCE-IT
- Reduction of Cardiovascular Events with Icosapent Ethyl - Intervention Trial
- STRENGTH
- Long-term Outcomes Study to Assess Statin Residual Risk with Epanova in High Cardiovascular Risk Patents with Hypertriglyceridemia
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.123.066213.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
For Sources of Funding and Disclosures, see page 34.
Circulation is available at www.ahajournals.org/journal/circ
This work was presented as an abstract at the American Heart Association Scientific Sessions, November 11–13, 2023.
Contributor Information
Lei Lei, Email: llei@esperion.com.
Michael J. Louie, Email: mlouiemd@yahoo.com.
Tariq Haddad, Email: thaddad@virginiaheart.com.
Stephen J. Nicholls, Email: stephen.nicholls@monash.edu.
A. Michael Lincoff, Email: lincofa@ccf.org.
Peter Libby, Email: plibby@bwh.harvard.edu.
Steven E. Nissen, Email: nissens@ccf.org.
REFERENCES
- 1.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401 [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202 [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 4.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388 [DOI] [PubMed] [Google Scholar]
- 5.Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu X-F, Ireland MA, Lenderink T, et al. ; LoDoCo2 Trial Investigators. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838–1847. doi: 10.1056/NEJMoa2021372 [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Bhatt DL, Pradhan AD, Glynn RJ, MacFadyen JG, Nissen SE; PROMINENT, REDUCE-IT, and STRENGTH Investigators. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomized trials. Lancet. 2023;401:1293–1301. doi: 10.1016/S0140-6736(23)00215-5 [DOI] [PubMed] [Google Scholar]
- 7.Fiolet ATL, Opstal TSJ, Mosterd A, Eikelboom JW, Jolly SS, Keech AC, Kelly P, Tong DC, Layland J, Nidorf SM, et al. ; on behalf of the Colchicine Cardiovascular Trialists Collaboration. Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials. Eur Heart J. 2021;42:2765–2775. doi: 10.1093/eurheartj/ehab115 [DOI] [PubMed] [Google Scholar]
- 8.Nelson K, Fuster V, Ridker PM. Low-dose colchicine for secondary prevention of coronary artery disease: JACC review topic of the week. J Am Coll Cardiol. 2023;82:648–660. doi: 10.1016/j.jacc.2023.05.055 [DOI] [PubMed] [Google Scholar]
- 9.Nissen SE, Lincoff AM, Brennan D, Ray KK, Mason D, Kastelein JJP, Thompson PD, Libby P, Cho L, Plutzky J, et al. ; CLEAR Outcomes Investigators. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. 2023;388:1353–1364. doi: 10.1056/NEJMoa2215024 [DOI] [PubMed] [Google Scholar]
- 10.Nurmohamed NS, Navar AM, Kastelein JJP. New and emerging therapies for reduction of LDL-cholesterol and apolipoprotein B. J Am Coll Cardiol. 2021;77:1564–1575. doi: 10.1016/j.jacc.2020.11.079 [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Lei L, Ray KK, Ballantyne CM, Bradwin G, Rifai N. Effects of bempedoic acid on CRP, IL-6, fibrinogen and lipoprotein(a) in patients with residual inflammatory risk: a secondary analysis of the CLEAR Harmony trial. J Clin Lipidol. 2023;17:297–302. doi: 10.1016/j.jacl.2023.02.002 [DOI] [PubMed] [Google Scholar]
- 12.Stroes ESG, Bays HE, Banach M, Catapano AL, Duell PB, Laufs U, Mancini GBJ, Ray KK, Sasiela WJ, Zhang Y, et al. Bempedoic acid lowers high-sensitivity C-reactive protein and low-density lipoprotein: analysis of pooled data from four phase 3 clinical trials. Atherosclerosis. 2023;373:1–9. doi: 10.1016/j.atherosclerosis.2023.03.020 [DOI] [PubMed] [Google Scholar]
- 13.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin-6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327 [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05 [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Rane M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ Res. 2021;128:1728–1746. doi: 10.1161/CIRCRESAHA.121.319077 [DOI] [PubMed] [Google Scholar]
- 16.Nissen SE, Menon V, Nicholls SJ, Brennan D, Laffin L, Ridker P, Ray KK, Mason D, Kastelein JJP, Cho L, et al. Bempedoic acid for primary prevention of cardiovascular events in statin-intolerant patients. JAMA. 2023;330:131–140. doi: 10.1001/jama.2023.9696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al. ; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646 [DOI] [PubMed] [Google Scholar]