Abstract
Objective
Split-thickness skin grafting (STSG) is commonly used for wound closure in diabetic foot ulcers. In many cases, patients with diabetes present on long-term anticoagulation therapy. The complications associated with anticoagulants can be discouraging to surgeons considering STSG. The goal of this study was to evaluate STSG outcomes in the setting of chronic anticoagulation across a large, multicenter database.
Methods
We queried the TriNetX Network, which provides access to electronic medical records for over 75 million patients, for patients with a history of diabetic foot ulcers treated with STSG. We divided those found into two groups: long-term anticoagulant use prior to grafting and no long-term anticoagulant use. After matching, outcomes following STSG were evaluated after 1 month and 5 years. We identified 722 diabetic foot ulcer patients on chronic anticoagulation who were treated with STSG; 446 of these patients were matched to 446 patients with no prior anticoagulation.
Results
One month following STSG, the anticoagulated group showed no significant increase in death (p=1.0), graft failure (p=0.666), or regrafting (p=0.1865). At 5 years, we found no significant increase in mortality (p=0.6249), graft failure (p=1.0), regrafting (p=0.7892), or lower extremity amputation rates (p=1.0).
Conclusion
Chronic anticoagulation therapy does not lead to increased short-term or long-term post-operative complications such as graft failure, re-grafting, or increased amputation rates following STSG for wound closure. Negative outcomes following STSG for diabetic foot ulcers in chronically anticoagulated are minimal, and grafting should be performed without hesitation.
Keywords: anticoagulants, diabetic foot, foot ulcer, skin transplantation, wound healing
Introduction
Diabetes is a growing problem across the world, especially in the United States. According to the CDC, 34.2 million Americans had a diagnosis of diabetes in 2022.1 Of Americans over the age of 65, the prevalence of diabetes was 26.8%. As the rate of diabetes increases so does the prevalence of complications associated with the disease. Cardiovascular disease is the most common cause of morbidity and mortality in this group. Foot ulcers are also a relevant complication, which is present in 13% of the diabetic population of North America.2 In an effort to treat diabetes and its associated conditions, these patients take, on average, 6 different types of medications including anticoagulant therapy.3 The combination of diabetes, cardiovascular disease, and a long list of medications makes the treatment of diabetic foot ulcers a complex issue.
Minor foot ulcers are often treated with conservative management; unfortunately, this is not always successful for larger or advanced ulcers that persist despite proper treatment. One of the treatments for diabetic foot ulcers that has yielded good outcomes for resolution and long-term wound coverage is split-thickness skin grafting (STSG).4 However, the literature is scant on short and long-term outcomes of patients receiving STSG for diabetic foot ulcers in the setting of chronic anticoagulation. It is our experience with this procedure that short and long-term coverage can be accomplished with minimal morbidity or mortality. Chronic anticoagulation is common among patients with diabetes, and the effects of these medications on STSG healing is not yet well described. The aim of this paper is to evaluate outcomes of STSG in the setting of chronic anticoagulation across a national network of hospitals and providers.
Methods
The data used in this study was collected on May 30, 2022, from the TriNetX Network. TriNetX provided access to electronic medical records of over 75 million patients from 57 healthcare organizations. TriNetX is certified to the ISO 27001:2013 standard and maintains an Information Security Management System (ISMS) to ensure the protection of healthcare data and meets requirements of the HIPAA Security Rule. Patient level data provided in a data set generated by TriNetX Platform only contains de-identified data as defined in Section §164.514(a) of the HIPAA Privacy Rule. Because this study uses de-identified patient records and did not involve the collection, use, or transmittal of individually identifiable data, it was exempted from Institutional Review Board approval.
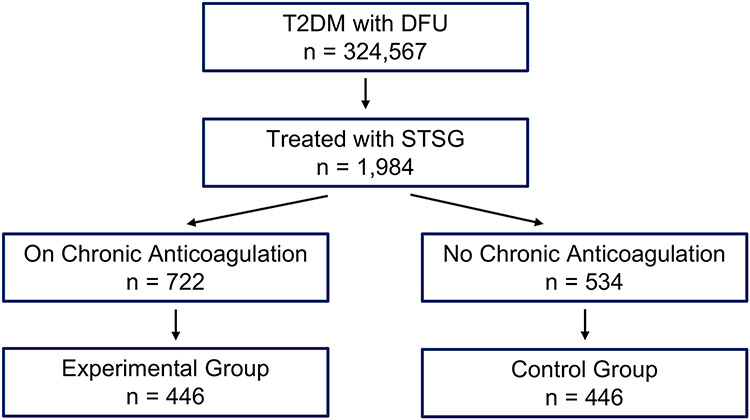
The TriNetX platform was used to evaluate the effect of long-term anticoagulation therapy on postoperative outcomes of diabetic patients receiving STSG for diabetic foot ulcers. Cohorts were divided into two comparison groups. The experimental group included patients on long-term anticoagulant therapy prior to grafting. The control group were patients who never received anticoagulation prior to grafting. Short-term outcomes were defined as occurring within 1 month of STSG. Long-term outcomes were defined as outcomes between 1 month and 60 months after STSG. Patients included in this study were those with a diagnosis of type 2 diabetes with a foot ulcer (ICD: E11.621) treated by STSG (ICD: 15100, 15101, 15121, or 15120). For the experimental group, the 5 most prescribed anticoagulants (rivaroxaban, warfarin, apixaban, enoxaparin, and dalteparin) were used as inclusion criteria. These same medications were used as exclusion criteria in the control group. Antiplatelet medications were used as exclusion criteria in both groups, but aspirin was not included in the inclusion or exclusion criteria due to the widespread use of aspirin in patients of all health statuses (Figure 1). The two cohorts were propensity score matched by age, gender, ethnicity, and comorbidities (Table 1). Patients were analyzed for both short and long-term outcomes. Short-term outcomes included mortality, graft failure (ICD: T86.821), re-grafting rates (ICD: 15120 or 15100), hematoma (ICD: T14) or seroma (ICD: L76.3) formation, and infections including cellulitis (ICD: L03) or localized infections of the skin (ICD: L08.9). Graft failure (ICD: T86.821) is used in cases of skin graft failure including anastomotic failure of the flap, complete flap loss, flap failure, flap loss, full thickness flap loss, or mechanical complication due to skin graft failure and/or rejection. Long-term outcomes included mortality, graft failure (ICD: T86.821), re-grafting rates (ICD: 15120 or 15100), amputation rates (ICD: S98.322A, 27880, 28825, Z89.6, or Z89.43), angiography (ICD: 75710, or 75716), and angioplasty (ICD: 37224, 37228, 37232). The primary outcome was amputations. The secondary outcomes were graft failure, re-grafting, hematoma formation, seroma formation, angiography, and angioplasty.
Figure 1: Stratification of Cohorts:
Medications included the top 5 most prescribed anticoagulants: rivaroxaban, warfarin, apixaban, enoxaparin, dalteparin; T2DM = Type 2 Diabetes Mellitus; DFU = Diabetic Foot Ulcer
Table 1:
Cohort Propensity Matching and Demographics
| Prior to Propensity Match | Post Propensity Match | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (n = 722) | Control (n = 534) | p-Value | Treatment (n = 446) | Control (n = 446) | p-Value | |||||
| Age | 57 +/− 13 | 55 +/− 13.1 | < 0.0064* | 55.7 +/− 13.1 | 55.9 +/− 13.1 | 0.8058 | ||||
| Ethnicity | ||||||||||
| White | 536 | 74.24% | 374 | 70.04% | 0.0995 | 314 | 70.40% | 308 | 69.06% | 0.6619 |
| African American | 126 | 17.45% | 111 | 20.79% | 0.1353 | 91 | 20.40% | 99 | 22.20% | 0.513 |
| Hispanic/Latino | 113 | 15.65% | 157 | 29.40% | < 0.0001* | 104 | 23.32% | 97 | 21.75% | 0.5748 |
| Asian | 10 | 1.39% | 10 | 1.87% | 0.4949 | 10 | 2.24% | 10 | 2.24% | 1 |
| Native American | 10 | 1.39% | 10 | 1.87% | 0.4949 | 10 | 2.24% | 10 | 2.24% | 1 |
| Gender | ||||||||||
| Male | 482 | 66.76% | 407 | 76.22% | 0.0003* | 326 | 73.09% | 328 | 73.54% | 0.8797 |
| Female | 240 | 33.24% | 127 | 23.78% | 0.0003* | 120 | 26.91% | 118 | 26.46% | 0.8797 |
| Diagnoses | ||||||||||
| Essential HTN | 622 | 86.15% | 395 | 73.97% | < 0.0001* | 355 | 79.60% | 361 | 80.94% | 0.6137 |
| Hyperlipidemia | 455 | 63.02% | 238 | 44.57% | < 0.0001* | 221 | 49.55% | 225 | 50.45% | 0.7888 |
| CKD | 288 | 39.89% | 182 | 34.08% | 0.04 | 157 | 35.20% | 163 | 36.55% | 0.6753 |
| Ischemic Heart Disease | 208 | 28.81% | 95 | 17.79% | < 0.0001* | 93 | 20.85% | 92 | 20.63% | 0.9342 |
| Thrombosis | 154 | 21.33% | 32 | 5.99% | < 0.0001* | 34 | 7.62% | 32 | 7.18% | 0.7981 |
| PAD | 277 | 38.37% | 168 | 31.46% | 0.01 | 151 | 33.86% | 155 | 34.75% | 0.7779 |
| Venous Stasis | 118 | 16.34% | 54 | 10.11% | 0.00 | 50 | 11.21% | 52 | 11.66% | 0.8333 |
| Type 2 Diabetes | 511 | 70.78% | 347 | 64.98% | 0.03 | 303 | 67.94% | 294 | 65.92% | 0.5218 |
This figure shows stratifications of the study and the number of STSG patients in each cohort both before and after matching for ethnicity, race, age, and significant comorbidities. HTN = hypertension; CKD = chronic kidney disease; PAD = peripheral arterial disease
Univariate statistical analysis of outcomes was performed using analytical tools provided by TriNetX. This included tests for measures of association (i.e. z-test with odds ratio and 95% confidence interval). Significance was defined as a p-value of < 0.05.
Results
Prior to matching, we identified 722 patients in the experimental group (anticoagulation group) and 534 patients in the control group (non-anticoagulation group). The ethnic compositions prior to matching were 74.24% (536 patients) and 70.04% (374 patients) Caucasian, 17.45% (126) and 20.79% (111) African American, 15.65% (113) and 29.40% (157) Hispanic or Latino, 1.39% (10) and 1.87% (10) Asian, and 1.39% (10) and 1.87% (10) Native American, respectively, for both cohorts. The experimental group had an average age of 57 +/− 13y while the control group had an average age of 55 +/− 13.1y (p = 0.05). The experimental group contained 66.76% (482) males and 33.24% (240) females, while the control group was made up of 76.22% (407) male and 23.78% (127) female, which was a statistically significant difference. Prior to propensity matching, the two cohorts had significant differences in prevalence of essential hypertension (HTN), hyperlipidemia, chronic kidney disease (CKD), ischemic heart disease, thrombosis, peripheral artery disease (PAD), venous stasis, and type 2 diabetes mellitus (T2DM). After propensity matching, 446 patients were included in both the experimental and control groups with no statistical differences in ethnicity, age, gender, or comorbidities (Table 1).
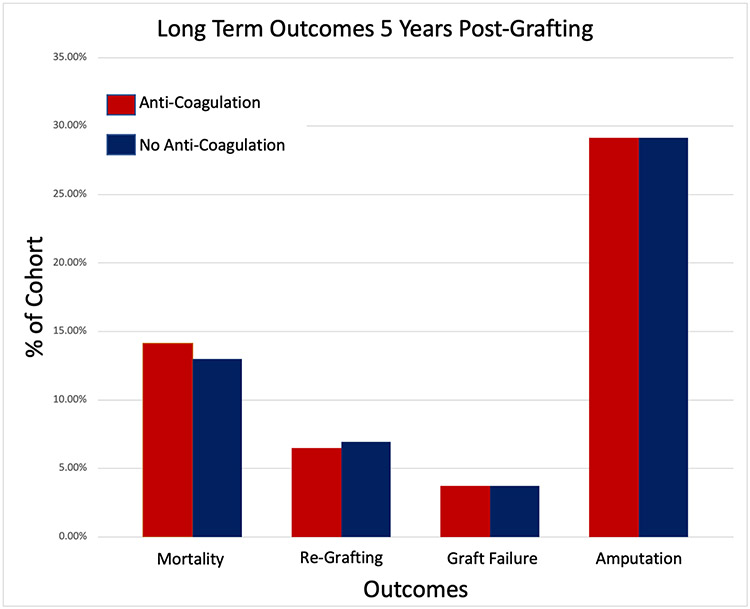
For the short term outcomes, the experimental group showed no significant increase in graft failure (p=0.666, OR 1.21, 95% CI: [0.515, 2.819]) or regrafting rates (p=0.1865, OR 1.663, 95% CI: [0.776, 3.561]) within 30 days of the procedure. As to long-term outcomes, we found no significant increase in graft failure (p=0.2797, OR 1.0, 95% CI: [0.494, 2.025]) or regrafting rates (p=0.3524, OR 0.931, 95% CI: [0.551, 1.573]) in the second to sixtieth months after STSG. The experimental group did not have a significant long-term increase in the occurrence of endovascular procedures (p=0.3751, OR 1.373, 95% CI: [0.68, 2.774]), amputations (p=1.0, OR 1, 95% CI: [0.747, 1.339]), or mortality (p=0.6249, Odds Ratio: 1.1, 95% CI: [0.75, 1.615]).
Full results are found in Table 2 and Table 3.
Table 2:
Short-Term Outcomes Following STSG
| Outcome | Treatment (n=446) |
Control (n=446) |
p-value | Odds Ratio |
Odds CI | ||
|---|---|---|---|---|---|---|---|
| Death | 10 | 2.20% | 10 | 2.20% | 1 | 1 | (0.412,2.426) |
| Graft Failure | 12 | 2.64% | 10 | 2.20% | 0.666 | 1.21 | (0.515,2.819) |
| Re-grafting | 18 | 3.97% | 11 | 2.42% | 0.1865 | 1.663 | (0.776,3.561) |
| Infection | 52 | 11.45% | 84 | 18.50% | 0.0029* | 0.57 | (0.392,0.828) |
| Hematoma | 19 | 4.19% | 18 | 3.97% | 0.8667 | 1.058 | (0.548,2.043 |
Short-term was defined as being within the first month following STSG.
Table 3:
Long-Term Outcomes Following STSG
| Outcome | Treatment (n=446) |
Control (n=446) |
p-value | Odds Ratio |
Odds CI | ||
|---|---|---|---|---|---|---|---|
| Death | 63 | 14.13% | 58 | 13.00% | 0.6249 | 1.1 | (0.75,1.615) |
| Graft Failure | 16 | 3.59% | 16 | 3.59% | 1 | 1 | (0.494,2.025) |
| Re-grafting | 29 | 6.50% | 31 | 6.95% | 0.7892 | 0.931 | (0.551,1.573) |
| Amp - MTP | 18 | 4.04% | 18 | 4.04% | 1 | 1 | (0.513,1.948) |
| Amp - Boyd/Symes | 91 | 20.40% | 95 | 21.30% | 0.7416 | 0.947 | (0.686,1.308) |
| Amp - Below Knee | 19 | 4.26% | 12 | 2.69% | 0.2007 | 1.609 | (0.772,3.356) |
| Amp - Above Knee | 20 | 4.48% | 15 | 3.36% | 0.3886 | 1.349 | (0.682,2.67) |
| Pooled Amputations | 125 | 28.03% | 125 | 28.03% | 1 | 1 | (0.747,1.339) |
| STSG - Foot | 14 | 3.14% | 14 | 3.14% | 1 | 1 | (0.471,2.123) |
| STSG - Leg | 17 | 3.81% | 19 | 4.26% | 0.7337 | 0.891 | (0.457,1.737) |
| Angiography | 19 | 4.26% | 14 | 3.14% | 0.3751 | 1.373 | (0.68,2.774) |
| Balloon Angioplasty | 12 | 2.69% | 10 | 2.24% | 0.6659 | 1.206 | (0.515,2.82) |
Long-term was defined as being between 1 month and 5 years after STSG. Amp = amputation; MTP = metatarsophalangeal joint; IP = interphalangeal joint
Discussion
This study found that long-term anticoagulation therapy has no correlation with clinical outcomes following STSG in either the short or long-term. In the experimental group, no significant difference was found in graft failure or re-grafting rates within the first month, or within the first sixty months (5 years). Our study also found no significant increase in amputations, endovascular procedures, or mortality rates between the experimental group and the control group.
The literature is scant regarding the effects of chronic anticoagulation on STSG healing; however, a few studies evaluated the impact of chronic anticoagulation in the wound healing process. One study found that antiplatelet and anticoagulant agents delay wound healing more than any other medications in their study.5 It was also found that not only do anticoagulant therapies increase the bleeding risk following low impact trauma, but they also contribute to wound healing complications including bleeding, excessive wound exudate, and the development of deep dissecting hematomas.5,6 Unlike this literature, our study did not find a significant increase in hematoma formation or any other reported major complication in patients on chronic anticoagulation therapy. We also found no significant difference in overall re-grafting with a total of 47 patients (10.54%) on chronic anticoagulation and a total of 42 patients (9.42%) not on chronic anticoagulation needing to undergo regrafting within 5 years following grafting.
Diabetes is a common and high-impact disease associated with a wide array of complications, two of the most morbid being diabetic foot ulcer (DFU) and subsequent amputation. Nonetheless, data are limited on these conditions in the setting of patient polypharmacy. It is well known that chronic DFU is associated with severe outcomes that affect quality of life and compromise the patient’s life span. To such a point, patients with resistant DFU have 1-, 2-, and 5-year survival rates of 80.80%, 69.01%, and 28.64%, respectively.7 Surprisingly, the magnitude of the effect of DFU on survival is greater than coronary artery disease, peripheral arterial disease, or stroke.7 The decline in 5-year survival is attributed to the compounding effect of complications caused by DFU with a major emphasis in amputations. A recent study showed that major amputation occurred in 26.2% of diabetic foot wound patients.8 On top of this, a second study found that patients with a previous lower extremity amputation (LEA) have higher rates of re-ulceration, re-amputation, and mortality.9 These aforementioned studies support the evidence of previous data, which show DFU often leads to higher complication rates of the lower extremity, which in turn leads to an even greater risk of more severe complications, further compounding the severity of the disease progression.
Achieving proper wound healing is paramount in preventing progression of DFU to amputation, and eventually death, which is why more effective treatment options such as STSG are necessary. Performing STSG on patients with diabetes who have DFU refractory to other treatment options has shown decreased incidence of future complications when compared to more conservative care. One meta-analysis found that 2 years after grafting, the ulcer recurrence rate was 4.2%, the regrafting rate was 12.1%, and the infection rate was 4.4%, which were superior results compared to those not undergoing STSG.10 In recent studies, STSG has shown promising results in the healing of non-progressing ulcers of the lower extremity while also showing low rates of graft failure and complications.
Because of the growing evidence that STSG should be used to treat therapy resistant DFU, it is important to understand how chronic anticoagulation therapy may affect these results as many patients with diabetes tend to also suffer from other comorbidities such as cardiovascular disease. The primary concern with the chronic use of anticoagulants is the inherent bleeding risk associated with these medications. Warfarin has been shown to have a range of 2% to 13% increased risk of major bleeding in the general population, and this percentage rises to 19.3% in patients with ischemic heart disease.11 Similarly, two related antiplatelet medications, prasugrel and ticagrelor, have shown increased bleeding events in both vascular access site locations and coronary artery bypass graft related bleeding.10,12 Of most concern, this same study also found that bleeding risk increased alongside increased duration of therapy, making chronic medication a concern for surgical procedures such as STSG.12 Taking this information into consideration, it is unsurprising that concern is raised for performing STSG in patients while on long-term anticoagulation therapy, with the major concern being poor outcomes and increased complication rates secondary to bleeding-related issues in the wound bed. Any buildup of fluid, including hematoma formation, between the STSG and the wound bed jeopardizes skin graft take.13 However, our study found no significant increase in mortality, re-grafting rates, hematoma formation, or graft failure. These findings suggest that, in general, long-term anticoagulation therapy should not be a contraindication for STSG in DFU.
One interesting finding seen in our study was decreased infections rates during the first month following grafting in the treatment group when compared to the control group. While not fully understood, there seems to be an ill-defined connection between coagulation and inflammation that could explain the differences seen between the two cohorts. Studies have been done suggesting that inhibition of coagulation, such as through anticoagulants, reduces the inflammatory reaction to some microbes.14 This information has further been supported by the discovery of protease-activated receptors (PARs) which are also known to activate platelets during clotting.14 It is possible that long term anticoagulation prior to grafting decreased the initial levels of inflammatory products, which in turn decreased the inflammatory response to microbes following the procedure. Another possible explanation is differences in perfusion between the two groups. The anticoagulated group develops decreased rates of blood clots forming in the microvasculature which allows for a more reliable blood supply to the wound location.14 Enhanced perfusion may reduce infection via decreased levels of blood stagnation which allows the tissue repair process to occur more efficiently. While our study does not allow us to determine the cause of decreased infection rates in those on chronic anticoagulation, this observation is worthy of further study in the future.
As this study is retrospective and based on administrative data, inherent limitations exist as we are dependent on accurate coding of patient diagnoses and treatments. This is especially true with ICD codes such as graft failure as these are not specifically defined and may be subject to variability between organizations. Variability may also exist between physicians once a patient progresses to the outpatient setting as ICD coding is often more regulated and consistent in the inpatient setting. We are also unable to determine whether anticoagulants were temporarily held during the STSG procedure, so we cannot comment on whether anticoagulant cessation is helpful in improving outcomes following grafting in patients with DFU. Furthermore, as we are unable to determine the duration of anticoagulant therapy for each patient, we are unable to include this in matching criteria or eliminate this as a possible confounder. It is also important to note that in order to protect patient identity, the TriNetX database automatically rounds all patient cohorts below 10 up to 10, thus diluting and underestimating the effect of very small groups of patients; however, this practice does not impact any of our primary outcomes. Finally, while we attempted to thoroughly match for any confounding comorbidities, it is possible that any unidentified comorbidity resulted in cohort characteristics that differ from the general population. It is also possible that variables not accounted for could mask a significant relationship between chronic anticoagulation therapy and adverse outcomes after grafting.
Conclusions
Chronic anticoagulation therapy does not increase short-term post-operative complications such as graft failure, re-grafting, or increased amputation rates after STSG for wound closure. Furthermore, long-term complications and disease progression remain low for this group as well. Short-term and long-term outcomes of STSG in the chronically anticoagulated patient with diabetic foot ulcers are well within acceptable ranges, and grafting should be performed without hesitation.
Figure 2: Primary Long-Term Outcomes Following STSG:
Long-term was defined as being between 1 month and 5 years after STSG. There was no significant difference in mortality, re-grafting rates, graft failure, or pooled amputations between the two cohorts.
Acknowledgments
Source of funding
Remembering the 15 Burn Research and Education Endowment, NIH (UL1 TR001439)
Abbreviations
- CDC
Centers for Disease Control and Prevention
- CKD
chronic kidney disease
- DFU
diabetic foot ulcer
- HTN
hypertension
- LEA
lower extremity amputation
- PAD
peripheral artery disease
- STSG
split-thickness skin graft
- T2DM
type 2 diabetes mellitus
Footnotes
Conflict of interest disclosure statement
The authors report no disclosures relevant to the manuscript.
References
- 1.Yammine K, Assi C. A Meta-Analysis of the Outcomes of Split-Thickness Skin Graft on Diabetic Leg and Foot Ulcers. Int J Low Extrem Wounds. 2019;18(1):23–30. [DOI] [PubMed] [Google Scholar]
- 2.Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global Dpidemiology of Diabetic Foot Ulceration: A Systematic Review and Meta-Analysis. Ann Med. 2017;49(2):106–116. [DOI] [PubMed] [Google Scholar]
- 3.Brennan MB, Hess TM, Bartle B, et al. Diabetic Foot Ulcer Severity Predicts Mortality Among Veterans With Type 2 Diabetes. J Diabetes Complications. 2017;31(3):556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose JF, Giovinco N, Mills JL, Najafi B, Pappalardo J, Armstrong DG. Split-Thickness Skin Grafting the High-Risk Diabetic Foot. J Vasc Surg. 2014;59(6):1657–1663. [DOI] [PubMed] [Google Scholar]
- 5.Kaya G, Saurat JH. Dermatoporosis: A Chronic Cutaneous Insufficiency/Fragility Syndrome. Clinicopathological Features, Mechanisms, Prevention and Potential Treatments. Dermatology. 2007;215(4):284–294. [DOI] [PubMed] [Google Scholar]
- 6.Windy Cole D, CWSP. The Impact of Oral Anticoagulants On Wound Healing and Development in an Aging Population. Podiatry Today. 2021. [Google Scholar]
- 7.Kim SY, Kim TH, Choi JY, et al. Predictors for Amputation in Patients with Diabetic Foot Wound. Vasc Specialist Int. 2018;34(4):109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rathnayake A, Saboo A, Malabu UH, & Falhammar H Lower Extremity Amputations and Long-Term Outcomes in Diabetic Foot Ulcers: A systematic review. World journal of diabetes. 2020;11(9), 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wigston C, Hassan S, Turvey S, et al. Impact of medications and lifestyle factors on wound healing: A pilot study. Wounds UK. 2013;9(1):22–28. [Google Scholar]
- 10.Montalescot G, Wiviott SD, Braunwald E, et al. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009;373(9665):723–731. [DOI] [PubMed] [Google Scholar]
- 11.Levine MN, Raskob G, Beyth RJ, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):287S–310S. [DOI] [PubMed] [Google Scholar]
- 12.Yorkgitis BK, Ruggia-Check C, Dujon JE. Antiplatelet and anticoagulation medications and the surgical patient. Am J Surg. 2014;207(1):95–101. [DOI] [PubMed] [Google Scholar]
- 13.Braza ME, Fahrenkopf MP. Split-Thickness Skin Grafts. [Updated 2022 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Jan. [PubMed] [Google Scholar]
- 14.Kumar V, Abbas AK, Aster JC, Turner JR, & Perkins JA Inflammation and Repair. In: Robbins & Cotran Pathologic Basis of Disease (Ninth Edition). Elsevier, 2015: 89–108. [Google Scholar]