Abstract
Introduction
Many athletes use short-acting inhaled β2-agonists multiple times weekly during training sessions to prevent exercise-induced bronchoconstriction, but it is unclear if treatment impairs training outcomes. Herein, we investigated performance adaptations in well-trained females and males training with prior inhalation of salbutamol.
Methods
19 females and 21 males with maximal oxygen uptake (V′O2max) of 50.5±3.3 and 57.9±4.9 mL·min−1·kg−1, respectively, participated in this double-blinded, placebo-controlled, parallel-group study. We randomised participants to placebo or salbutamol inhalation (800–1600 µg·training day−1) for 6 weeks of combined endurance (1× per week) and high-intensity interval training (2× per week). We assessed participants’ body composition, V′O2max and muscle contractile function, and collected vastus lateralis muscle biopsies.
Results
Salbutamol induced a sex-specific loss of whole-body fat mass (sex×treatment: p=0.048) where only salbutamol-treated females had a fat mass reduction compared to placebo (–0.8 kg at 6 weeks; 95% CI: −0.5 to −1.6; p=0.039). Furthermore, salbutamol-treated females exhibited a repartitioning effect, lowering fat mass while gaining lean mass (p=0.011), which was not apparent for males (p=0.303). Salbutamol negatively impacted V′O2max in both sexes (treatment main effect: p=0.014) due to a blunted increase in V′O2max during the initial 4 weeks of the intervention. Quadriceps contractile strength was impaired in salbutamol-treated females (−39 N·m; 95% CI: −61 to −17; p=0.002) compared to placebo at 6 weeks. Muscle electron transport chain complex I–V abundance increased with salbutamol (treatment main effect: p=0.035), while content of SERCAI, β2-adrenoceptor and desmin remained unchanged.
Conclusion
Inhaled salbutamol appears to be an effective repartitioning agent in females but may impair aerobic and strength-related training outcomes.
Tweetable abstract
Prolonged treatment with inhaled salbutamol induces leanness only in female athletes and concurrently impairs aerobic and strength-related training outcomes in both sexes. Athletes with uncontrolled asthma should avoid overreliance on inhaled salbutamol. https://bit.ly/3uXfQUc
Introduction
Many athletes use short-acting inhaled β2-agonists multiple times weekly during training sessions to prevent exercise-induced bronchoconstriction − a condition characterised by airway narrowing in conjunction with exercise [1–7]. This is particularly true for athletes engaged in sports with high ventilatory loads, training volumes and aerobic demands [1–3, 7, 8]. However, it remains unclear whether inhaled β2-agonists affect training outcomes when used in conjunction with training.
Short-acting β2-agonist salbutamol is the most common drug used by elite athletes to counter exercise-induced bronchoconstriction. While the World Anti-Doping Agency (WADA) restricts use of salbutamol and other β2-agonists in- and out-of-competition, athletes are permitted to inhale salbutamol at a daily maximum dose of 1600 µg not to exceed 600 µg in any 8-h period [9]. The dosing limit is in place to prevent misuse at supratherapeutic inhaled doses and via systemic routes, as salbutamol can impose performance-enhancing effects. Early findings by Martineau et al. [10] in the 1990s showed that daily ingestion of oral salbutamol for 2–3 weeks effectively increased muscle strength by 10–27%. And several studies have since underpinned the acute ergogenic effects of oral salbutamol on sprint performance and muscle strength as well as chronic effects in promoting muscle hypertrophy and power development [11–15] – independent of concurrent resistance training programmes [12, 16]. Although systemic effects are not as apparent at therapeutic inhaled doses, a significant fraction of the inhaled dose reaches the systemic circulation [17–19] and could therefore influence the training response. For example, 0.4–0.6 mg inhaled salbutamol (equivalent to 2–3 puffs of a standard 0.2-mg salbutamol inhaler device) is sufficiently high to induce systemic effects, as reflected by increased heart rate, ventilation and metabolic rate [20–22].
A few studies have shown that daily inhalation of β2-agonists leads to adaptive changes during periods of exercise training in moderately trained males. Terbutaline, a short-acting β2-agonist like salbutamol, attenuated aerobic exercise outcomes when used daily at inhaled doses (8 puffs of 0.5 mg) during 4 weeks of endurance-based training [23]. While the 4-week training period augmented maximum oxygen uptake (V′O2max) and incremental exercise capacity by 5% and 12%, respectively, in the placebo group, the group randomised to terbutaline experienced no significant changes in either parameter. The same pattern was apparent for a range of exercise-responsive proteins involved in oxidative muscle metabolism [23]. Using a similar dosing regimen, terbutaline was also demonstrated to lower V′O2max and muscle oxidative capacity during 4 weeks of resistance training [24]. And while inhaled salbutamol at doses of 1.6 mg did not affect performance outcomes when administered daily for 5 weeks during a period of strength and power training programme three times weekly, daily inhalation of long-acting β2-agonists formoterol and salmeterol for 5 weeks was shown to enhance sprint performance [25].
Although the above studies indicate that daily inhalation of β2-agonists can affect adaptations during periods with training, the treatment was administered on a daily basis and not in conjunction with training [23, 24, 26] as would be normal practice for an athlete with exercise-induced bronchoconstriction [7]. Furthermore, the studies with inhaled salbutamol and terbutaline utilised high doses and were conducted with moderately trained males only [23, 24, 26]. Potential sex-specific effects of β2-agonists are important to consider, especially because Lee et al. [27] showed that females have a higher anabolic response to oral formoterol than males. Thus, there is a need for studies illuminating sex-specific effects of inhaled β2-agonists when used in conjunction with training by endurance-trained individuals. Such studies will not only help guide anti-doping regulations but also practices if treatment is associated with detrimental effects. Indeed, recent studies highlight that chronic use of inhaled β2-agonists may impose detrimental effects on aerobic exercise outcomes, including in V′O2max and incremental exercise capacity [23, 28].
Herein, we investigated sex-specific effects of 6 weeks of aerobic exercise training with or without inhalation of salbutamol on training days on body composition, V′O2max and muscle function in well-trained males and females. Furthermore, we examined putative mechanisms in skeletal muscle. We hypothesised that females would respond to a greater extent than males in terms of lean mass gain and muscle adaptations with inhaled salbutamol, while adaptations to aerobic exercise training would be blunted with salbutamol treatment in both sexes.
Methods
Study design and ethics approval
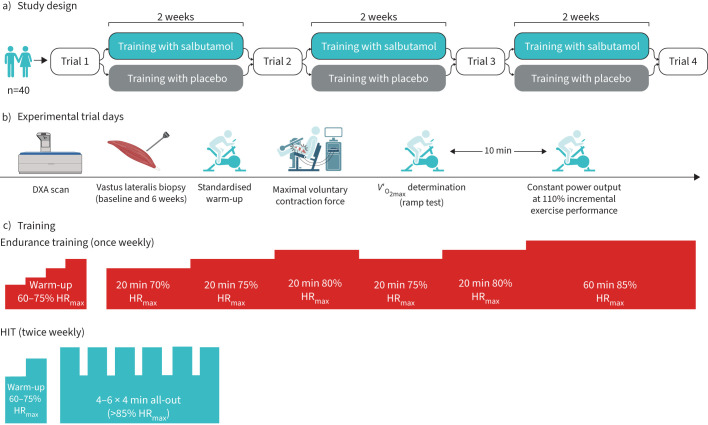
The study was a randomised, double-blinded, placebo-controlled, parallel-group study. Participants completed experimental trial days at baseline, and following 2 weeks, 4 weeks, and 6 weeks of training with concurrent inhalation of salbutamol or placebo (figure 1a). The study was registered in Clinicaltrials.gov (Trial identifier: NCT03902106). The study was conducted in accordance with the 2013 Declaration of Helsinki and was approved by the regional ethics committee of Copenhagen (H-18007889). All participants were informed about possible risks involved and gave their oral and written consent before inclusion.
FIGURE 1.
Experimental overview. a) Study design, b) experimental trial days and c) training days. DXA: dual-energy X-ray absorptiometry; V′O2max: maximal oxygen uptake; HRmax: maximal heart rate; HIT: high-intensity interval training.
Participants and eligibility criteria
All trials were conducted at the Department of Nutrition, Exercise and Sports, University of Copenhagen, Denmark between April 2019 and December 2020. Inclusion criteria were age 18–45 years, body mass index <26 kg·m−2 and V′O2max >50 and >55 mL·min−1·kg−1 body weight for females and males, respectively. Exclusion criteria were chronic use of β2-agonist or allergy towards β2-agonist, serious adverse effects to β2-agonist, chronic disease deemed by the study responsible medical doctor to interfere with any part of the study (such as asthma or exercise-induced bronchoconstriction), smoking, chronic use of prescription medicine (excluding contraceptives) deemed by the study responsible medical doctor to interfere with any part of the study, or pregnancy (for females).
Assessment of eligibility criteria
We assessed eligibility criteria during a medical examination with electrocardiography (ECG-2150; Nihon Kohden, Rosbach, Germany), blood pressure measurements (M7 Intellisense, OMRON, Kyoto, Japan) and lung function (EasyOne® Air, NDD Medical Technologies, Andover, MA, USA). Participants also completed a ramp test on a bicycle ergometer (Monark LC6; Monark, Vansbro, Sweden) for determination of V′O2max by indirect calorimetry (Oxycon Pro; CareFusion, San Diego, CA, USA). The ramp test was preceded by 3× 4-min bouts at 75, 100 and 125 W and 100, 150 and 200 W for females and males, respectively. Participants performed lung function testing of forced expiratory volume in 1 s (FEV1) 5, 10, 15 and 20 min after conclusion of the ramp test to exclude exercise-induced bronchoconstriction (defined as a >10% decrease in FEV1 from pre-test values). After the last FEV1 measurement, participants inhaled 800 µg salbutamol (onset of action ≈5 min [29]), and FEV1 was assessed once more 10 min after inhalation to test for reversibility defined as a 12% and 200-mL increase in FEV1 according to European Respiratory Society (ERS) guidelines [30].
Randomisation and blinding
Upon inclusion, participants were allocated into two groups receiving either salbutamol or placebo during training days. Randomisation was performed by minimisation for sex, V′O2max and lean body mass to ensure group homogeneity at baseline for the main outcome measures [31, 32]. Allocation was performed by personnel not involved in enrolment or conducting experimental trials. Salbutamol and placebo were administered in identical-looking inhalers, and the intervention was identical for both treatments, and thus allocation was concealed to assessors and participants throughout the whole intervention.
Outcome measures and sample size
The main response outcome measure was change in body composition (i.e., fat and lean mass) measured by dual energy X-ray absorptiometry (DXA) and V′O2max during a ramp test to exhaustion on a bike ergometer. Other outcome measures were changes in muscle function. All measurements were conducted on experimental trial days at baseline and after 2 weeks, 4 weeks and 6 weeks.
Sample size was determined for the main outcome measure in GPower 3.1.9.3 with an α-level of 0.05 and β-level of 0.8 for a linear mixed model repeated measures design which resulted in a required sample size of 18 participants per treatment group with an equal distribution of males and females. Effect size and standard deviation (sd) were estimated from a previous study [33].
Training and study drugs
The intervention period consisted of 6 weeks of exercise training (three sessions per week) consisting of a high-intensity interval training (HIT; two sessions per week) and endurance exercise (one session per week) on an indoor spinning bike (Body Bike indoor cycle, W014, Body Bike International, Frederikshavn, Denmark). Intensity was monitored by heart rate sensors (Polar Team Pro, Polar, Kempele, Finland) and an instructor supervised all training sessions.
HIT sessions consisted of a 5-min warm-up at 65% and 5 min at 75% of heart rate max (HRmax) followed by intervals of 4 min >85% of HRmax interspersed by 2 min active recovery. The participants were encouraged to achieve the highest possible HR during each interval. Strong verbal encouragement was given during the training sessions. During the first week, sessions consisted of four repetitions and increased by one repetition every second week, resulting in six repetitions during the last 2 weeks.
Endurance training sessions consisted of a 20 min warm-up at 60–75% HRmax, followed by 3×20 min at 70%, 75% and 80% HRmax, respectively, 20 min at 75% HRmax and finally 40 min at 85% HRmax for a total exercise duration of 3 h (figure 1c).
At each training session, participants were administered either salbutamol (Ventoline®, GlaxoSmithKline, Brentford, UK) or placebo (lactose monohydrate). Salbutamol and placebo inhalers were delivered by the regional pharmacy of Copenhagen, Denmark. During the intervention, participants were instructed to maintain their physical activity levels and not to make major changes in their nutrition regimens and training outside of the intervention. In addition, participants were asked not to donate blood during the intervention. Participants were asked to report their physical activity levels and any potential illness during the intervention at the post-intervention trial. On trial days, participants kept a detailed nutritional log and replicated nutritional intake to minimise inter-day variation in study outcome measurements.
At training sessions, the administered dose of salbutamol was 800 µg as a single dose immediately prior to training on HIT training days and 1600 µg as split doses on endurance training days. These doses were chosen to be within those maximally allowed by WADA at the time of the study conception [34], and to reflect the dose that could conceivably be used by athletes in the duration of the respective training sessions. To simulate a dosing regimen during the endurance training sessions, salbutamol was administered before the warm-up (200 µg), after the warm-up (400 µg) and immediately after the training session (200 µg). In addition, 2×400 µg was administered in the evening after the training session for a total dose of 1600 µg in 24 h. All inhalations were supervised during the training sessions, and via video monitoring (e.g., Skype/FaceTime) for the evening inhalations.
Experimental trial days
At all experimental trial days, we measured body composition using DXA (Lunar DPX-IQ, Version 4.7 E; Lunar Corporation, Madison, WI, USA) using the mean of two separate scans, followed by participants completing a standardised warm-up at 3×4 min at 30%, 50% and 70% of V′O2max, respectively, as calculated from the screening ramp test.
After the warm-up, participant's contractile properties of the quadriceps muscle were measured using a maximal voluntary contraction setup (MVC) with electrical percutaneous muscle stimulations delivered on top of the plateau of each MVC as well as 1 s before and after each bout. Participants completed three MVCs of 3–4 s duration interspersed by 60 s recovery.
Then, participants completed a bike ergometer ramp test to task failure for determination of V′O2max and incremental exercise performance, starting from 125 and 150 W for females and males, respectively, with workload continuously increasing every second for an increase of 25 and 30 W·min−1 for females and males, respectively, until task failure, which was defined as a drop below 70 rpm despite strong verbal encouragement for >3 s. After the ramp test, participants rested for 10 min before completing a constant power output test at 110% incremental exercise performance from the prior ramp test to exhaustion (as suggested by Poole and Jones [35] for more accurate V′O2max assessment). In addition, we collected a muscle biopsy from the left vastus lateralis muscle under local anaesthesia at the baseline and 6 weeks experimental trial day.
V′O2max was determined as the highest mean value recorded in any consecutive 30-s period during either the ramp test or constant power output test at 110% incremental exercise performance. Incremental exercise performance was determined as the highest power output attained during the ramp test.
All trials were carried out at the same time of day for each participant, to minimise the influence of circadian hormones. Participants were instructed to standardise meal and fluid intake on the day of each experimental trial, and to refrain from alcohol, caffeine and exercise 24 h before testing. A flowchart depicting the experimental trials is shown in figure 1b.
Experimental procedures
Detailed descriptions of experimental procedures are available in the supplementary material.
Statistics
Statistical analysis was performed in SPSS version 27.0 (IBM, Armonk, NY, USA). Data were tested for normality using the Shapiro–Wilk test and Q-Q plots. Normally distributed data are presented as mean±sd and outcome statistics as delta mean-change with 95% confidence intervals (CI). To determine within-sex changes with the intervention we employed a two-factor mixed linear model on delta change from baseline from each sex separately with treatment (salbutamol/placebo) and time (2, 4 and 6 weeks) as fixed factors and participant as a random factor. To determine between-sex changes with the intervention we employed a three-factor mixed linear model on delta change from baseline with treatment (salbutamol/placebo), time (2, 4 and 6 weeks) and sex (male/female) as fixed factors and participant as a random factor. For Western blot and gel electrophoresis data, we employed a mixed linear model as described above but on absolute pre- and post-6-week values. To estimate between-treatment differences in body composition repartitioning for each sex, we performed a multivariate mixed linear model with treatment (salbutamol/placebo) and composition (lean/fat) as fixed factors.
Results
Participants
In total, 40 healthy well-trained participants (19 females and 21 males) completed the study distributed to either a salbutamol or placebo group (table 1). None of the participants presented with post-exercise reductions in FEV1, airflow obstruction or a positive bronchodilator reversibility test after exercise (table 1). Outside of the intervention, participants were engaged mainly in running, cycling and cross-fit with a weekly training volume of 3–7 h. Mean compliance with the training protocol was 99.6% and compliance with the inhalation regimen was 100%. During the intervention, six participants in the salbutamol group reported side-effects (light-headedness n=1, insomnia n=1, tachycardia n=2, tiredness n=2). Upon completion of the last trial visit, seven participants in the salbutamol group thought they had received salbutamol (32%), eight thought they had received placebo (36%) and seven participants did not know (32%).
TABLE 1.
Baseline characteristics of study participants
| Male# | Female ¶ | |||
| Salbutamol | Placebo | Salbutamol | Placebo | |
| Participants n | 12 | 9 | 10 | 9 |
| Age years | 26±5 | 25±4 | 24±3 | 25±2 |
| Height cm | 182±4 | 182±5 | 169±7 | 165±5 |
| Weight kg | 77.8±10.1 | 76.6±7.9 | 63.6±7.9 | 59.9±5.7 |
| Whole-body lean mass kg | 62.7±6.8 | 63.6±7.9 | 46.1±4.7 | 43.4±4.6 |
| Whole-body fat mass kg | 12.2±3.8 | 10.1±2.6 | 15.2±4.5 | 14.1±3.3 |
| Whole-body fat % | 16.1±3.5 | 13.8±3.7 | 24.6±5.0 | 24.4±5.1 |
| V′O2max mL·min−1 | 4589±602 | 4592±658 | 3424±348 | 3096±306 |
| V′O2max mL·min−1·kg−1 | 59.2±5.6 | 59.9±6.1 | 54.2±5.4 | 52.8±4.7 |
| Incremental exercise performance W | 378±48 | 375±28 | 282±28 | 262±18 |
| FVC L | 5.8±0.7 | 5.9±0.5 | 4.3±0.6 | 3.7±0.2 |
| FEV1 L | 4.8±0.6 | 4.8±0.3 | 3.4±0.4 | 3.1±0.2 |
| FEV1/FVC | 0.83±0.05 | 0.81±0.08 | 0.81±0.11 | 0.85±0.06 |
| Change in FEV1 after exercise % | −3.9±3.6 | −1.2±5.9 | −1.8±4.5 | 0.3±2.7 |
| Change in FEV1 after β2-agonist % | 0.5±3.0 | 3.9±4.3 | 2.6±4.2 | 2.7±2.2 |
Values are presented as mean±sd. Incremental exercise performance is the highest power output achieved during the ramp test to exhaustion. V′O2max : maximal oxygen uptake. FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s. #: n=21; ¶: n=19.
Body composition
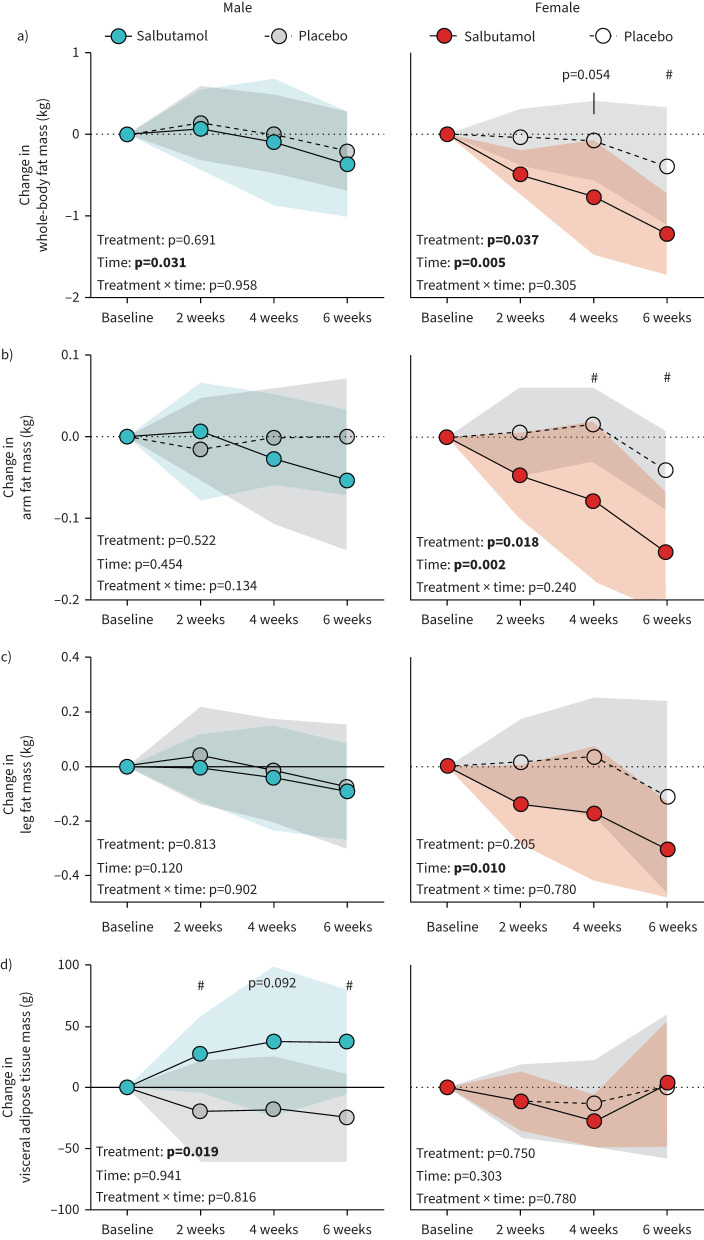
Salbutamol exhibited a sex-specific effect on whole-body fat mass (sex×treatment: p=0.048), for which females lost more fat than males. Specifically, salbutamol-treated females had lost 0.6 kg (95% CI: 0.0 to 1.3; p=0.054) and 0.8 kg (95% CI: 0.5 to 1.6; p=0.039) more fat than placebo after 4 and 6 weeks of treatment, while no treatment differences were apparent in males (figure 2a). The sex-specific effect of salbutamol on fat mass was mainly apparent as a reduction in fat mass of the upper extremities (figure 2b and c), where the salbutamol-treated females lost 0.1 kg (95% CI: 0.0 to 0.2; p=0.020) and 0.1 kg (95% CI: 0.0 to 0.2; p=0.014) of fat mass after 4 and 6 weeks of treatment, respectively, compared to placebo.
FIGURE 2.
Change in body composition during 6 weeks of exercise training with concurrent salbutamol inhalation in moderately trained males (n=21; left panels) and females (n=19; right panels). a) Whole-body fat mass, b) arm fat mass, c) leg fat mass and d) visceral adipose tissue mass. All values are changes relative to baseline. Symbols are means and shaded areas are 95% confidence intervals. p-values in bold denote statistical significance. #: between-group difference (p<0.05).
Visceral adipose tissue increased in a sex-specific manner (sex×treatment: p=0.003), but in contrast to the whole-body changes this was related to an increase in salbutamol-treated males compared to placebo-treated males at weeks 2 and 6 (p=0.047 and p=0.017, respectively), while no changes were apparent in females (figure 2d).
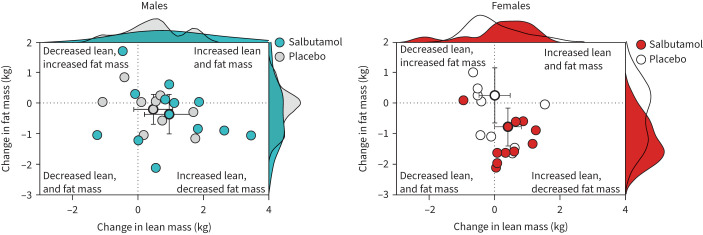
There was no apparent effect of salbutamol treatment on whole-body lean mass when analysing pooled data (treatment main effect: p=0.165) or when analysing data for each sex independently (treatment main effect, females: p=0.714; males: p=0.135). However, in a multivariate mixed model, females treated with salbutamol exhibited a repartitioning effect, lowering fat mass while gaining lean mass, compared to placebo (p=0.011), whereas no such effect was apparent for males (p=0.303; figure 3).
FIGURE 3.
Relationship between change in fat mass and lean mass after 6 weeks of exercise training with concurrent salbutamol inhalation in moderately trained males (n=21; left panels) and females (n=19; right panels). Plots on outer edges of panels denote the distributions of data points on the respective axes and were created in R with base density function using Gaussian distribution. Symbols with bold border are means and error bars are 95% confidence intervals.
V′O2max and incremental exercise performance
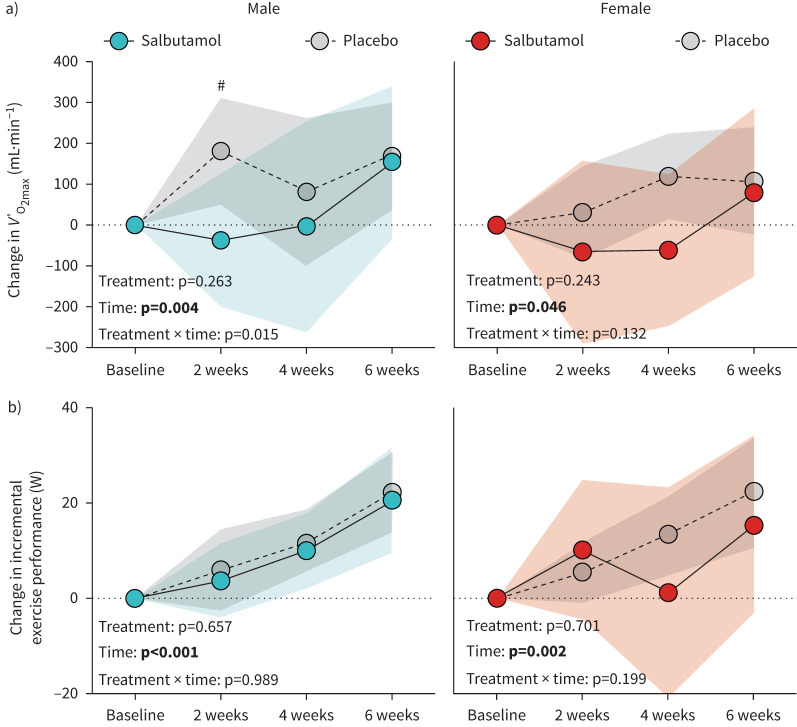
Salbutamol treatment had an overall negative effect on changes in V′O2max (treatment main effect: p=0.014), which was independent of sex (treatment×sex interaction: p=0.964) and mainly attributed to an initially blunted increase in V′O2max in salbutamol-treated participants (figure 4a). After 6 weeks, increases in V′O2max were of similar magnitude between treatments in both males and females.
FIGURE 4.
Changes in a) maximum oxygen uptake (V′O2max) and b) incremental exercise performance during 6 weeks of exercise training with concurrent salbutamol inhalation in moderately trained males (n=21; left panels) and females (n=19; right panels). All values are changes relative to baseline. Symbols are means and shaded areas are 95% confidence intervals. p-values in bold denote statistical significance. #: between-group difference (p<0.05).
Salbutamol treatment had no effect on training-induced increases in incremental exercise performance (figure 4b).
Muscle function
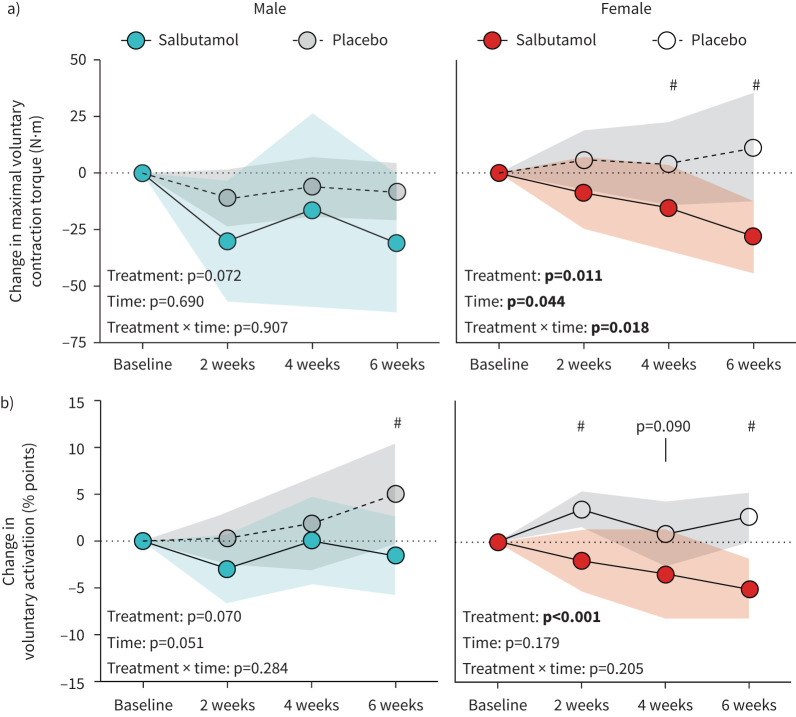
Salbutamol exhibited an overall negative effect on changes in MVC (treatment main effect: p<0.001) independent of sex (treatment×sex interaction: p=0.517). However, the effect of salbutamol treatment on MVC was only significant in females, where the change in MVC was lower compared to placebo by 22 N·m (95% CI: −43 to −1; p=0.039) and 39 N·m (95% CI: −61 to −17; p=0.002) after 4 and 6 weeks of treatment, respectively (figure 5a). The decrease in MVC was paralleled by an overall decrease in voluntary activation level (main effect of treatment: p<0.001), which was not different between sexes (treatment×sex interaction: p=0.517). After 6 weeks of treatment, change in voluntary activation levels was 6 percentage points (95% CI: 12 to 0; p=0.036) lower in males, and 8 percentage points (95% CI: 11 to 4; p<0.001) in females, compared to placebo, respectively.
FIGURE 5.
Change in a) maximal voluntary contraction torque and b) voluntary activation level during 6 weeks of exercise training with concurrent salbutamol inhalation in moderately trained males (n=21; left panels) and females (n=19; right panels). All values are changes relative to baseline. Symbols are means and shaded areas are 95% confidence intervals. p-values in bold denote statistical significance. #: between-group difference (p<0.05).
Time to peak tension after percutaneous electrical stimulation was shortened by 21.5% in females treated with salbutamol compared to placebo (treatment main effect: p=0.016), whereas there was no effect in males (treatment main effect: p=0.263), but with no difference between sexes (treatment×sex interaction: p=0.254). Time to half-relaxation was prolonged in a sex-specific manner (treatment×sex interaction: p=0.007), as females treated with salbutamol exhibited a 34.6% prolonged half relaxation time (treatment main effect: p=0.045), while this effect was not apparent in males (treatment main effect: p=0.530).
Muscle fibre type distribution
Salbutamol had no effect on muscle fibre type distribution with the intervention (treatment×time interaction: p=0.820), and the response was not influenced by sex (sex×treatment×time interaction: p=0.956) (table 2).
TABLE 2.
Muscle fibre type distribution before and after 6 weeks of exercise training with concurrent salbutamol inhalation in moderately trained males (n=21) and females (n=19)
| Male | Female | |||||||
| Placebo# | Salbutamol ¶ | Placebo+ | Salbutamol § | |||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| MHCI % | 62.1±15 | 59.0±12.5 | 61.2±13.3 | 59.5±9 | 64.2±12 | 60.5±9.9 | 70.1±9.1 | 67.3±10.7 |
| MHCII % | 37.9±15 | 41.0±12.5 | 38.8±13.3 | 40.5±9 | 35.8±12 | 39.5±9.9 | 29.9±9.1 | 32.7±10.7 |
Values are presented as mean±sd. MHC: myosin heavy chain. #: n=9; ¶: n=12; +: n=9; §: n=10.
Muscle protein content
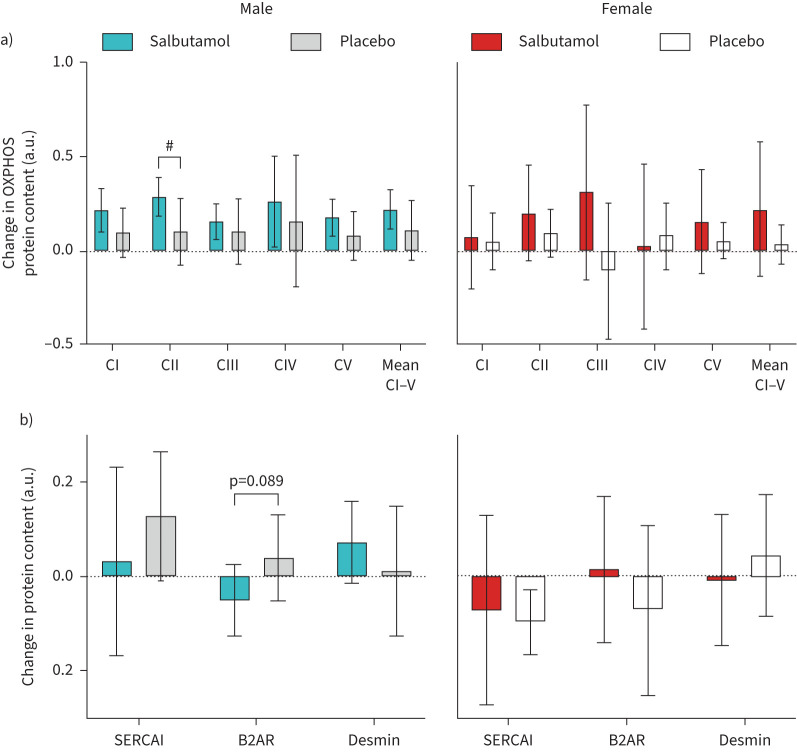
Training-induced increases in electron transport chain complexes were not affected by salbutamol treatment except for complex II, which increased more in males treated with salbutamol compared with placebo (treatment×time: p=0.044) (figure 6a). However, mean muscle complex I–V expression was increased with salbutamol treatment (treatment main effect: p=0.035).
FIGURE 6.
Change in muscle individual and mean muscle content of a) mitochondrial electron transport chain complexes, and b) SECAI, β2-adrenoceptor and desmin content after 6 weeks of exercise training with concurrent salbutamol inhalation in moderately trained males (n=21; left panels) and females (n=19; right panels). Bars are means with 95% confidence intervals. B2AR: β2-adrenoceptor. #: between-group difference (p<0.05).
Muscle protein content of desmin and SERCAI was not affected by salbutamol treatment, whereas β2-adrenergic receptor content tended to be downregulated in males (treatment×time: p=0.089) but not in females (treatment×time: p=0.287) with no between-sex difference (treatment×time×sex interaction: p=0.195).
Discussion
The key takeaway from this study was that inhaled salbutamol had a sex-specific effect on body composition when used by well-trained individuals in conjunction with a period of aerobic training. Specifically, salbutamol lowered fat mass, while retaining or even increasing lean mass in females, but not in males. Another key finding was that inhaled salbutamol had a sex-independent detrimental effect on isometric strength of the quadriceps muscle.
Females in the salbutamol group had a progressive fat mass loss from 0.4 kg at week 2 reaching 1.2 kg at week 6 versus only 0.4 kg at week 6 in the placebo group. Fat mass loss around the arms and legs accounted for a combined 0.4 kg, meaning that the predominant fat loss occurred in the abdominal and thoracic region during the 6-week intervention. This occurred concomitantly with an increase in lean mass in nine out of 10 female participants and highlights the efficacy of salbutamol as a repartitioning agent – even at therapeutic inhaled doses in well-trained lean females. On the other hand, no such effect was evident in males. While the mechanism underlying this sex-dependent difference is not apparently clear, Lee et al. [27] did observe a greater effect of β2-agonist on protein synthesis in females than males. And although we, and others, have previously found β2-agonists to induce leanness in males [15, 28, 33, 36–38], concomitant endurance training was shown to blunt this effect [23, 33]. This collectively suggests that β2-agonists exert sex-specific effects on body composition and that such effects depend on habitual training patterns.
Several studies have demonstrated that prolonged β2-agonist use increases muscle mass and strength [14, 39]. Therefore, we were surprised to find a marked decline in isometric muscle torque in the salbutamol-treated participants. This likely reflects other factors than muscle cross-sectional area since leg lean mass remained stable throughout the study (data not shown). Furthermore, we observed no indication of a muscle fibre-type shift or sarcomeric re-organisation, as reflected by no changes in muscle fibre type composition or in protein abundance of SERCAI and desmin. The degree of voluntary activation declined by a few percentage points in the salbutamol-treated participants, mainly in females, which suggests that participants were not as able to voluntarily recruit as many motor units as before the intervention – hence explaining some of the decline in isometric muscle torque. Salbutamol treatment also revealed some other notable sex-specific changes in muscle function, including a shortened time to peak tension and prolonged relaxation time in females but not in males. This points to salbutamol inducing muscle adaptations pertaining to the on-and-off kinetics of Ca2+ binding to troponin C either by changes in the regulation and expression of troponin isoforms or its interaction with tropomyosin. This is supported by the observation that daily treatment with terbutaline has been shown to lower the abundance of both the α and β chains of tropomyosin during a period of endurance-based training in moderately trained males [23]. Nonetheless, our findings collectively indicate that aerobic training with concomitant salbutamol treatment can impose negative effects on muscle function.
Another notable effect was that the salbutamol-treated participants temporarily had a lower increase in V′O2max than placebo-treated participants during the first 4 weeks of the training intervention after which the salbutamol-treated participants approximated the change in V′O2max observed in the placebo-treated participants at week 6. Although it could be expected that mitochondrial content would also be compromised, as has been shown for prolonged treatment with formoterol [28], our data did not support such an effect. In fact, salbutamol seemed to increase mitochondrial content as determined by electron transport complex protein expression, where there was an overall increase with salbutamol treatment. This could suggest a compensatory mechanism of mitochondrial content, which may explain that incremental exercise performance was not impaired in parallel to V′O2max in the male participants. Although we cannot readily explain the temporal attenuated training response in V′O2max with salbutamol treatment, it is unlikely to be explained by desensitisation of the β2-adrenergic receptor from repeated salbutamol exposure as immunoblotting analysis showed no change in its abundance in muscle homogenates. Furthermore, the participants did not report any changes in exercise habits during the intervention. Consistent with a potential negative effect of inhaled β2-agonist, daily treatment with inhaled terbutaline attenuated V′O2max in moderately trained males subjected to 4 weeks of endurance or resistance training [23, 24], while daily therapeutic inhaled doses of formoterol for 6 weeks lowered V′O2max in well-trained females and males [28]. Thus, it seems clear that prolonged daily treatment with inhaled β2-agonists can negatively affect V′O2max – likely in a dose-dependent manner and dependent on weekly usage. For example, in the present study we only administered salbutamol during training days, in contrast to Jessen et al. 2023 [28] and Hostrup et al. 2017 [23], where β2-agonist was also administered on non-training days.
Few studies have investigated sex-specific adaptations to prolonged inhalation of β2-agonist. The observation that salbutamol may induce a sex-specific effect contrasts with a previous study where we administered inhaled formoterol to well-trained males and females and reported no sex-specific effects on exercise performance or body composition [28]. However, a key difference in the present study was that dosing occurred during training sessions, whereas in the previous study participants inhaled formoterol in the morning and evenings outside of their own training. Considering that concurrent training affects the hypertrophic response to prolonged β2-agonist treatment [33], it is possible that exercise may modulate the responsiveness to treatment between males and females.
Taken together, our results demonstrate that inhaled salbutamol may have several sex-specific effects when used in conjunction with aerobic training. Salbutamol had profound effects in females in terms of fat mass loss but also had negative effects on V′O2max and muscle function. Thus, this study provides new insights into the potential interactions between inhaled salbutamol and endurance exercise, which is of particular relevance to athletes who use inhaled salbutamol as part of their normal training routine.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00657-2023.SUPPLEMENT (543.4KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
This study is registered at www.clinicaltrials.gov with identifier number NCT03902106. Source data are available upon reasonable request to corresponding author.
Author contributions: M. Hostrup conceived the study. M. Hostrup, S. Jessen, C. Weinreich, M. Bjerre and D. Kohlbrenner collected the data. M. Hostrup, S. Jessen, C. Weinreich, D. Kohlbrenner and J. Bangsbo analysed the data. M. Hostrup and S. Jessen wrote the first draft. All authors critically revised the manuscript and approved the final version of the manuscript.
Conflicts of interest: The authors have no conflicting interests.
Support statement: D. Kohlbrenner was supported by a Postdoc Mobility Fellowship of the Swiss National Science Foundation (SNSF). The study was supported by Anti Doping Denmark (principal investigator: M. Hostrup). Funding information for this article has been deposited with the Crossref Funder Registry.
Ethics statement: The study was conducted in accordance with the 2013 Declaration of Helsinki and was approved by the regional ethics committee of Copenhagen (H-18007889). All participants were informed about possible risks involved and gave their oral and written consent before inclusion.
References
- 1.Burns J, Mason C, Mueller N, et al. Asthma prevalence in Olympic summer athletes and the general population: an analysis of three European countries. Respir Med 2015; 109: 813–820. doi: 10.1016/j.rmed.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 2.Selge C, Thomas S, Nowak D, et al. Asthma prevalence in German Olympic athletes: a comparison of winter and summer sport disciplines. Respir Med 2016; 118: 15–21. doi: 10.1016/j.rmed.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 3.Fitch KD. β2-Agonists at the Olympic Games. Clin Rev Allergy Immunol 2006; 31: 259–268. doi: 10.1385/CRIAI:31:2:259 [DOI] [PubMed] [Google Scholar]
- 4.Arie S. What can we learn from asthma in elite athletes? BMJ 2012; 344: e2556. [DOI] [PubMed] [Google Scholar]
- 5.Hsu E, Bajaj T. Be ta 2 Agonists. In: StatPearls. Treasure Island, StatPearls Publishing, 2020. [Google Scholar]
- 6.Morris MJ. Medscape: Asthma Medication. https://emedicine.medscape.com/article/296301-medication?form=fpf Date last updated: 31 July 2023. 2020.
- 7.Hostrup M, Hansen ES, Rasmussen SM, et al. Asthma and exercise-induced bronchoconstriction in athletes: diagnosis, treatment, and anti-doping challenges. Scand J Med Sci Sports 2023; in press [ 10.1111/sms.14358]. [DOI] [PubMed] [Google Scholar]
- 8.Dickinson JW, Whyte GP, McConnell AK, et al. Impact of changes in the IOC-MC asthma criteria: a British perspective. Thorax 2005; 60: 629–632. doi: 10.1136/thx.2004.037499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.W orld Anti-Doping Agency . Prohibited List 2023. www.wada-ama.org/sites/default/files/2022-01/2022list_final_en_0.pdf Date last updated: 1 January 2022. Date last accessed: 3 May 2023.
- 10.Martineau L, Horan MA, Rothwell NJ, et al. Salbutamol, a beta 2-adrenoceptor agonist, increases skeletal muscle strength in young men. Clin Sci (Lond) 1992; 83: 615–621. doi: 10.1042/cs0830615 [DOI] [PubMed] [Google Scholar]
- 11.Hostrup M, Kalsen A, Auchenberg M, et al. Effects of acute and 2-week administration of oral salbutamol on exercise performance and muscle strength in athletes. Scand J Med Sci Sports 2016; 26: 8–16. doi: 10.1111/sms.12298 [DOI] [PubMed] [Google Scholar]
- 12.Jessen S, Reitelseder S, Kalsen A, et al. β2-Adrenergic agonist salbutamol augments hypertrophy in MHCIIa fibers and sprint mean power output but not muscle force during 11 weeks of resistance training in young men. J Appl Physiol (1985) 2021; 130: 617–626. doi: 10.1152/japplphysiol.00553.2020 [DOI] [PubMed] [Google Scholar]
- 13.Sanchez AM, Collomp K, Carra J, et al. Effect of acute and short-term oral salbutamol treatments on maximal power output in non-asthmatic athletes. Eur J Appl Physiol 2012; 112: 3251–3258. doi: 10.1007/s00421-011-2307-3 [DOI] [PubMed] [Google Scholar]
- 14.Hostrup M, Jessen S, Backer V, et al. β2-adrenergic agonists can enhance intense performance and muscle strength in healthy individuals. Allergy 2021; 76: 2318–2319. doi: 10.1111/all.14735 [DOI] [PubMed] [Google Scholar]
- 15.Hostrup M, Jacobson GA, Jessen S, et al. Anabolic and lipolytic actions of β2-agonists in humans and antidoping challenges. Drug Test Anal 2020; 12: 597–609. doi: 10.1002/dta.2728 [DOI] [PubMed] [Google Scholar]
- 16.Caruso JF, Hamill JL, De Garmo N. Oral albuterol dosing during the latter stages of a resistance exercise program. J Strength Cond Res 2005; 19: 102–107. [DOI] [PubMed] [Google Scholar]
- 17.Dyreborg A, Krogh N, Backer V, et al. Pharmacokinetics of oral and inhaled terbutaline after exercise in trained men. Front Pharmacol 2016; 7: 150. doi: 10.3389/fphar.2016.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jessen S, Becker V, Rzeppa S, et al. Pharmacokinetics of salmeterol and its main metabolite alpha-hydroxysalmeterol after acute and chronic dry powder inhalation in exercising endurance-trained men: implications for doping control. Drug Test Anal 2021; 13: 747–761. doi: 10.1002/dta.2978 [DOI] [PubMed] [Google Scholar]
- 19.Haase CB, Backer V, Kalsen A, et al. The influence of exercise and dehydration on the urine concentrations of salbutamol after inhaled administration of 1600 microg salbutamol as a single dose in relation to doping analysis. Drug Test Anal 2016; 8: 613–620. doi: 10.1002/dta.1828 [DOI] [PubMed] [Google Scholar]
- 20.Crane J, Burgess C, Beasley R. Cardiovascular and hypokalaemic effects of inhaled salbutamol, fenoterol, and isoprenaline. Thorax 1989; 44: 136–140. doi: 10.1136/thx.44.2.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amoroso P, Wilson SR, Moxham J, et al. Acute effects of inhaled salbutamol on the metabolic rate of normal subjects. Thorax 1993; 48: 882–885. doi: 10.1136/thx.48.9.882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jartti T, Kaila T, Tahvanainen K, et al. The acute effects of inhaled salbutamol on the beat-to-beat variability of heart rate and blood pressure assessed by spectral analysis. Br J Clin Pharmacol 1997; 43: 421–428. doi: 10.1046/j.1365-2125.1997.00565.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hostrup M, Onslev J, Jacobson GA, et al. Chronic β2-adrenoceptor agonist treatment alters muscle proteome and functional adaptations induced by high intensity training in young men. J Physiol 2018; 596: 231–252. doi: 10.1113/JP274970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemminger AK, Jessen S, Habib S, et al. Effect of β2-adrenergic agonist and resistance training on maximal oxygen uptake and muscle oxidative enzymes in men. Scand J Med Sci Sports 2019; 29: 1881–1891. doi: 10.1111/sms.13544 [DOI] [PubMed] [Google Scholar]
- 25.Merlini M, Whyte G, Marcora S, et al. Improved sprint performance with inhaled long-acting beta2-agonists combined with resistance exercise. Int J Sports Physiol Perform 2019; 14: 1344–1349. doi: 10.1123/ijspp.2018-0921 [DOI] [PubMed] [Google Scholar]
- 26.Dickinson J, Molphy J, Chester N, et al. The ergogenic effect of long-term use of high dose salbutamol. Clin J Sport Med 2014; 24: 474–481. doi: 10.1097/JSM.0000000000000076 [DOI] [PubMed] [Google Scholar]
- 27.Lee P, Birzniece V, Umpleby AM, et al. Formoterol, a highly β2-selective agonist, induces gender-dimorphic whole body leucine metabolism in humans. Metabolism 2015; 64: 506–512. doi: 10.1016/j.metabol.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 28.Jessen S, Lemminger A, Backer V, et al. Inhaled formoterol impairs aerobic exercise capacity in endurance-trained individuals: a randomised controlled trial. ERJ Open Res 2023; 9: 00643-2022. doi: 10.1183/23120541.00643-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Noord JA, Smeets JJ, Maesen FP. A comparison of the onset of action of salbutamol and formoterol in reversing methacholine-induced bronchoconstriction. Respir Med 1998; 92: 1346–1351. doi: 10.1016/S0954-6111(98)90140-8 [DOI] [PubMed] [Google Scholar]
- 30.Louis R, Satia I, Ojanguren I, et al. European Respiratory Society guidelines for the diagnosis of asthma in adults. Eur Respir J 2022; 2101585. doi: 10.1183/13993003.01585-2021 [DOI] [PubMed] [Google Scholar]
- 31.Scott NW, McPherson GC, Ramsay CR, et al. The method of minimization for allocation to clinical trials. A review. Control Clin Trials 2002; 23: 662–674. doi: 10.1016/S0197-2456(02)00242-8 [DOI] [PubMed] [Google Scholar]
- 32.Treasure T, MacRae KD. Minimisation: the platinum standard for trials? Randomisation doesn't guarantee similarity of groups; minimisation does. BMJ 1998; 317: 362–363. doi: 10.1136/bmj.317.7155.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jessen S, Onslev J, Lemminger A, et al. Hypertrophic effect of inhaled β2-agonist with and without concurrent exercise training: a randomized controlled trial. Scand J Med Sci Sports 2018; 28: 2114–2122. doi: 10.1111/sms.13221 [DOI] [PubMed] [Google Scholar]
- 34.World Anti-Doping Agency . Prohibited List 2021. www.wada-ama.org/sites/default/files/resources/files/2021list_en.pdf Date last updated: 1 January 2021. Date last accessed: 25 April 2021.
- 35.Poole DC, Jones AM. Measurement of the maximum oxygen uptake Vo2max: Vo2peak is no longer acceptable. J Appl Physiol (1985) 2017; 122: 997–1002. doi: 10.1152/japplphysiol.01063.2016 [DOI] [PubMed] [Google Scholar]
- 36.Hostrup M, Kalsen A, Onslev J, et al. Mechanisms underlying enhancements in muscle force and power output during maximal cycle ergometer exercise induced by chronic β2-adrenergic stimulation in men. J Appl Physiol (1985) 2015; 119: 475–486. doi: 10.1152/japplphysiol.00319.2015 [DOI] [PubMed] [Google Scholar]
- 37.Acheson KJ, Ravussin E, Schoeller DA, et al. Two-week stimulation or blockade of the sympathetic nervous system in man: influence on body weight, body composition, and twenty four-hour energy expenditure. Metabolism 1988; 37: 91–98. doi: 10.1016/0026-0495(88)90035-2 [DOI] [PubMed] [Google Scholar]
- 38.Hostrup M, Onslev J. The β2-adrenergic receptor: a re-emerging target to combat obesity and induce leanness? J Physiol 2022; 600: 1209–1227. doi: 10.1113/JP281819 [DOI] [PubMed] [Google Scholar]
- 39.Breenfeldt Andersen A, Jacobson GA, Bejder J, et al. An abductive inference approach to assess the performance-enhancing effects of drugs included on the World Anti-Doping Agency prohibited list. Sports Med 2021; 51: 1353–1376. doi: 10.1007/s40279-021-01450-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00657-2023.SUPPLEMENT (543.4KB, pdf)