Abstract
A patient who had been diagnosed with infantile retinal dystrophy developed renal failure in his twenties, at which time the diagnosis was revised to Senior–Loken syndrome. He was poorly compliant. At 36 years old, he experienced a sudden drop in visual acuity in the setting of cramping and fatigue and was found to be in uremic crisis. Six months after the event and its treatment, his vision failed to improved. Optic nerve pallor was out of proportion to the retinal dystrophy, and the presumed reason for his new visual loss was uremic optic neuropathy. The patient’s younger sister also had been diagnosed with infantile retinal dystrophy, and metabolic screening confirmed subclinical renal dysfunction that was to be carefully followed going forward. Infantile retinal dystrophy can be associated with later systemic disease. Early detection of such disease can potentially decrease morbidity. Patients with retinal dystrophy can develop new visual loss from causes other than the retinopathy itself.
Keywords: Optic neuropathy, renal failure, retinal dystrophy, Senior–Loken syndrome, uremia
INTRODUCTION
Early childhood-onset retinal dystrophies are phenotypically and genetically heterogeneous.[1,2] Some cases are the first manifestation of systemic disease, and associated extraocular features may not become evident until years later. While progressive visual loss is often from the retinal dystrophy itself, other causes for visual loss need to be ruled out. The subject of this report is a patient with infantile retinal dystrophy who developed renal failure in his twenties, at which time he was diagnosed with Senior–Loken syndrome.[3] He subsequently experienced sudden bilateral loss of vision in his thirties in the setting of uremic crisis. When evaluated for his new visual complaint 6 months after the episode, he was diagnosed with presumed uremic optic neuropathy.[4]
CASE REPORT
A 36-year-old male was evaluated for sudden bilateral visual loss that occurred 6 months before presentation and had not since improved. Soon after birth, he was diagnosed with infantile retinal dystrophy. At 24 years old, he was evaluated for skin allergy and was found to be in renal failure. His diagnosis was then revised to Senior–Loken syndrome. He underwent a right renal transplant and was started on dialysis. However, he was not always compliant with his dialysis treatments. He was not taking any of his prescribed oral medications. At 36 years old, approximately 6 months before presentation, he experienced sudden bilateral significant visual loss (over seconds, as he recalls) from his baseline, as well as cramping and fatigue. He was evaluated within 1 day and found to be in severe renal failure with uremia. By history, he was not in hypertensive crisis. After dialysis treatments and metabolic optimization, he felt much better, but his new visual loss persisted. Details of treatment at this time were not available for review.
Visual acuity was hand-motion at 1 foot. There was rapid pendular nystagmus and a moderate exotropia. Pupillary reaction was sluggish in both eyes without a relative afferent pupillary defect. Anterior segment examination was unremarkable. The posterior segment examination was significant for bilateral retinal pigment epithelium mottling and atrophy, scattered bone spicule pigmentation, vascular attenuation, and optic nerve pallor [Figure 1]. Cycloplegic refraction was +5.00 in either eye. Full-field electroretinography was nonrecordable. Magnetic resonance imaging of the brain and orbits with and without contrast was within normal limits and confirmed no evidence of compressive optic neuropathy. There were also no soft signs for increased intracranial pressure (i.e., no posterior flattening of the globe, distension of optic nerve sheath, or empty sella). Based on the patient’s history and findings, the presumed explanation for his new visual loss was uremic optic neuropathy. A retinal dystrophy gene panel (G42 Healthcare Abu Dhabi 329-gene panel, January 2023) did not uncover a molecular cause for his syndromic retinal dystrophy.
Figure 1.
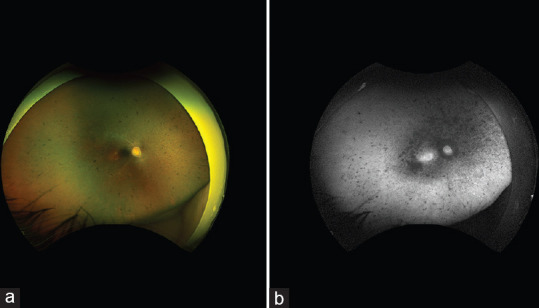
(a) Wide-field retinal imaging of the right eye shows retinal pigment epithelium mottling and atrophy, scattered bone spicule pigmentation, vascular attenuation, and optic nerve pallor. The left eye (not shown) was similar; (b) Wide-field short-wave autofluorescence of the right eye shows confluent areas of hypo-autofluorescence centrally, increased hyper-autofluorescence in the central macula, and scattered areas of hypo-autofluorescence in the mid and far periphery. The left eye (not shown) was similar.
The proband’s younger sister, 26 years old, had also been diagnosed with infantile retinal dystrophy. Because of the proband’s history of associated renal failure, she underwent renal assessment and was found to have subclinical renal dysfunction, for which she began medical management.
DISCUSSION
This case highlights how infantile retinal dystrophy can be the first sign of systemic disease that might not become clinically significant until adulthood and how its earlier detection could potentially decrease morbidity. This case also highlights how new visual loss in patients with retinal dystrophy can be from other cause.
Infantile retinal dystrophy is typically isolated but can be a sign of systemic disease.[1,2] Obtaining a molecular genetic diagnosis for a child’s infantile retinal dystrophy is useful in helping to determine if there is such risk.[2] A common mechanism for syndromic retinal dystrophy is ciliopathy, i.e., mutations in genes responsible for primary cilia function.[5] Primary cilia are microtubule-based organelles that can serve different cellular functions, such as transduction of molecular signals from the extracellular environment, and are present in most human cells. Because the outer segment of the photoreceptor is a modified primary cilium, ciliopathy can result in isolated or syndromic retinal dystrophy. Ciliary signal transduction also plays a critical role in kidney development and homeostasis.[6] When a ciliopathy that results in retinal dystrophy affects other organ functions, the kidney is often affected, resulting in nephronophthisis. Juvenile or adult-onset nephronophthisis is more common than infantile nephronophthisis. Senior–Loken syndrome refers to the association between retinal dystrophy and nephronophthisis.[3] Genetic testing can be helpul in identifying causative mutations, but not finding a mutation does not rule out the syndrome Finding causative mutations in a non-syndromic retinal dystrophy gene reassures that the retinal dystrophy is isolated.
Most infantile retinal dystrophies are progressive. However, when visual loss occurs in a patient with retinal dystrophy, other cause must be ruled out. In the current case, the patient apparently suffered from bilateral optic neuropathy in the setting of uncontrolled uremia, making the most likely diagnosis uremic optic neuropathy. Other causes for optic neuropathy in the setting of kidney failure include increased intracranial pressure (no history of this in the patient), medication toxicity (the patient was not taking any medications at the time of the event), and ischemic optic neuropathy (the patient was young for this diagnosis, which is also less likely to occur simultaneously bilaterally, and, by history, he was not in hypertensive crisis). Uremic optic neuropathy is a rare but recognized phenomenon that can show improvement with urgent dialysis and steroid treatment.[4,7] The current patient was poorly compliant with prescribed medications and follow-up appointments, which likely contributed to his outcome. In the setting of diffuse retinopathy that includes the macula, the diagnosis of optic neuropathy can only be presumed clinically as visual electrophysiology is confounded. This case appears to be the first report of presumed uremic optic neuropathy in the setting of Senior–Loken syndrome. An unrestricted search of English language articles in PUBMED (June 2023) for “Senior–Loken syndrome” did not reveal a similar case.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Patel N, Aldahmesh MA, Alkuraya H, Anazi S, Alsharif H, Khan AO, et al. Expanding the clinical, allelic, and locus heterogeneity of retinal dystrophies. Genet Med. 2016;18:554–62. doi: 10.1038/gim.2015.127. [DOI] [PubMed] [Google Scholar]
- 2.Khan AO. Phenotype-guided genetic testing of pediatric inherited retinal disease in the United Arab Emirates. Retina. 2020;40:1829–37. doi: 10.1097/IAE.0000000000002675. [DOI] [PubMed] [Google Scholar]
- 3.Tsang SH, Aycinena AR, Sharma T. Ciliopathy:Senior-Løken syndrome. Adv Exp Med Biol. 2018;1085:175–8. doi: 10.1007/978-3-319-95046-4_34. [DOI] [PubMed] [Google Scholar]
- 4.Korzets Z, Zeltzer E, Rathaus M, Manor R, Bernheim J. Uremic optic neuropathy. A uremic manifestation mandating dialysis. Am J Nephrol. 1998;18:240–2. doi: 10.1159/000013344. [DOI] [PubMed] [Google Scholar]
- 5.Chen HY, Kelley RA, Li T, Swaroop A. Primary cilia biogenesis and associated retinal ciliopathies. Semin Cell Dev Biol. 2021;110:70–88. doi: 10.1016/j.semcdb.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stokman M, Lilien M, Knoers N. Nephronophthisis-related ciliopathies. In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, Gripp KW, et al., editors. GeneReviews®. Seattle (WA): University of Washington, Seattle; 1993. [Last accessed on 2023 Jun 26]. Available from:https://www.ncbi.nlm.nih.gov/books/NBK368475/ [PubMed] [Google Scholar]
- 7.Winkelmayer WC, Eigner M, Berger O, Grisold W, Leithner C. Optic neuropathy in uremia:An interdisciplinary emergency. Am J Kidney Dis. 2001;37:E23. doi: 10.1053/ajkd.2001.22101. [DOI] [PubMed] [Google Scholar]


