Abstract
Pediatric ocular examinations are often a challenge in the outpatient setting due to limited cooperation of the child. Hence an evaluation under anesthesia (EUA) or sedation is important for a holistic ophthalmic examination. It can be combined with short procedures, such as suture removal and corneal scrappings, both for diagnosis and for the management of several ophthalmic disorders. It can also be performed before planning a surgical intervention to record the baseline characters and formulate or refine a surgical plan. Every EUA must be used as a chance to perform a complete ophthalmic examination rather than perform a single task such as recording the intraocular pressure. This article aims to provide a protocol that can be followed for a complete EUA.
Keywords: Congenital cataract, congenital glaucoma, cycloplegic refraction, electrophysiological tests, examination under anesthesia, intraocular pressure, retinal dystrophies
Pediatric ocular examination is often challenging due to limited cooperation by children. Sometimes, despite best efforts, a child may not cooperate, and examination under anesthesia (EUA) or sedation becomes essential for a comprehensive ophthalmic evaluation. Anesthesia is needed for small procedures such as suture removal or diagnostic procedures like applanation tonometry, gonioscopy, detailed fundus examination, anterior and posterior segment imaging, and electrophysiological tests.[1] It can be a part of surgery to record baseline parameters which are also the part of postoperative follow-up regime.
It is important to remember that every EUA that is planned must be used as an opportunity to do a holistic eye evaluation and diagnostic testing rather than performing a single task. Effective EUA is quick as well as comprehensive. If a multidisciplinary approach is required, coordination is essential to get the right equipment and personnel available at the time of the procedure. It is equally important in such a scenario for one person to takeup the leadership role to coordinate with the rest of the team to avoid confusion. It establishes a clear and unambiguous communication channel with the parents regarding diagnosis, prognosis, and plan of management. This article aims to elaborate the guidelines practiced at our institute and provide insight to make EUAs more comprehensive.
Indications for examination under anesthesia
Pediatric ocular examinations in an office can be incomplete when dealing with very young children, those with special needs, and with complex problems.[1] The various common indications for EUA are enlisted in Table 1.
Table 1.
Indications for examination under anesthesia
| Anterior Segment Indications | Posterior Segment Indications | Miscellaneous |
|---|---|---|
| Congenital and childhood glaucomas | Retinoblastoma and other ocular tumors | Systemic disorders with ophthalmic manifestations such as albinism, neurofibromatosis spectrum, etc. |
| Congenital and childhood cataract | ROP sequelae | Unexplained visual loss, low vision |
| Microphthalmia and other global developmental anomalies | Optic nerve head disorders | Developmental delay and children with special needs |
| Anterior segment dysgenesis (including congenital corneal opacities) |
Inherited retinal disorders | Follow-up after intraocular surgery in children |
| Comprehensive examination of a one-eyed child | Performing diagnostic tests such as Electrophysiology, OCT, UBM, etc. | |
| Chemical and thermal injuries Infants with aphakia |
The examination cart
Comprehensive EUA often requires the use of multiple instruments, as enlisted in Table 2. It is convenient if these instruments are arranged around the head of the examination table and appropriate electrical outlets are readily available to enable a smooth and quick examination.[2] Instruments that require charging must be charged fully before the procedure. Other requirements like dilatation and dark adaptation for electroretinography (ERG) must be rechecked. The team of personnel including anesthetists, nurses, ophthalmologists, optometrists, and technicians should be available. A quick check-in among the team to establish the goal of EUA helps to avoid missing any part of the examination. A surgical cart that serves the dual purpose of storage as well as a working table during anesthesia is the ideal choice.[2]
Table 2.
Equipment needed for examination under anesthesia
| Tonometer: Perkins Tonometer, Tonopen, Rebound tonometer | Indirect Ophthalmoloscope with 20 D and 28 D lens* Direct gonioscopy Lens-Koeppe's Lens (small and medium-sized), Swan Jacob Lens* |
Specialized examination like ERG equipment, Retcam, ultrasound biomicroscopy, handheld OCT cameras, photography equipment |
| Calipers* | Self Illuminating retinoscope | Handheld keratometer |
| Portable slit lamp | Trial lenses, contact lens trial set | Pachymeter, AL biometer |
| Equipment for GA administration such as laryngoscope, LMA, ET tubes, etc. | Basic instrument sets with plain and tooth forceps, eye speculum, and disposable needles* | Topical anesthetics, antibiotics, antiseptics, sterile saline solution, and dilatation drops (labeled with name and date if needed) |
| Lasers and probes | Pre-labelled sample bottles and tubes (in case if lab work is needed) | Specialized pre-prepared medications including antimetabolites etc |
*Sterilized and kept separately
An ideal cart is made of stainless steel and designed according to the operating room specifications with inbuilt wheels, wheel brakes, and handles to enable easy maneuverability and stability.
Anesthetic agents and anesthesia protocol
Sedatives and anesthetic agents are known to affect the intraocular pressure (IOP) measurement in a dose or time-dependent manner. The method of airway access also may interfere with precise IOP recordings. The other factors that affect the IOP are blood pressure[3] (by affecting the episcleral venous pressure), carbon dioxide,[4] oxygen levels,[5] and to a lesser extent body temperature and volume status.[5,6]
Pediatric anesthesiologists’ choice of the induction process are inhalational agents before obtaining an intravenous (IV) line and airway device placement. Inhalational agents act by suppressing the diencephalon, by augmenting signals to chloride channels (neurotransmitter gamma-aminobutyric acid receptors) and potassium channels while depressing neurotransmission pathways, which reduces the IOP by suppressing aqueous production, increasing outflow, and relaxing the extraocular muscles.[7] Fluorinated agents and the older halogenated agents all reduce the IOP sometimes even up to 4 mmHg after induction; however, the newer fluorinated agents such as sevoflurane and desflurane affect the IOP to a lesser extent as they have low blood gas partition coefficients.[8] Among inhalational anesthetics, sevoflurane is the preferred agent during ophthalmic examination via mask ventilation. Desflurane hasn’t been a practical option due to its unsuitability for volatile induction during ophthalmic examination and diagnostic procedures. Propofol and ketamine are other commonly used IV agents used for induction. Their effect on IOP has mixed results, some authors suggest it causes a sharp drop in IOP, while others suggest that it does not affect the IOP.[9,10,11]
The various methods of obtaining airway access are facemasks (administration of inhalational agents for induction), intubation, and laryngeal mask airway (LMA). The facemask, if inappropriately sized, may inadvertently raise the IOP by applying pressure to the adnexa and the manipulation of the jaw.[12] It may cause difficulty for the ophthalmologist to take an accurate IOP reading using an applanation tonometry.
Intubation also increases the IOP stimulating the sympathetic system, which usually lasts for about 2–5 minutes after intubation.[13] Few authors suggest the concurrent use of IV lidocaine, opioids before intubation as it blunts the expected increase in IOP after intubation.[14,15]
An increasing number of pediatric anesthesiologists now use the LMA during general anesthesia (GA) as the hemodynamic responses are less pronounced compared with endotracheal intubation.[16] The use of LMA doesn’t require neuromuscular blocking agents before intubation. It also reduces the time under anesthesia.[17] Studies suggest that LMA use leads to a variable change in the IOP measurement under GA.[18] In this context, it is suggested to measure IOP before the plane of anaesthesia becomes deeper for intubation or insertion of LMA.[19]
The anesthesia for ophthalmic examination and diagnostic procedures also requires appropriate monitoring. Standard monitoring includes visual observation, pulse oximetry, ECG, noninvasive blood pressure, capnography, and respiratory rate. IV access is essential. Oculo-cardiac reflex may rarely be observed during ophthalmic examination. Whenever presented, it is mostly due to traction of the extraocular muscles during a forced duction test or while scleral depression during fundus examination.[10] Several ophthalmic examinations require room lights to be switched off, and this adds to the challenges faced by the anesthetist. A simple table lamp setting at the side might take care of such issues.
Examination protocol
A clear protocol for ocular examination makes the process quick and comprehensive. For example, if a child has glaucoma or is a glaucoma suspect, IOP should be recorded as soon as the child is under plane 2 (surgical) anesthesia and not during deeper planes of anesthesia to minimize the effect of anesthetic agents on IOP.[20] Rest of the eye examination can be done comfortably after intubation.[20]
An examination should have a natural progression starting with retinoscopy, external eye examination, and anterior and posterior segment examination. Repeat examinations can be customized depending on clinical diagnosis. Therapeutic procedures like laser photocoagulation or suture removal are usually done at the end.[21]
IOP evaluation
Several tonometers are available for use, Perkins or similar applanation tonometers are the gold standard for IOP. Careful use of fluorescein stain avoids corneal toxicity in children and maintains good visualization. IOP should be checked without the application of the speculum by gently opening the eyelids and avoiding any pressure on the globe to avoid errors in readings.[22] Two to three readings of IOP should be taken. It is also advisable to use another tonometer such as a tonopen or a rebound tonometer to record IOP in case of corneal irregularity to allow comparison. Many practices use rebound tonometer, which should be discouraged except in clinical settings for screening on normal children (with normal central corneal thickness), as children with ocular conditions often have thicker or irregular corneas, leading to variable values. It is important to correlate corneal thickness and applanation tonometry values while making treatment decisions. Often conditions such as corneal edema, aniridia, or aphakia have dynamic effects on corneal thickness and biomechanics, causing variable effects on IOP.[23] The normal ranges of IOP for age are given in Table 3.[24]
Table 3.
Normal parameters according to age[23]
| Intraocular pressure | Age | Applanation IOP (mmHg) | Pneumotonometer (mmHg±SD) |
|---|---|---|---|
| Premature (26–37 weeks) | 18.3 | ||
| 0–1 year | 4.6 (± 0.5) | 14.5 (± 0.5) | |
| 1–2 years | 4.9 (± 0.5) | 14.6 (± 0.6) | |
| 2–3 years | 5.8 (± 1.0) | 15.3 (± 1.4) | |
|
| |||
| Corneal diameters | Age | Normal (mm) | Possible Glaucoma (mm) |
|
| |||
| Newborn | 9.5–10.5 | 11.5–12 | |
| 1 year | 10–11.5 | 12–12.5 | |
| 2 years | 11.5–12 | 12.5–13.0 | |
| >3 years | >12 | 13–14 | |
|
| |||
| Axial length | Age | Normal (mm) | Possible Glaucoma (mm) |
|
| |||
| Newborns | 16–17 | >20 | |
| 1 year | 20.1 | >22.5 | |
| 2 years | 21.3 | >23 | |
| 3 years | 22.1 | >24 | |
| >3 years | 23 | >25 | |
Retinoscopy and contact lens (CL) trial
A cycloplegic retinoscopy should be performed in all children before any manipulation of the eye if indicated. The timing of the instillation of the cycloplegic drops is important here. The timing of retinoscopy depends on the initial examination or follow-up. The drops can be instilled after the anterior segment evaluation is done, especially if it is the first evaluation. On follow-up examinations, it may be done as the first procedure. Pre-dilated pupils may interfere with visualization of the angle during gonioscopy. Cyclopentolate 1% or homatropine 2% may be used to achieve the desired cycloplegic effect and tropicamide may be used additionally to achieve mydriasis that facilitates easier retinoscopy as well as fundus examination.
The retinoscopy should be performed at least 45 min after the instillation of the cycloplegic drugs.[25] The guidelines for the prescription of glasses according to age and ocular alignment are given in Table 4.[26]
Table 4.
Various syndromes and their anesthetic and ocular implications
| Syndrome | Ocular Features | Anesthetic implication | ||
|---|---|---|---|---|
| Apert syndrome | Glaucoma, cataract, strabismus, proptosis | Possible difficult intubation and choanal atresia, cervical spine fusion, CHD# | ||
| Crouzon syndrome | Glaucoma, cataract, strabismus and proptosis | Possible difficult intubation and elevated intracranial pressure | ||
| Cystinosis | Corneal opacities, retinal degeneration | Chronic renal failure, diabetes mellitus, esophageal varices, hyperthermia | ||
| Downs syndrome | Cataract, strabismus | Airway obstruction, atlantoaxial instability, CHD, more sensitive to atropine | ||
| Ehlers's Danlos syndrome | Retinal detachment, blue sclera, ectopia lentis, keratoconus | Laryngeal trauma possible with intubation, careful positioning, avoid arterial and central lines | ||
| Goldenhar syndrome | Glaucoma, cataracts, dermoids, lacrimal drainage defects | Hemifacial microsomia, possible cervical spine abnormalities, possible difficult intubation, and mask ventilation | ||
| Hallermann-Strieff syndrome | Congenital cataracts, coloboma, microphthalmia, glaucoma | Major craniofacial abnormalities with likely difficult intubation, upper airway obstruction, chronic lung disease | ||
| Hunters’ syndrome | Retinal detachment, optic atrophy | Often difficult intubation, copious secretions, macroglossia, Stiff temporomandibular joint, limited neck mobility, Possible ischemic and valvular heart disease | ||
| Hurlers syndrome | Corneal clouding, retinal degeneration, optic atrophy | Often difficult intubation and mask ventilation, possible cervical spine instability, possible ischemic or valvular heart disease | ||
| Juene syndrome | Retinal degeneration | Limited thoracic excursion, pulmonary hypoplasia, possible renal and hepatic insufficiency | ||
| Lowes syndrome | Cataracts, glaucoma | Renal failure, renal tubular acidosis | ||
| Marfan syndrome | Ectopia lentis, glaucoma, retinal detachment, cataract | Aortic or pulmonary root dilatation, mitral valve prolapse, pectus excavatum, risk for pneumothorax | ||
| Myotonic dystrophy | Cataract, ptosis, strabismus | Prone to myotonic and succinylcholine induces contractions, cardiac conduction abnormalities, sensitive to CNS depressants | ||
| Rubella spectrum | Cataract, microphthalmia, optic atrophy, glaucoma, | Neonatal anemia, pneumonia, thrombocytopenia, CHD, hypopituitarism | ||
| Sticklers’ syndrome | Vitreous degeneration, retinal detachment, cataract | Possible difficult intubation, micrognathia, pulmonary hyperplasia, CHD | ||
| Sturge–Weber syndrome | Choroidal hemangioma, glaucoma | Airway angiomas, CHD, High output failure, seizure disorder, Hyperkalemic response to succinylcholine | ||
| Treacher collins syndrome | Lid defects, microphthalmia, dermoids | Often difficult intubation, mandibular hypoplasia, CHD | ||
| Turners’ syndrome | Ptosis, strabismus, cataracts, corneal scars, blue sclera | Possible difficult intubation and IV access, CHD | ||
| Von Hippel-Lindau syndrome | Retinal hemangioma | Possible increased intracranial pressure | ||
| Von Recklinghausen's disease | Ptosis, proptosis, optic nerve glioma, meningioma, optic atrophy, glaucoma, lisch nodules | Possible difficult mask ventilation and intubation, possible airway tumors, restrictive lung disease, renovascular hypertension, sensitivity to neuromuscular blockers | ||
| Zellweger syndrome | Glaucoma, cataracts, optic atrophy, optic nerve hypoplasia | Micrognathia, possible CHD, renal and adrenal insufficiency | ||
| CHD: Congenital heart disease, CNS: Central nervous system | ||||
CL trials can be combined with EUA to ensure a proper fit. If a CL fitting is required keratometry should be done before IOP recording to get clear mires. Fluorescein dye and a handheld slit lamp may be used to assess the proper fit of CL.[27]
External eye examination
It is important to inspect the ocular adnexa, especially in cases of suspected ocular tumors such as retinoblastoma, gliomas, and hemangiomas to ascertain the orbital involvement. Syringing and probing in cases of neonatal lacrimal duct obstruction (NLDO) may also be done using LMA.[28] In cases where NLDO is confirmed, probing and syringing may also be performed in the same setting.[28]
Anterior segment evaluation
Limbus is an important anatomical landmark that needs to be evaluated in detail. Signs such as limbal stretch should be looked for in congenital and developmental glaucoma. In addition, palisades of Vogt should be examined in case of suspected limbal stem cell deficiency as seen in aniridia, microcornea, post-chemical injuries, and ocular surface disorders.
Corneal diameter is measured using calipers. Automated corneal calipers (used in Implantable collamer lens (ICL) procedures) can also be used in certain situations.
Both vertical and horizontal diameters should be documented. The normal range for corneal diameters according to age is given in Table 3.[29] Corneal diameter is a deciding factor while implanting intraocular lenses (IOLs) in infants.[30] It is also an important parameter to monitor IOP control in children with glaucoma who are on medication or post-surgery. Digital calipers are available for accurate and repeatable measurements.
Slit lamp examination is done using a portable slit lamp or on operating microscope under adequate illumination and magnification. Corneal opacities, Haab's Striae, or breaks in Descemet's membrane should be documented.[31] The level and extent of corneal scarring must be documented in reference to the visual axis in children. Details such as quadrant involved, depth of the opacity, and associated features such as corneal vascularization, presence of associated features such as hair follicles, and fat cell debris as in cases of dermoid should be documented. It should be noted if pupil dilates beyond the corneal opacity, an appropriate management like mydriatic drops or optical iridectomy can be planned.[31]
Pachymetry should be done in all children undergoing EUA before cataract surgery or aphakic children, pseudophakic children, and children with glaucoma as a routine procedure. An average of ten readings along with the maximum and minimum values should be taken. Additional attention should be paid to the standard deviation (SD). An SD of <0.3–0.5 indicates reliable measurements. Any outliers in readings should be interpreted with caution.
Staining of the cornea and conjunctiva (ocular surface) can be done using a sterilized 0.1% sodium fluorescein strip to look for associated epithelial defects while examining a case of chemical injury or corneal ulcer. Eversion of the lids, including double eversion of the upper lid must be done in cases of suspected foreign bodies or chemical injuries. Areas of ocular surface and limbal ischemia in the cases of chemical injuries should be noted.[32]
Smartphones with attachments can be used along with a microscope to get high-resolution good quality photographs. Several photography techniques have been described. Corneal scraping samples can be taken during an EUA. However, this needs to be done in an operation theatre designated for infected cases and sample tubes or strips need to be kept ready.
Iris structure and the pupil should be examined in detail in cases of suspected anterior segment dysgenesis.[31] The pattern of the iris may be abnormal in cases of anterior segment dysgenesis associated with secondary glaucomas. Other features such as iris neovascularization and iris cysts should be documented.
In children, post-glaucoma surgery attention should be paid to bleb health, tube position, corneal clarity, and myopic shift [in terms of both refraction and axial length (AL) and pachymetry]. Releasable suture removal can also be performed. Similarly, in children who have undergone corneal grafting, evaluation of the graft health and clarity, suture placement or revision or removal can be done. Glaucoma and the anterior segment evaluation are summarised in Figs. 1 and 2 respectively.
Figure 1.
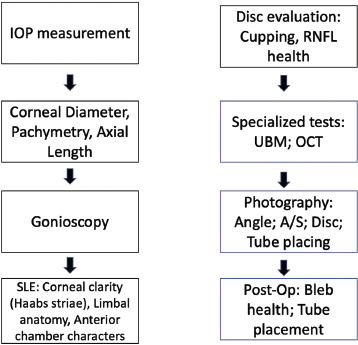
Flowchart for glaucoma evaluation
Figure 2.
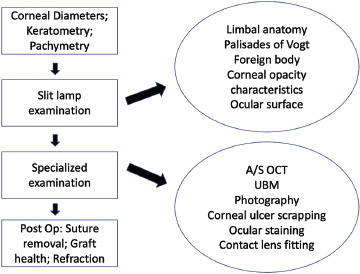
Flowchart for the evaluation of anterior segment
Gonioscopy
A direct gonioscopy is done using a Koeppe lens (which provides almost 160° field of view). The Koeppe diagnostic gonioscopy lens is available in various sizes[33] (commonly used are small – 12 mm, medium – 14 mm, and large – 16 mm contact diameters), and an appropriate size of the lens should be used (referencing from horizontal corneal diameter). It is advisable to use a hydroxypropyl methylcellulose gel over saline as a coupling medium. It is a superior coupling medium and prevents inadvertent injury to the cornea. After placing the Koeppe's lens over the cornea, the angle is examined with the help of a handheld slit lamp. An indirect ophthalmoscope (IDO) and a 20 D aspheric lens to help with magnification can be a reasonable alternative. Microscope which allows tilting of the eyepiece to see the angle can also be used. A 360° examination should be done and documented. Status of angles whether open or closed should be noted along with the presence or absence of iris processes. A note should also be made about the iris insertion whether it is normal, anterior insertion, or a wraparound iris configuration. In cases of trauma look for recession of angle or cyclodialysis cleft. Gonioscopy must always be done in both eyes and compared. In cases of anterior segment dysgenesis, the presence of iridocorneal, irido lenticular, and irido-corneo-lenticular adhesions should be looked for. The presence of iris and ciliary body cysts should also be seen. Photography of angle structures can be done using a Retcam (Eyecam) with an anterior segment module.[34]
Posterior segment evaluation
Examination under anesthesia offers an opportunity to examine the entire retina with scleral depression and to document the findings[35] for future reference.
The optic nerve size, shape, color, and any rim changes must be noted. A complete evaluation including careful scleral depression should be performed and all findings documented on the fundus drawing chart.
If ERG is planned, the fundus examination is performed after the completion of the tests. The cornea can be kept moist with lubricants to avoid distortion of the image. It is also important to wash off residual fluorescein stains that could interfere with accurate examination.
It is possible to get good fundus photos with mobile cameras with appropriate attachments to the microscope without requiring expensive equipment.[35] If available, Retcam fundus photography provides excellent photographs which can be used for documentation as well as diagnosis. This is especially helpful where serial follow-up is required such as in tumors or in retinopathy of prematurity (ROP).
This is the time to decide on and perform investigations like fluorescein angiography that are invaluable to diagnose vascular abnormalities involving the periphery, such as familial exudative vitreoretinopathy (FEVR), ROP, Coats disease, and intraocular tumors. A summary of the posterior segment evaluation is shown in Fig. 3.
Figure 3.
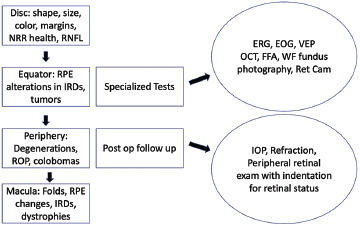
Flowchart for the evaluation of posterior segment
Additional therapeutic interventions such as laser photocoagulation, cryopexy, surgery, and tumor management can be performed after discussion with the parents, keeping the child under anesthesia, and taking appropriate consent.
Additional Procedures
Anterior and posterior segment photography
Ocular photography using handheld cameras or a high-resolution mobile camera is an essential part of EUA. It is an opportunity to document clinical findings as part of follow-up and to avoid medico-legal issues. Simple macro photography setting in a mobile camera (preferably dedicated) is often sufficient and cost-effective.[35] However, it is important to choose high magnification, flash ON with high-resolution setting with good exposure on the area of interest.[36,37,38,39] Images should be transferred to the patient's records as per institutional policies.
Ophthalmic imaging systems such as the RetCam EnvisionTM enable quick and easy capture of wide-field images of the anterior and posterior segments.[40] It comes with a handpiece with an inbuilt camera and barrier filter for fluorescein angiography. It comes with two lens pieces, one used for retinal imaging in newborn or premature infants capturing up to 130° field of view, and the other is a portrait lens used to capture the external eye and facial imaging. A coupling agent such as hydroxy propyl methyl cellulose gel or viscoelastic agent is used. It is recommended that time exposure for imaging per eye should not exceed more than 10 min at a stretch to prevent adverse events such as corneal drying and retinal phototoxicity.[40]
Biometery
This is often done as a part of follow-up or to calculate IOL power in children undergoing cataract surgery. AL measurements can be done using an ultrasound A-scan biometer. The head position should be straight to prevent any errors in measurement. Readings with corresponding reliable A-scan spikes should be considered as well as a SD of 0.2 and less overall.
Handheld spectral domain optical coherence tomography (SD-OCT) for anterior and posterior segment
Various portable, noncontact, handheld SD-OCT devices are available, such as the Bioptigen Inc., OptoVue iVue, and so on.[41] It consists of an imaging handpiece connected via a 1.3 m flexible cable to an SD-OCT engine. The probe has a wide focus range of +10 D to −12 D. The time to image a single eye should not be more than 5 min. A separate coupling agent need not be used; artificial lubricants or saline drops generally serve the purpose.[41] Some deep focus models are also newly available which enable better visualization of the angles.[41] This is useful in the diagnosis of macular conditions such as foveal hypoplasia, inherited and pigmentary retinal dystrophies, macular dystrophies, and their prognostication.
Electrophysiology
ERG is an important diagnostic tool in suspected retinal dystrophies which often requires GA in very young or uncooperative children. The pupils should be fully dilated using a mydriatic agent. The pupil size at the time of testing should be recorded. Since the ERG requires dark adaptation, eyes can be patched 30 min before induction to save time. The electrode should be inserted under dull illumination or using a red-light illumination. In case there has been exposure to bright light, the child should be re-dark adapted for at least five more minutes before starting the test[42,43,44,45].
Fundus photography and wide-field angiogram, if planned, should be done after the ERG, as pre-exposure of the retina to the high-intensity flashes may interfere with ERG recordings. Pediatric speculum containing electrode models are preferred for infants and children. Electrode position should be monitored to avoid artifacts in the recording. GA can attenuate the ERG recording; hence results must be interpreted with care.[46,47]
Ultrasound biomicroscopy (UBM)
UBM is a contact procedure and time-consuming in children; however, it is still valuable equipment to assess lens iris diaphragm, especially in trauma, lens zonular status, anterior segment tumors, and anterior segment dysgenesis. It is valuable to assess the extent of iris cysts congenital or acquired. Caution should be exercised to avoid penetrating injuries or infective conditions. Sterile cups should be used, and smaller cups may need to be kept ready for infants and children.[48]
Documentation and recordings
It is important to document all the findings in a systematic manner with dates and signatures, for future follow-up as well as for medico-legal purposes. It also works as a checklist for the clinical team during follow-up to compare. Appropriate drawings and photographs as well as recordings must be incorporated in patients’ medical records as electronic files or hard copies.
Complications and precautions
GA-related complications should be explained in detail to the parents while taking consent. Preanesthetic evaluation of systemic disorders in syndromic conditions needs to be done as per the requirement to avoid complications. Table 3 enumerates various syndromes and their anesthetic and ocular implications. Special consent may be necessary, especially in stand-alone ophthalmic facilities while planning EUA in high-risk children. During the procedure, corneal exposure or corneal abrasions occur if the ocular surface is not kept moist. Inadvertent leaks may occur during suture removal, and equipment for re-suturing should be kept readily available.
Conclusion
Examination under anesthesia is an extremely important service in uncooperative children and to those who require a multidisciplinary approach. It allows high-quality eye examination and documentation in a short period. Proper planning and coordination can make the process smooth and stress-free for both clinicians and the patient's family.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mahmoud AO, Ayanniyi AA, Oyedepo OO. Pediatric ophthalmic indications for examination under anesthesia in IIorin, Nigeria. Ann Afr Med. 2010;9:181–3. doi: 10.4103/1596-3519.68357. [DOI] [PubMed] [Google Scholar]
- 2.Shields MB, Jarrell JA. Instrument cart for examinations with patients under anaesthesia. Arch Ophthalmol. 1980;98:1850–1. doi: 10.1001/archopht.1980.01020040702022. [DOI] [PubMed] [Google Scholar]
- 3.Klieen BEK, Klien R, Knudtson MA. Intraocular pressure and systemic blood pressure: Longitudinal prespective: The beaver dam eye study. Br J Ophthalmol. 2005;89:284–7. doi: 10.1136/bjo.2004.048710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hvidberg A, Kessing S, Fernades A. Effect of changes in PCO2 and body position on intraocular pressure. Acta Ophthalmol. 1981;59:465–75. doi: 10.1111/j.1755-3768.1981.tb08331.x. [DOI] [PubMed] [Google Scholar]
- 5.Kergoat H, Faucher C. Effects of oxygen and carbogen breathing choroidal hemodynamics in humans. Investig Ophthalmol Vis Sci. 1999;40:2906–11. [PubMed] [Google Scholar]
- 6.Gofman N, Cohen B, Matot I, Cattan A. Do intraocular pressure measurements under anaesthesia reflect the awake conition? J Glaucoma. 2017;26:299–302. doi: 10.1097/IJG.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 7.Ebert T, Trotier T, Arian S, Uhrich T, Barneyy J. High concentrations of isoflurane do not block the sympathetic nervous system activation from desflurane. Can J Annaesth. 2001;48:133–8. doi: 10.1007/BF03019725. [DOI] [PubMed] [Google Scholar]
- 8.Park JT, Lim HK, Jang KY, Um DJ. The effects of desflurane and sevoflurane on the intraocular pressure associated with endothracheal intubation in pediatric Ophthalmic surgery. Korean J Anesthesiol. 2013;64:117–21. doi: 10.4097/kjae.2013.64.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Persson J. wherefore ketamine? Curr Opin Anaesthesiol. 2010;23:455–60. doi: 10.1097/ACO.0b013e32833b49b3. [DOI] [PubMed] [Google Scholar]
- 10.Mirakhur RK, Shepherd WFI, Darrah WC. Propofol or thiopentone: Effects of intraocular pressure associated with induction of anaesthesia and tracheal intubation (facilitated with suxamethonium) Br J Anaesthesia 1987; 2011;95:1102–5. doi: 10.1093/bja/59.4.431. [DOI] [PubMed] [Google Scholar]
- 11.Hanna SF, Ahmad F, Pappas AL, Mikat-Stevens M, Jellish WS, Kleinman B, et al. The effect of propofol/remifentanil rapid-induction technique without muscle relaxants on intraocular pressure. J Clin Anesth. 2010;22:437–42. doi: 10.1016/j.jclinane.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 12.France N. 2nd. New York: Churchill Livingston; 1989. Anesthesia for Pediatric Ophthalmologic Surgery; pp. 1065–95. [Google Scholar]
- 13.Johnsson M, Dabrowski M, Eriksson L. Pharmalogical characteristics of the inhibition of neuromuscular blocking agents at human adult muscle nicotine acetylcholine receptor. Anaesthesiology. 2009;110:1124–52. doi: 10.1097/ALN.0b013e31819fade3. [DOI] [PubMed] [Google Scholar]
- 14.Prys-Roberts C, Greene L, Meloche R, Foex P. Studies of anaesthesia in relation to hypertension. II. Haemodynamic consequences of induction and endotracheal intubation. Br J Anaesth. 1971;43:531–47. doi: 10.1093/bja/43.6.531. [DOI] [PubMed] [Google Scholar]
- 15.Shribman A, Smith G, Achola K. Cardiovascular and catecholamine response to laryngoscopy with and without tracheal intubation. Br J Anaesth. 1987;59:295–9. doi: 10.1093/bja/59.3.295. [DOI] [PubMed] [Google Scholar]
- 16.Patel A, Clark SR, Schiffmiller M, Schoenberg C, Tewfik G. A survey of practice patterns in the use of laryngeal mask by pediatric anesthesiologists. Pediatr Anesth. 2015;25:1127–31. doi: 10.1111/pan.12727. [DOI] [PubMed] [Google Scholar]
- 17.Cone F, Steinhart M, Oglesby E, Kalesnykas G, Pease ME, Quigley HA, et al. The effects of anesthesia, mouse strain, and age on intraocular pressure and an improved murine model of experimental glaucoma. Exp Eye Res. 2012;99:27–35. doi: 10.1016/j.exer.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watts P, Lim M, Gandhewar R. The effect of laryngeal mask airway insertion on intraocular pressure measurement in children receiving general anesthesia. Am J Ophthalmol. 2007;144:507–10. doi: 10.1016/j.ajo.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Watts P, Lim MK, Gandhewar R, Mukherjee A, Wintle R, Armstrong T, et al. The effect of laryngeal mask airway insertion on intraocular pressure measurement in children receiving general anesthesia. Am J Ophthalmol. 2007;144:507–10. doi: 10.1016/j.ajo.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Pirlich N, Grehn F, Mohnke K, Maucher K, Schuster A, Wittenmeier E, et al. Anaesthetic protocol for paediatric glaucoma examinations: The prospective EyeBIS Study protocol. BMJ Open. 2021;11:e045906. doi: 10.1136/bmjopen-2020-045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liebermann M, Hetherington J. Managament of infantile glaucoma. In: Koch D, Park D, Paton D, editors. Current management in Ophthalmology. New York: Churchill Livingston; 1983. [Google Scholar]
- 22.Epley KD, Tychesn L, Lueder GT. The effect of an eyelid speculum on intraocular pressure measurement in children. Am J Ophthalmol. 2002;134:926–7. doi: 10.1016/s0002-9394(02)01793-2. [DOI] [PubMed] [Google Scholar]
- 23.Stamper L., Lierbermann MF, Drake MV. Philadelphia: Elseivier; 2009. Becker-Shaffer's diagnosis and therapy of the glaucomas. Chapter 16- Developmental and Childhood Glaucomas. [Google Scholar]
- 24.Pensiero S, Da Pozzo S, Perissutti P, Cavallini GM, Guerra R. Normal intraocular pressure in children. J Pediatr Ophthalmol Strabismus. 1992;29:79–84. doi: 10.3928/0191-3913-19920301-05. [DOI] [PubMed] [Google Scholar]
- 25.Mjor E, Dutson T, Moshirfar M. Cycloplgia in children: An optometrist's perspective. Clin Optom (Auckl) 2020;12:129–33. doi: 10.2147/OPTO.S217645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace DK, Morse CL, Melia M, Sprunger DT, Repka MX, Lee KA, Christiansen SP; American Academy of Ophthalmology Preferred Practice Pattern Pediatric Ophthalmology/Strabismus Panel Pediatric Eye Evaluations Preferred Practice Pattern®: I. Vision Screening in the Primary Care and Community Setting; II. Comprehensive Ophthalmic Examination. Ophthalmology. 2018;125:184–227. doi: 10.1016/j.ophtha.2017.09.032. doi:10.1016/j.ophtha.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhary Z, Vanathi M. New Delhi: Jaypee Brothers; 2012. Post Graduate ophthalmology-Volume 1. Essentials in Contact lens Practice. [Google Scholar]
- 28.Chaudhary Z, Vanathi M. New Delhi: Jaypee Brothers; 2012. Post Graduate ophthalmology-Volume 2. Anomalies of the lacrimal System. [Google Scholar]
- 29.Kiskis AA., Markowitz SN, Morin JD. Corneal diameter and axial length in congenital glaucoma. Can J Ophthalmol. 1985;20:93. [PubMed] [Google Scholar]
- 30.Self JE, Taylor R, Solebo AL, Biswas S, Parulekar M, Dev Borman A, et al. Cataract management in children: A review of the literature in children and current practice across five large UK centres. Eye. 202;34:2197–218. doi: 10.1038/s41433-020-1115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krachmer JH, Mannis MJ, Holland EJ. Cornea: Fundamentals, Diagnosis and Management. 3rd. Mosby Elsevier; 2011. Congenital corneal opacities: Diagnosis and management; pp. 239–65. [Google Scholar]
- 32.Nguyen AYM, Chamberlain K, Holland AJA. Pediatric chemical burns: A clinical review. Eur J Pediatr. 2021;180:1359–69. doi: 10.1007/s00431-020-03905-z. [DOI] [PubMed] [Google Scholar]
- 33.Clark RA, Ely A, Wong Mom, Freedman SF, Kozak A, Epley D, et al. Am Acad Ophthalmol. EyeWIKI; 2022. Primary congenital glaucoma. [Google Scholar]
- 34.Allengham RR, Damji KF, Freedmann S. Shields Textbook for Glaucoma. 6th. Delphialphia, US: Lippincott Williams and Wilkins; 2010. The basic aspects of glaucoma, gonioscopy and other techniques for assessing the anterior segment; pp. 72–80. [Google Scholar]
- 35.Perera SA, Baskaran M, Friedman DS, Tun TA, Htoom HM, Kumar RS, et al. Use of EyeCam for imaging the anterior chamber angle. Invest Ophthalmol Vis Sci. 2010;51:2993–7. doi: 10.1167/iovs.09-4418. [DOI] [PubMed] [Google Scholar]
- 36.Yip IVM, Tacea F, Dodeja R, Pendlebury J, Yeo DCM. Ophthalmology examinations under anaesthesia carried out on non-ophthalmology lists in a specialist children's hospital. Eye. 2022;36:669–70. doi: 10.1038/s41433-021-01478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pujari A, Saluja G, Agarwal D, Selvan H, Sharma N. Clinically useful smartphone ophthalmic imaging techniques. Graefes Arch Clin Exp Ophthalmol. 2021;259:279–87. doi: 10.1007/s00417-020-04917-z. [DOI] [PubMed] [Google Scholar]
- 38.Chandrakanth P, Ravichandaran R, Nischal NG, Subhashini M. Trash to treasure retcam. Indian J Opthalmol. 2019;67:541–4. doi: 10.4103/ijo.IJO_1524_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puthalath AS, Gupta N, Samanta R, Singh A, Kumawat D, Mittal SK. Cobalt blue light unit filter- A smartphone attachemetn for blue light photography. Indian J Ophthalmol. 2021;69:2841–3. doi: 10.4103/ijo.IJO_3697_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berrocal AM. Digital imaging for screening, diagnosis and treatment: The RetCam enables clinicians to improve care in pediatric ophthalmology. Retina Today; 2009:60–3. [Google Scholar]
- 41.Sayeram S, Buckland E. Technology and Services Section. Boptigen, Inc; Research Triangle Park, MC, US: Bioptigen high-resolution spectral domain optical coherence tomography imaging for clinical and pre-clinical research application. [Google Scholar]
- 42.Hahn P, Migacz J, Connell RO, Maldonado RS, Izatt JA Toth CA. The use of optical coherence tomography in intraoperative ophthalmic imaging. Ophthalmic Surg Lasers Imaging. 2011;42:S85–94. doi: 10.3928/15428877-20110627-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marmor MF, Arden GB, Nilsson SE, Zrenner E. Standard for clinical electroretinography. Arch Ophthalmol. 1989;107:816–9. [Google Scholar]
- 44.Marmor MF, Zrenner E. Standard for Clinical Electroretinography (1994 Update) Doc Ophthalmol. 1995;89:199–210. doi: 10.1007/BF01203373. [DOI] [PubMed] [Google Scholar]
- 45.Marmor MF, Zrenner E. Standard for clinical electro-oculography. Arch Ophthalmol. 1993;111:601–4. doi: 10.1001/archopht.1993.01090050035023. [DOI] [PubMed] [Google Scholar]
- 46.Marmor MF, Zrenner E. Standard clinical electroretinography (1999 update) Doc Ophthalmol. 199;97:143–561. doi: 10.1023/a:1002016531591. [DOI] [PubMed] [Google Scholar]
- 47.Lin SL, Shiu WC, Liu PC, Cheng FP, Lin YC, Wang WS. The effects of different anesthetic agents on short electroretinography protocol in dogs. J Vet Med Sci. 2009;71:763–8. doi: 10.1292/jvms.71.763. [DOI] [PubMed] [Google Scholar]
- 48.Janssens R, van Rijn LJ, Eggink CA, Jansonius NM, Janssen SF. Ultrasound biomicroscopy of the anterior segment in patients with primary congenital glaucoma: A review of the literature. Acta Ophthalmol. 2022;100:605–13. doi: 10.1111/aos.15082. [DOI] [PubMed] [Google Scholar]


