Abstract
Parasitic plants invade their host through their invasive organ, the haustorium. This organ connects to the vasculature of the host roots and hijacks water and nutrients. Although parasitism has evolved independently in plants, haustoria formation follows a similar mechanism throughout different plant species, highlighting the developmental plasticity of plant tissues. Here, we compare three types of haustoria formed by the root and shoot in the plant parasites Striga and Cuscuta. We discuss mechanisms underlying the interactions with their hosts and how different approaches have contributed to major understanding of haustoria formation and host invasion. We also illustrate the role of auxin and cytokinin in controlling this process.
Keywords: Auxin, Cuscuta, cytokinin, haustoria, host, invasion, lateral roots, parasitic plant, Striga
Parasitic plants invade their host through the formation of haustoria. Here we discuss the development of these organs and highlight the role of the phytohormones auxin and cytokinin.
Introduction
Unlike animals, where organogenesis takes place during embryogenesis, plants form organs post-embryonically and continue generating new ones throughout their life cycle. This developmental modularity allows plants to adapt to changes in their environment. Parasitic plants invade their host and deprive it of water and nutrients, drastically reducing the host’s fitness and performance, impacting the yield, and causing a severe loss in agricultural fields (Berner et al., 1995; Mishra and Kogan, 2009; Rodenburg et al., 2016). One percent of flowering plants are considered parasitic plants (Westwood et al., 2010). They interact with the host plants by forming a haustorium, a specialized organ used to penetrate the root or shoot tissues of the host, which acts as an interface for the exchange of water and nutrients between the host and parasite (Yoshida et al., 2016).
Parasitic plants can be categorized into different types, including holoparasites, which are unable to perform photosynthesis and are fully dependent on the host to obtain water and nutrients. On the other hand, hemiparasitic plants do not fully depend on the host, because they are photosynthetically active (Westwood et al., 2010).
The best-studied examples of root and shoot parasites are those of the families Orobanchaceae and Convolvulaceae (Yoshida et al., 2016). Parasites from the Orobanchaceae family, including the genera Orobanche and Striga, invade the root system (Table 1). These plants produce small seeds (between 0.2 mm and 0.5 mm) that can remain in the soil for up to 10 years and germinate when a host is nearby (Musselman, 1980).
Table 1.
Summary of parasitic plant species mentioned in this review
| Plant species | Family | Target, type of parasitism | Host | Haustorium type |
|---|---|---|---|---|
| Orobanche spp. (broomrape) | Orobanchaceae | Root, obligate holoparasite (Musselman, 1980) |
Tomato, tobacco, potato, hemp, sunflower, peas, lentils (Musselman, 1980) |
Terminal haustoria, lateral haustoria (Musselman, 1980; Westwood et al., 2010) |
| Striga spp. (witchweed) | Orobanchaceae | Root, obligate hemiparasite (Musselman, 1980) |
Maize, sorghum, sugarcane, rice, millet (Musselman, 1980) |
Terminal haustoria, lateral haustoria (Musselman, 1980; Westwood et al., 2010) |
| Phtheirospermum ssp. | Orobanchaceae | Root, facultative hemiparasite (Ishida et al., 2011) |
Medicago, Arabidopsis (Cui et al., 2016; Irving et al., 2019) |
Lateral haustoria (Cui et al., 2016) |
| Triphysaria versicolor | Orobanchaceae | Root, facultative hemiparasite (Honaas et al., 2019) |
Medicago, Arabidopsis, tomato, maize, rice (Honaas et al., 2019) |
Lateral haustoria (Matvienko et al., 2001) |
| Cuscuta spp. (dodder) | Convolvulaceae | Stem, obligate holoparasite (Kaiser et al., 2015) |
Alfalfa, potato, sweet pepper, tomato (Kaiser et al., 2015) |
Haustoria derived from stem (Vaughn, 2002) |
Striga, also known as witchweed, is an obligate parasite that grows predominantly in Africa, India, and Southeast Asia and infests crop plants such as maize, sorghum, rice, and millet, causing enormous yield losses (Musselman, 1980). Striga seeds germinate only in close proximity to the host roots, as they can sense compounds released from host roots, such as strigolactone. The root of the Striga seedling then grows towards the host roots and invades its tissues (Fig. 1A–C) (Musselman, 1980).
Fig. 1.
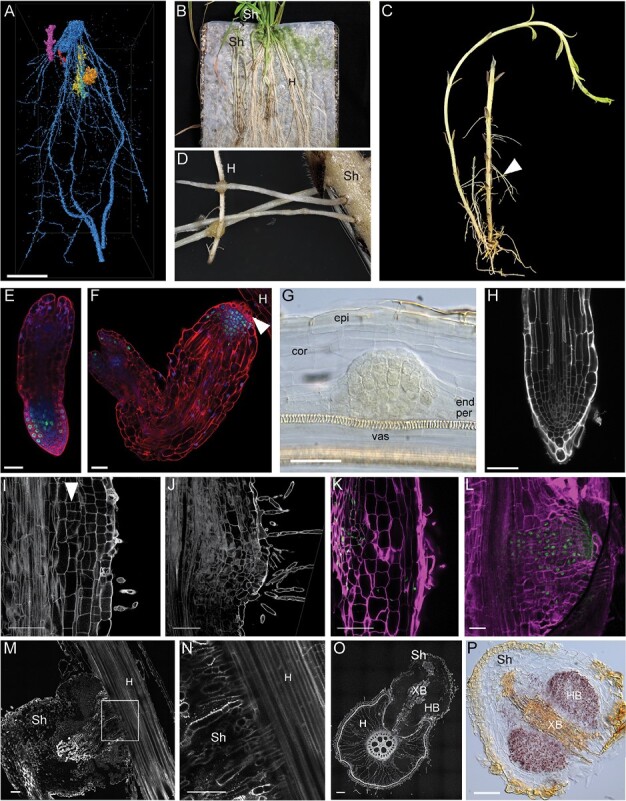
Terminal and lateral haustorium development in Striga hermonthica. (A) Micro-CT 3D reconstruction and post-processed image segmentation depicting the association of Striga seedlings (orange, yellow, and purple) attached to the rice roots (blue). (B) Macrophotograph showing Striga plants grown on rice as a host plant on top of nylon mesh. (C) Macrophotograph showing the Striga plant isolated from the host plants. Note the emerging adventitious roots (arrowhead). (D) Attachment of Striga adventitious roots to the host roots by lateral haustoria. (E and F) Confocal image showing the root meristem of Striga seedlings directly after germination (E) and before attachment to the host root (F). Roots were stained with modified pseudo-Schiff propidium iodide (mPS-PI) (red) (Truernit et al., 2008), dividing cells were visualized by 5-ethynyl-2ʹ-deoxyuridine (EdU) staining (green), and nuclei were stained with Hoechst (blue); the arrowhead points to haustorial hairs. (G) Lateral root primordium of Striga roots stained with Lugol, cleared with chloral hydrate, and visualized with Nomarski microscopy. (H) Confocal image of a Striga lateral root tip; cell walls stained with mPS-PI. (I and J) Confocal images of longitudinal vibratome sections of a Striga root developing a lateral haustorium; cell walls and starch granules were stained with mPS-PI; the arrowhead points to periclinal cell divisions in the inner cortex upon haustorium initiation. (K and L) Confocal longitudinal sections showing cell divisions in developing Striga lateral haustoria. EdU-stained nuclei are green and cell walls stained with SCRI Renaissance are in magenta. (M) Longitudinal section of a Striga lateral haustorium during attachment to the host plant; (N) magnification of the area marked in (M). (O) Cross-sections of a Striga lateral haustorium attached to a host root; cell walls stained with mPS-PI. (P) Starch granules accumulation in the Striga lateral haustorium, visualized by Lugol’s staining. cor, cortex; end, endodermis; epi, epidermis; H, host; HB, hyaline body; per, pericycle; Sh, Striga hermonthica; vas, vasculature; XB, xylem bridge. Scale bars 20 μm (A, B), 50 μm (E, F, G, H, I, J, K, L, M, N, O, P).
The stem parasite Cuscuta spp. (Cuscuta) from the Convolvulaceae family, also called dodder, is an obligate stem holoparasite that originates from North America and has spread all over the world (Table 1). Cuscuta causes enormous economic loss due to its wide host range, which includes many important crops, such as alfalfa, potato, sweet pepper, and tomato. Most plants of the genus Cuscuta do not have chlorophyll, and therefore completely depend on the host plants (Kaiser et al., 2015). After seed germination, the parasite develops a stem that grows and extends shootward. To maximize the chance of host detection, attachment, and invasion, the Cuscuta stem performs circular movements and, once a host is found, the parasite winds around its stem and forms the haustoria at the attachment sites (Fig.1A, B) (Runyon et al., 2006).
For both parasites, attaching to a host must occur within the first days after germination to ensure their survival, and haustorium formation is vital for a successful invasion. In this review, we aim to highlight developmental programs in both root and shoot parasitic plants, focusing on Striga and Cuscuta as model systems for parasitic plants species. We will discuss their commonalities and differences and how the interplay between the two hormones auxin and cytokinin modulates host invasion, haustorium formation, and establishment in the host plants. We will also compare their developmental programs with those of lateral root initiation and emergence.
The haustorium as an invasive organ
The haustorium is the organ with which parasitic plants invade their hosts. Two types of haustoria can be recognized based on their origins: the terminal haustorium, which results from the differentiation of the root apical meristem of the parasitic plant and is only found in some obligate parasites, predominantly in the Orobanchaceae family; and the lateral haustorium, which can be found in all facultative and some obligate parasites and originates from differentiated tissues of the parasite root or stem (Yoshida et al., 2016). Evolutionarily, lateral haustoria originated first in the transition from non-parasitic to parasitic plants, and only later did the terminal haustoria evolve (Westwood et al., 2010).
Terminal haustoria: differentiate and invade
Terminal haustoria develop from the differentiation of the root apical meristem a few days after germination. After emerging from the seed coat, the Striga root meristem has a similar structure to that of the model plant Arabidopsis. The meristem contains a quiescent center marked by a low cell division rate; the vasculature cells lay in the center, surrounded by tissue files consisting of one endodermal layer, one to two cortical layers, and one epidermal layer (Fig. 1E) (Xiao et al., 2022). Within 24–48 h after emerging from the seed coat, the roots elongate and, upon sensing the haustorium-inducing factors (HIFs) produced by the host, the Striga root meristem differentiates and forms the terminal haustorium (Hood et al., 1998). During the formation of the terminal haustorium, the cell division rate in the meristem decreases, the meristematic cells differentiate, and root hairs, also termed haustorial hairs, emerge from the epidermis (Fig. 1H) (Xiao et al., 2022). These cells elongate in proximity to the host root and penetrate the host root tissues (Hood et al., 1998). Once they reach the host endodermis, the outermost cells elongate, undergo anticlinal division, and form a palisade arrangement. Then the vascular elements in the haustorium differentiate into xylem elements and establish the vascular connection to the host. After the establishment of the xylem–xylem connection, the cotyledons of the parasite grow out of the seed coat (Hood et al., 1998).
Lateral haustoria: maximizing invasion
After initial formation of the terminal haustorium as an attachment point to the host plant, the obligate parasites grow out shoots underground, and secondary adventitious roots emerge, from which lateral haustoria can form to enhance the nutrient uptake from the host (Fig. 1B, D, G, H) (Cai et al., 1993). Facultative parasites of the Orobanchaceae and the Scrophulariaceae families form lateral haustoria that emerge at the root elongation zone, permitting continuous root growth and the formation of multiple haustoria (Matvienko et al., 2001; Ishida et al., 2011; Wakatake et al., 2018).
Lateral haustoria formation involves activation of cell division in multiple root tissue layers, including the pericycle, endodermis, cortex, and epidermis (Figs 1I–L, 3A–C). At the initial stage, anticlinal cell divisions are induced in the epidermis and outer cortex, while periclinal cell divisions are induced in the pericycle, endodermis, and inner cortex (Fig. 1I, K). After the initial anticlinal cell divisions of the epidermis, cells become densely protoplasmic and contain enlarged nuclei, followed by elongation of the cells and emergence of root hairs (Fig. 1J, M, N) (Musselman and Dickison, 1975). Upon attachment to the host, cells continue to divide within the center of the haustorium primordia to form the future vasculature and the hyaline body (Fig. 1L, M, O, P). The hyaline body is the center of the lateral haustorium, consisting of parenchymatic cells with high cytoplasm density and large nuclei, and is proposed to have high metabolic activity and to act as a sink for the host metabolites (Visser et al., 1984; Yoshida et al., 2019).
Fig. 3.
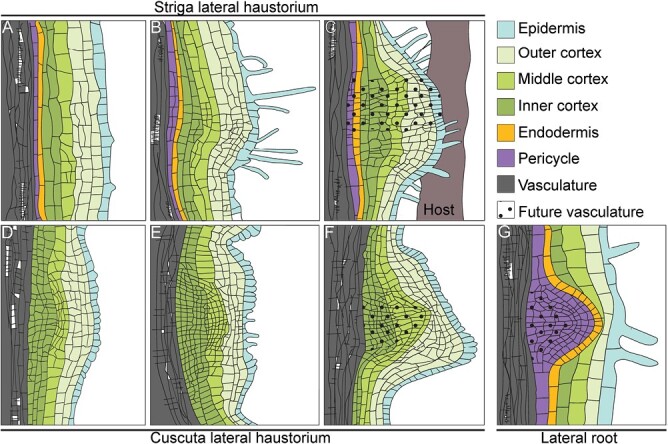
Organogenesis of lateral haustoria and lateral roots. (A–C) Striga lateral haustorium development. (D–F) Cuscuta lateral haustorium development. (G) Lateral root initiation in Arabidopsis; tissues are color-coded according to the key.
During terminal and lateral haustoria initiation, starch granules accumulate in the cortical cells of the root (Fig. 1P) (Joel and Losner-Goshen, 1994). Interestingly, no starch granules form during the lateral root formation of the parasitic plant (Fig. 1G). Accumulation of starch granules in the hyaline body supports the idea that the hyaline body in haustoria serves as a host sink tissue (Visser et al., 1984).
Invading stems: Cuscuta haustoria
After germination, the Cuscuta shoot grows and comes into contact with the host. The Cuscuta shoots twine around the host plant stems and initiate haustorium formation (Fig. 2A, B). The process begins with a swelling of stem areas in close proximity to the host tissue (Fig. 2B) (Vaughn, 2002). Anatomically, Cuscuta stems consist of one layer of epidermis and 6–7 layers of cortex cells surrounding the vasculature in the center (Fig. 2C) (Lee, 2007). At the haustorium initiation, the stems form a plate-like organ, termed the ‘holdfast’, which is formed by anticlinal divisions and elongation of epidermal cells (Fig. 2D, E). The epidermal cells form finger-like structures and develop into unicellular secretory-type trichomes that secrete adhesive compounds (Fig. 2E) (Vaughn, 2002). At the same time, cortex cells divide to create multiple cell layers and contribute to the swelling of the stem (Fig. 2E, G). Then they start to elongate towards the host contact side (Fig. 2F) (Lee, 2007). In the subsequent intrusive phase, the primordium, made up of divided cortex cells, breaks through the outer cortex cells files and the epidermal layer of the parasite, and then through the epidermis of the host and the host cortex (Lee, 2007). The cells at the apex of the haustorium, the so-called ‘searching hyphae’, elongate and search for the vascular tissue of the host plant (Vaughn, 2002). Subsequently, the haustorium develops into conductive vascular cells and the xylic hyphae differentiate into xylem, establishing the haustorial bridge, while the phloic hyphae develop into phloem (Fig. 2G, H) (Vaughn, 2006).
Fig. 2.
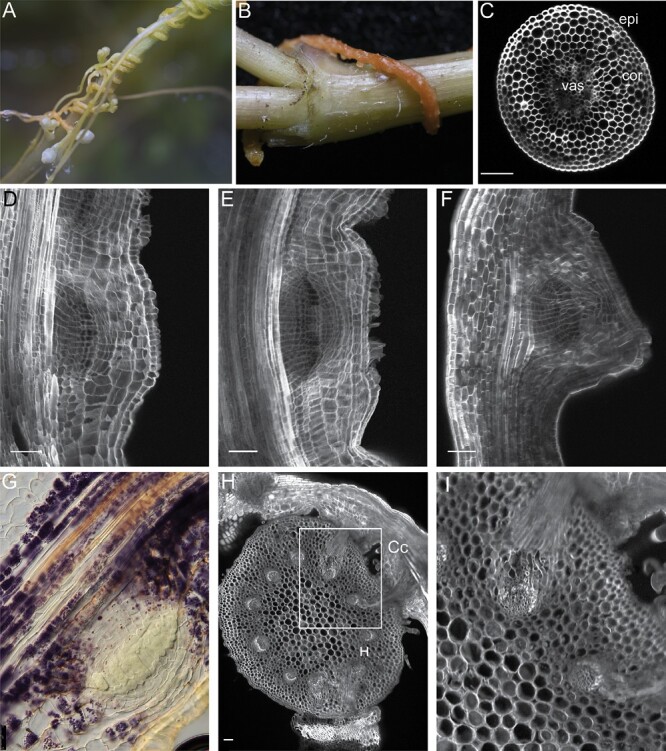
Cuscuta haustorium development. (A and B) Cuscuta plants grown around Sphagneticola trilobata as host plants. (C) Confocal image of a vibratome cross-section of a Cuscuta stem; cell walls are stained with SCRI Renaissance. (D–F) Longitudinal vibratome sections of a Cuscuta stem developing a haustorium; cell walls stained are with SCRI Renaissance. (G) Accumulation of starch granules during haustorium formation, visualized by Lugol’s staining. (H and I) Vibratome cross-sections of the host stem with Cuscuta attached to it with haustoria; cell walls stained with mPS-PI (Truernit et al., 2008); (I) magnification of the area marked in (H). Cc, Cuscuta campestris; H, host; cor, cortex, epi, epidermis, vas, vasculature; scale bars 50 μm.
Similarities and differences between Striga and Cuscuta haustoria formation
Although parasitism within the Orobanchaceae and in the Cuscuta genus has evolved independently of each other (Westwood et al., 2010), both families deploy a similar mode of host colonization. In both Striga and Cuscuta, haustoria derive from differentiated tissue, either from the root in the case of Striga or from the stem in the case of Cuscuta. The initiation of lateral haustoria in Striga involves cell division within multiple tissues, including the epidermis, cortex, endodermis, and pericycle (Fig. 3A–C). In contrast, in Cuscuta, lateral haustoria formation involves divisions mainly from the cortex (Fig. 3D, E) (Lee, 2007).
Another common feature between root and stem haustorium formation is the remodeling of cell walls by the emerging haustorium. In Cuscuta, genes encoding cell wall-modifying enzymes such as pectin lyases, cellulases, and expansins are up-regulated during the infective stages (Ranjan et al., 2014). In the haustoria, the finger-like epidermal cells secrete cell wall-loosening complexes from Golgi-derived vesicles, which are deposited into the cell wall. The tips of the finger-like cells then bend backward into the cytoplasm, and the cell wall-loosening complexes are prominent in the areas of the cell walls in contact with the host and the areas of infoldings, probably to make the Cuscuta trichomes malleable to form a tight host connection (Vaughn, 2002). Additionally, the secretion of cell wall-loosening enzymes leads to changes in the host cell walls in close proximity to the parasitic haustorium (Johnsen et al., 2015). In Striga, the intrusive cells of the lateral haustoria force their way towards the host vasculature during haustorium invasion of the host tissue. In this process, secretion of substances from the haustorial papillae might play a role in forming an adhesive surface (Neumann et al., 1999). It has been speculated early on that the parasites use enzymatic activity to penetrate between host cortical cells (Musselman and Dickison, 1975). This idea is supported by the up-regulation of catalytic activity-related gene expression in the terminal haustoria during host penetration, with a high proportion of genes categorized as carbohydrate active enzymes (CAZymes) being differentially expressed during the invasion stages of the parasite. These include pectin-degrading enzymes that target primary cell wall components, and proteases (Yoshida et al., 2019). A similar up-regulation of cell wall-related genes was observed in late stages of lateral haustorium development in the facultative parasitic plant Phtheirospermum japonicum (Kokla et al., 2022).
Thus, both for stem haustoria and for root-derived haustoria, the remodeling of cell walls by enzymatic activity might have an effect on the parasite cell wall to form an adhesive interaction with the host, as well as on the host cell wall, to facilitate penetration of the haustorium.
Reprogramming involves recruitment of lateral root developmental modules
Lateral haustoria formation in Striga is initiated by HIFs derived from the host roots. These include 2,6-dimethoxy-p-benzoquinone (DMBQ) and its structural analogs (Yoshida et al., 2016). Cuscuta haustoria are induced by the tactile stimulus (i.e. coiling around the host stem) and supplementation with a far-red light stimulus (Bernal-Galeano et al., 2022). HIFs induce a dedifferentiation of root or stem tissue in the parasitic plants, resembling the lateral roots, which originate from the dedifferentiation of the pericycle (Fig. 3G) (Malamy and Benfey, 1997). Indeed, it was found that terminal haustorium development in Striga recruits genes involved in lateral root development (Yoshida et al., 2019). It is plausible that lateral haustorium development uses the same genetic program, given that both terminal and lateral haustoria derive from multiple differentiated cell layers. Similarly, in Cuscuta, haustorium formation recruits genes that are involved in root development in closely related plant species (Sun et al., 2018). The transcriptome during haustorium development in Striga was compared with the genetic program governing lateral root development in Arabidopsis (Yoshida et al., 2019). However, the Arabidopsis lateral root development, originating from the pericycle exclusively, presents an exception rather than the norm, and lateral root formation in other plant species involves more cell files (Xiao et al., 2019). Additionally, lateral root development in Arabidopsis is initiated with anticlinal cell divisions of the pericycle (Malamy and Benfey, 1997), whereas Striga lateral haustoria formation is initiated with periclinal cell divisions in the pericycle, endodermis, and inner cortex (Fig. 1I) (Musselman and Dickison, 1975). Similarly, lateral haustorium development in P. japonicum starts with periclinal cell division in the pericycle (Wakatake et al., 2018). In Cuscuta, the cortical cells divide both periclinally and anticlinally for haustorium initiation (Fig. 2D) (Lee, 2007). Striga itself is able to form adventitious roots from the shoot, and higher order lateral roots from them (Fig. 1B, D) (Wolf and Timko, 1991; Cai et al., 1993). Therefore, rather than comparing haustoria with Arabidopsis lateral root formation, it would be more informative to compare the transcriptomal changes during lateral root development and haustorium development in Striga itself to understand the developmental changes. Another module for comparison is the nitrogen-fixing root nodule, which is a lateral root organ formed through the dedifferentiation of pericycle and cortex cells (Xiao et al, 2014).
Auxin and cytokinin balance during haustorium initiation
The balance between phytohormones is important for plant growth, organogenesis, callus induction, and regeneration (i.e. for genetic reprogramming) (Skoog and Miller, 1957; Christianson and Warnick, 1985). Local accumulation of auxin is generally considered as prerequisite for early plant organogenesis (Benková et al., 2003).
Transcriptomic studies in P. japonicum showed that haustoria induction by HIFs correlates with the activation of the auxin biosynthesis gene YUCCA3 in the root epidermis. Furthermore, the reduction of YUCCA3 activity by gene silencing results in a reduction of haustorium formation (Ishida et al., 2016). In the facultative parasite Triphysaria versicolor, exposure to HIFs also leads to auxin accumulation in the root tip, while exogenous application of auxin in addition to HIFs increases the frequency of haustorium formation (Tomilov et al., 2005). In Cuscuta, genes related to polar auxin transport were found to be up-regulated in haustoria (Ranjan et al., 2014). This indicates a functional role for local auxin biosynthesis in lateral haustorium formation. In terminal haustoria of Striga, SOLITARY ROOT (SLR) (INDOLE-3-ACETIC ACID INDUCIBLE 14/IAA14) and AUXIN RESPONSE FACTOR 19 (ARF19) orthologs are specifically expressed in the early stage of haustorium development (Yoshida et al., 2019). In Arabidopsis, these genes work in concert to regulate the expression of an auxin influx carrier that facilitates auxin accumulation during lateral root development (Swarup et al., 2008). Application of auxin to Striga radicles leads to prolonged meristem maintenance with active cell division. In contrast, applying an auxin biosynthesis inhibitor increases the rate of haustoria formation and a cessation of cell division (Xiao et al., 2022). Assuming that terminal and lateral haustorium formation follow a similar mechanism, the increase in auxin concentration at haustorium initiation sites could lead to a reactivation of cell division in the differentiated tissue, which leads to lateral haustorium initiation. However, there is evidence that initiation of haustorium formation between terminal and lateral haustoria actually follows a different pattern: cytokinins trigger the induction of a pre-haustorium in Striga but not in P. japonicum, indicating that the processes are influenced by different phytohormone concentrations (Aoki et al., 2022). In T. versicolor, the application of cytokinin leads to localized swelling and epidermal hair proliferation near the root tips, resembling the formation of Striga terminal haustoria (Wrobel and Yoder, 2001). Importantly, cytokinin can act as a HIF, hence the application of cytokinin to the parasitic plants would mimic the secretion of HIFs by the host plant (Aoki et al., 2022). Cytokinin, in its role as a HIF, most probably acts through cytokinin receptors in the parasite and partially activates the same transcriptional pathways downstream of the HIF DMBQ (Aoki et al., 2022). A combination of HIFs including cytokinins in host root exudates promotes the expression of cytokinin biosynthesis and signaling genes, leading to an elevated cytokinin concentration in the pre-haustorium, which could further promote haustorium formation (Aoki et al., 2022; Xiao et al., 2022). Cytokinin promotes the formation of the terminal pre-haustorium in Striga and T. versicolor, but is not able to induce a lateral haustorium in P. japonicum, suggesting that it only acts as a HIF for terminal haustoria. For obligate parasitic plants that form terminal haustoria as the first tool to attach to the host plant, the formation of this organ is crucial for survival. Therefore, it is possible that obligate parasitic plants are sensitive to a broader spectrum of substances that serve as HIFs. Cytokinin signaling reporter lines or knockout mutants of cytokinin pathway components would shed light on how an internal balance of the phytohormones regulates haustorium formation. In summary, the correct concentration of auxin in conjunction with cytokinin and/or other phytohormones is necessary to initiate cell division and de-differentiation of cells involved in the formation of lateral haustoria. Like haustoria, cytokinin and auxin play crucial roles in nodulation. In certain legume species, cytokinin can function as a nodulation factor, inducing nodule formation even in the absence of Rhizobia (Gamas et al., 2017). This shared regulation between parasitic haustoria and nitrogen-fixing root nodules represents yet another common characteristic.
Parasitic plants manipulate their host by modulating auxin and cytokinin levels
During Striga terminal haustorium development, auxin concentration decreases while cytokinin concentration increases. The reduction of auxin concentration correlates with basal localization of the auxin efflux carriers PIN1 and PIN2 at the epidermis, which suggests that auxin is secreted from the tip of the haustorium to the environment, leading to an increase in auxin response in the host plants (Xiao et al., 2022). Similar processes have been observed for the haustorium development in Cuscuta: during attachment to the host, the content of free IAA in the contact zone increases in both the host and the parasitic tissue (Löffler et al., 1999). This is correlated with an elongation of the haustorial epidermis cells and enlargement of the host cortical cells. It is hypothesized that the auxin is excreted from the haustorium to the host (Löffler et al., 1999). However, it is yet to be determined how the auxin that is produced by the parasite is perceived by the host. The host response will most probably be through the canonical auxin signaling pathway involving the auxin receptors TIR1 and AFB proteins. Recently, it has been shown that nuclear TIR1 mediates slow responses to auxin while AFB1 was found to be important for its rapid response (Chen et al., 2023). With TIR1 and AFB1 having a distinct function in Arabidopsis, it remains to be determined how the parasite modulates the host auxin response, transport, and signaling. The molecular tools that are continuously generated in crops [e.g. reporter lines and clustered regularly interspaced palindromic repeats (CRISPR)/CRISPR-associated protein (Cas) mutants] combined with the advances in imaging technologies, allowing long-term imaging of dynamic interactions (von Wangenheim et al., 2017), will provide a mechanistic understanding on how the parasite impinges on the host roots to secure a successful invasion.
The increase in auxin concentration could lead to cell wall modification in both the haustorium and the host, to form an adhesive surface or facilitate penetration of the host tissue. Auxin is known to regulate the expression of genes encoding cell wall modification enzymes directly (Swarup et al., 2008), and could thereby indirectly influence the cell wall composition, or modify the cell wall directly by acidification of the apoplast, as described by the Acid Growth Theory (Rayle and Cleland, 1992). Evidence for the influence of auxin on changes in cell wall structure was shown recently in lupin, where auxin triggers homogalacturonan demethylesterification in cluster roots and induces the expression of a polygalacturonase in the tissue outside of the outgrowing root primordia, to facilitate outgrowth without damage in the overlying cortex and epidermis cells (Jobert et al., 2022).
Similar to auxin, it was also shown that haustoria are able to manipulate the host phytohormone balance by cytokinin secretion. In P. japonicum, genes associated with cytokinin metabolism are up-regulated in the haustorium upon infection, cytokinin levels increase, and cytokinins are transported to host plants via the haustorium (Spallek et al., 2017; Kokla et al., 2022). Cytokinin biosynthesis is likely to take place in the intrusive cells of the P. japonicum haustorium, as shown by the expression of the cytokinin biosynthesis gene PjISOPENTENYLTRANSFERASEa, and mutation of this gene leads to a loss of cytokinin response in the host roots (Greifenhagen et al., 2021). Cytokinins produced in Cuscuta can also be transferred to host plants and trigger a cytokinin response there (Furuhashi et al., 2014). The cytokinins as mobile signals might induce morphological changes in host roots, such as hypertrophy (i.e. plant tissue overgrowth and an increase in the number of vascular bundles), facilitating tissue penetration of the parasitic plant and formation of a haustorial bridge between parasite and host.
Controlling phytohormones levels as a strategy against parasitic plant infestation
Strategies to control parasitic plant infestation nowadays comprise the creation of parasite-resistant crop plants (Kaiser et al., 2015; Mutuku et al., 2019), suicidal germination (Kountche et al., 2019), or application of myco-herbicides that target the parasite, but not the host (Rebeka et al., 2013). With the importance of hormone interplay in parasite–host interactions, and with the currently available genomic resources in parasitic plants (Table 2; Yoshida and Kee, 2021), it is important to develop molecular and genetic tools to understand their specific role in this process, to be able to manipulate the interaction to fight parasitic plant infestation. These tools can target both the parasite to make it less infective and the host by increasing its resistance. Application of specific phytohormone signaling inhibitors could prevent haustorium formation and thereby attachment to host plants. Here, a detailed knowledge of the nature of the phytohormone signaling in the parasite is needed to ensure that inhibitors only target processes in the parasite wihout interference with host phytohormones. Conversely, phytohormone signaling components of the host are a potential target, in order to interrupt the tissue adaptations in favor of the parasite. For example, mutants in cytokinin signaling genes in Arabidopsis are resistant to the hypertrophy induced by P. japonicum (Spallek et al., 2017). Another possible target is manipulating the cell wall composition of the host to make it inaccessible for cell wall modifications caused by the auxin release of the parasite, and thereby prevent attachment and penetration of the parasite. It has been suggested that the resistance against parasites in host plants is triggered by modifications in the cell wall structure at the infection sites: in Cuscuta-resistant tomato, plant cells walls undergo secondary modifications involving phenylpropanoids and long-chain components that can become cross-linked within the cell wall to prevent infection with the parasite (Kaiser et al., 2015). In Striga-resistant rice cultivars, the resistance is associated with an increase in lignin, and lignin- and glucan-derived compound deposition at the infection site (Mutuku et al., 2019). Interfering with auxin transport, response, and signaling of the parasite, on the other hand, could prevent the auxin-mediated cell wall modifications necessary for the penetration of the haustorium.
Table 2.
Recent advances in genomes and transcriptomes of the species mentioned in this review
| Plant | Genome | Transcriptome |
|---|---|---|
| Striga asiatica | Yoshida et al. (2019) | Yoshida et al. (2019) |
| Striga hermontica | Qiu et al. (2022) | Yang et al. (2015); Wicket et al. (2011); Yoshida et al. (2019) |
| Phtheirospermum japonicum | Kado and Innan (2018); Yoshida and Kee (2021) | Ogawa et al. (2021); Cui et al. (2020); Kurotani et al. (2020); Ishida et al. (2016) |
| Triphysaria versicolor | Not available | Wicket et al., 2011; Yang et al., 2015 |
| Cuscuta australis | Sun et al., 2018 | Hettenhausen et al. (2017) |
| Cuscuta campestris | Vogel et al. (2018) | Zhang et al. (2021); Kim et al. (2014); Shahid et al. (2018); Kaga et al. (2020) |
Genomes and transcriptomes of parasitic plants not mentioned in this study are described in Yoshida and Kee (2021).
The analysis of phytohormonal responses in parasitic plants at the cellular level is still hampered by the lack of suitable molecular biological tools. To analyze phytohormone content in a spatial manner, antibodies against auxin and cytokinin can be used (Xiao et al., 2022); however, they do not allow for monitoring the temporal dynamic of haustoria development in vivo. Hairy root transformation in P. japonicum represents an important breakthrough to express reporter constructs transiently and create gene knockouts by CRISPR/Cas9; however, no stably transformed reporter lines have been reported to date (Spallek et al., 2017; Greifenhagen et al., 2021).
Although Cuscuta does not develop roots, a protocol for hairy root transformation was recently used to transform the adhesive disk cells of the haustorium. Unfortunately, the transformation events are so far restricted to the adhesive disk, so that analysis of early haustorium formation events by reporter gene expression remains impossible (Lachner et al., 2020).
In conclusion, understanding cellular and molecular mechanisms controlling haustorium formation will remain a challenging task. The availability of genome and transcriptome resources (Table 2; Yoshida and Kee, 2021) and the use of single-cell omics might be promising to deceipher the molecular interplay of de novo organogenesis from differentiated tissues. However, it will not be possible, without the development of basic tools for functional biology studies such as a stable transformation protocol, to validate the candidate genes involved in the interaction and communication between the host and the parasite. These tools will also be useful to develop biotechnological approaches that will help in combating parasitic infestations.
Contributor Information
Gwendolyn K Kirschner, BESE Division, Plant Cell and Developmental Biology, King Abdullah University of Science and Technology, Thuwal, Kingdom of Saudi Arabia.
Ting Ting Xiao, BESE Division, Plant Cell and Developmental Biology, King Abdullah University of Science and Technology, Thuwal, Kingdom of Saudi Arabia.
Muhammad Jamil, BESE Division, The BioActives Lab, King Abdullah University of Science and Technology, Thuwal, Kingdom of Saudi Arabia.
Salim Al-Babili, BESE Division, The BioActives Lab, King Abdullah University of Science and Technology, Thuwal, Kingdom of Saudi Arabia.
Vinicius Lube, BESE Division, Plant Cell and Developmental Biology, King Abdullah University of Science and Technology, Thuwal, Kingdom of Saudi Arabia.
Ikram Blilou, BESE Division, Plant Cell and Developmental Biology, King Abdullah University of Science and Technology, Thuwal, Kingdom of Saudi Arabia.
Lucia Strader, Duke University, USA.
Conflict of interest
The authors declare no conflict of interest.
Funding
This study was supported by King Abdullah University of Science and Technology (KAUST) baseline funding (BAS/1/1081-01-01) to IB and by the Bill & Melinda Gates Foundation grant no (OPP1136424) to SA-B.
Author contributions
GKK, TTX, and IB: conceptualization; GKK and IB: writing the initial draft; TTX, MJ, SA-B, and VL: revising the manuscript. All the authors read and approved the final version of the manuscript.
References
- Aoki N, Cui S, Yoshida S.. 2022. Cytokinins induce prehaustoria coordinately with quinone signals in the parasitic plant Striga hermonthica. Plant and Cell Physiology 63, 1446–1456. [DOI] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J.. 2003. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602. [DOI] [PubMed] [Google Scholar]
- Bernal-Galeano V, Beard K, Westwood JH.. 2022. An artificial host system enables the obligate parasite Cuscuta campestris to grow and reproduce in vitro. Plant Physiology 189, 687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner DK, Kling JG, Singh BB.. 1995. Striga research and control—a perspective from Africa. Plant Disease 79, 652–660. [Google Scholar]
- Cai T, Babiker AG, Ejeta G, Butler LG.. 1993. Morphological response of witchweed (Striga asiatica) to in vitro culture. Journal of Experimental Botany 44, 1377–1384. [Google Scholar]
- Chen H, Li L, Zou M, Qi L, Friml J.. 2023. Distinct functions of TIR1 and AFB1 receptors in auxin signalling. Molecular Plant 16, 1117–1119. [DOI] [PubMed] [Google Scholar]
- Christianson ML, Warnick DA.. 1985. Temporal requirement for phytohormone balance in the control of organogenesis in vitro. Developmental Biology 112, 494–497. [Google Scholar]
- Cui S, Kubota T, Nishiyama T, Juliane K, Shigenobu S, Shibata TF, Toyoda A, Hasebe M, Shirasu K, Yoshida S.. 2020. Ethylene signaling mediates host invasion by parasitic plants. Science Advances 6, eabc2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S, Wakatake T, Hashimoto K, Saucet SB, Toyooka K, Yoshida S, Shirasu K.. 2016. Haustorial hairs are specialized root hairs that support parasitism in the facultative parasitic plant Phtheirospermum japonicum. Plant Physiology 170, 1492–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi T, Kojima M, Sakakibara H, Fukushima A, Hirai MY, Furuhashi K.. 2014. Morphological and plant hormonal changes during parasitization by Cuscuta japonica on Momordica charantia. Journal of Plant Interactions 9, 220–232. [Google Scholar]
- Gamas P, Brault M, Jardinaud MF, Frugier F.. 2017. Cytokinins in symbiotic nodulation: when, where, what for? Trends in Plant Science 22, 792–802. [DOI] [PubMed] [Google Scholar]
- Greifenhagen A, Braunstein I, Pfannstiel J, Yoshida S, Shirasu K, Schaller A, Spallek T.. 2021. The Phtheirospermum japonicum isopentenyltransferase PjIPT1a regulates host cytokinin responses in Arabidopsis. New Phytologist 232, 1582–1590. [DOI] [PubMed] [Google Scholar]
- Hettenhausen C, Li J, Zhuang H, et al. 2017. Stem parasitic plant Cuscuta australis (dodder) transfers herbivory-induced signals among plants. Proceedings of the National Academy of Sciences, USA 114, E6703–E6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaas LA, Jones S, Farrell N, Kamerow W, Zhang H, Vescio K, Altman NS, Yoder JI, Depamphilis CW.. 2019. Risk versus reward: host dependent parasite mortality rates and phenotypes in the facultative generalist Triphysaria versicolor. BMC Plant Biology 19, 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood ME, Condon JM, Timko MP, Riopel JL.. 1998. Primary haustorial development of Striga asiatica on host and nonhost species. Phytopathology 88, 70–75. [DOI] [PubMed] [Google Scholar]
- Irving LJ, Kim D, Schwier N, Vaughan JKE, Ong G, Hama T.. 2019. Host nutrient supply affects the interaction between the hemiparasite Phtheirospermum japonicum and its host Medicago sativa. Environmental and Experimental Botany 162, 125–132. [Google Scholar]
- Ishida JK, Wakatake T, Yoshida S, Takebayashi Y, Kasahara H, Wafula E, Depamphilis CW, Namba S, Shirasu K.. 2016. Local auxin biosynthesis mediated by a YUCCA flavin monooxygenase regulates haustorium development in the parasitic plant Phtheirospermum japonicum. The Plant Cell 28, 1795–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida JK, Yoshida S, Ito M, Namba S, Shirasu K.. 2011. Agrobacterium rhizogenes-mediated transformation of the parasitic plant Phtheirospermum japonicum. PLoS One 6, e25802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobert F, Soriano A, Brottier L, Casset C, Divol F, Safran J, Lefebvre V, Pelloux J, Robert S, Péret B.. 2022. Auxin triggers pectin modification during rootlet emergence in white lupin. The Plant Journal 112, 1127–1140. [DOI] [PubMed] [Google Scholar]
- Joel DM, Losner-Goshen D.. 1994. The attachment organ of the parasitic angiosperms Orobanche cumana and O. aegyptiaca and its development. Canadian Journal of Botany 72, 564–574. [Google Scholar]
- Johnsen HR, Striberny B, Olsen S, Vidal-Melgosa S, Fangel JU, Willats WGT, Rose JKC, Krause K.. 2015. Cell wall composition profiling of parasitic giant dodder (Cuscuta reflexa) and its hosts: a priori differences and induced changes. New Phytologist 207, 805–816. [DOI] [PubMed] [Google Scholar]
- Kado T, Innan H.. 2018. Horizontal gene transfer in five parasite plant species in Orobanchaceae. Genome Biology and Evolution 10, 3196–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaga Y, Yokoyama R, Sano R, Ohtani M, Demura T, Kuroha T, Shinohara N, Nishitani K.. 2020. Interspecific signaling between the parasitic plant and the host plants regulate xylem vessel cell differentiation in haustoria of Cuscuta campestris. Frontiers in Plant Science 11, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser B, Vogg G, Fürst UB, Albert M.. 2015. Parasitic plants of the genus Cuscuta and their interaction with susceptible and resistant host plants. Frontiers in Plant Science 6, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, LeBlanc ML, Wafula EK, DePamphilis CW, Westwood JH.. 2014. Genomic-scale exchange of mRNA between a parasitic plant and its hosts. Science 345, 808–811. [DOI] [PubMed] [Google Scholar]
- Kokla A, Leso M, Zhang X, Simura J, Serivichyaswat PT, Cui S, Ljung K, Yoshida S, Melnyk CW.. 2022. Nitrogen represses haustoria formation through abscisic acid in the parasitic plant Phtheirospermum japonicum. Nature Communications 13, 2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kountche BA, Jamil M, Yonli D, Nikiema MP, Blanco-Ania D, Asami T, Zwanenburg B, Al-Babili S.. 2019. Suicidal germination as a control strategy for Striga hermonthica (Benth.) in smallholder farms of sub-Saharan Africa. Plants, People, Planet, 1, 107–118. [Google Scholar]
- Kurotani K, Wakatake T, Ichihashi Y, Okayasu K, Sawai Y, Ogawa S, Cui S, Suzuki T, Shirasu K, Notaguchi M.. 2020. Host–parasite tissue adhesion by a secreted type of β-1,4-glucanase in the parasitic plant Phtheirospermum japonicum. Communications Biology 3, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachner LAM, Galstyan L, Krause K.. 2020. A highly efficient protocol for transforming Cuscuta reflexa based on artificially induced infection sites. Plant Direct 4, e00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KB. 2007. Structure and development of the upper haustorium in the parasitic flowering plant Cuscuta japonica (Convolvulaceae). American Journal of Botany 94, 737–745. [DOI] [PubMed] [Google Scholar]
- Löffler C, Czygan FC, Proksch P.. 1999. Role of indole-3-acetic acid in the interaction of the phanerogamic parasite Cuscuta and host plants. Plant Biology 1, 613–617. [Google Scholar]
- Malamy JE, Benfey PN.. 1997. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124, 33–44. [DOI] [PubMed] [Google Scholar]
- Matvienko M, Torres MJ, Yoder JI.. 2001. Transcriptional responses in the hemiparasitic plant Triphysaria versicolor to host plant signals. Plant Physiology 127, 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra JS, Kogan M.. 2009. Biology and management of Cuscuta species. Indian Journal of Weed Science 41, 1–11. [Google Scholar]
- Musselman LJ. 1980. The biology of Striga, Orobanche, and other root-parasitic weeds. Annual Review of Phytopathology 18, 463–489. [Google Scholar]
- Musselman LJ, Dickison W.. 1975. The structure and development of the haustorium in parasitic Scrophulariaceae. Botanical Journal of the Linnean Society 70, 183–212. [Google Scholar]
- Mutuku JM, Cui S, Hori C, et al. 2019. The structural integrity of lignin is crucial for resistance against Striga hermonthica parasitism in rice. Plant Physiology 179, 1796–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann U, Vian B, Weber HC, Sallé G.. 1999. Interface between haustoria of parasitic members of the Scrophulariaceae and their hosts: a histochemical and immunocytochemical approach. Protoplasma 207, 84–97. [Google Scholar]
- Ogawa S, Wakatake T, Spallek T, et al. 2021. Subtilase activity in intrusive cells mediates haustorium maturation in parasitic plants. Plant Physiology 185, 1381–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, BradleyJM, Zhang P, Chaudhuri R, Blaxter M, Butlin RK, Scholes JD.. 2022. Genome-enabled discovery of candidate virulence loci in Striga hermonthica, a devastating parasite of African cereal crops. New Phytologist 236, 622–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan A, Ichihashi Y, Farhi M, Zumstein K, Townsley B, David-Schwartz R, Sinha NR.. 2014. De novo assembly and characterization of the transcriptome of the parasitic weed dodder identifies genes associated with plant parasitism. Plant Physiology 166, 1186–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle DL, Cleland RE.. 1992. The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiology 99, 1271–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeka G, Shimelis H, Laing MD, Tongoona P, Mandefro N.. 2013. Evaluation of sorghum genotypes compatibility with Fusarium oxysporum under Striga infestation. Crop Science 53, 385–393. [Google Scholar]
- Rodenburg J, Demont M, Zwart SJ, Bastiaans L.. 2016. Parasitic weed incidence and related economic losses in rice in Africa. Agriculture, Ecosystems and Environment 235, 306–317. [Google Scholar]
- Runyon JB, Mescher MC, De Moraes CM.. 2006. Volatile chemical cues guide host location and host selection by parasitic plants. Science 313, 1964–1967. [DOI] [PubMed] [Google Scholar]
- Shahid S, Kim G, Johnson NR, et al. 2018. MicroRNAs from the parasitic plant Cuscuta campestris target host messenger RNAs. Nature 553, 82–85. [DOI] [PubMed] [Google Scholar]
- Skoog F, Miller CO.. 1957. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symposia of the Society for Experimental Biology 11, 118–130. [PubMed] [Google Scholar]
- Spallek T, Melnyk CW, Wakatake T, Zhang J, Sakamoto Y, Kiba T, Yoshida S, Matsunaga S, Sakakibara H, Shirasu K.. 2017. Interspecies hormonal control of host root morphology by parasitic plants. Proceedings of the National Academy of Sciences, USA 114, 5283–5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Xu Y, Liu H, et al. 2018. Large-scale gene losses underlie the genome evolution of parasitic plant Cuscuta australis. Nature Communications 9, 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R, et al. 2008. The auxin influx carrier LAX3 promotes lateral root emergence. Nature Cell Biology 10, 946–954. [DOI] [PubMed] [Google Scholar]
- Tomilov AA, Tomilova NB, Abdallah I, Yoder JI.. 2005. Localized hormone fluxes and early haustorium development in the hemiparasitic plant Triphysaria versicolor. Plant Physiology 138, 1469–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truernit E, Bauby H, Dubreucq B, Grandjean O, Runions J, Barthélémy J, Palauqui J-C.. 2008. High-resolution whole-mount imaging of three-dimensional tissue organization and gene expression enables the study of phloem development and structure in Arabidopsis. The Plant Cell 20, 1494–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn KC. 2002. Attachment of the parasitic weed dodder to the host. Protoplasma 219, 227–237. [DOI] [PubMed] [Google Scholar]
- Vaughn KC. 2006. Conversion of the searching hyphae of dodder into xylic and phloic hyphae: a cytochemical and immunocytochemical investigation. International Journal of Plant Sciences 167, 1099–1114. [Google Scholar]
- Visser JH, Dörr I, Kollmann R.. 1984. The ‘hyaline body’ of the root parasite Alectra orobanchoides benth. (Scrophulariaceae)—its anatomy, ultrastructure and histochemistry. Protoplasma 121, 146–156. [Google Scholar]
- Vogel A, Schwacke R, Denton AK, et al. 2018. Footprints of parasitism in the genome of the parasitic flowering plant Cuscuta campestris. Nature Communications 9, 2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wangenheim D, Hauschild R, Fendrych M, Barone V, Benková E, Friml J.. 2017. Live tracking of moving samples in confocal microscopy for vertically grown roots. eLife 6, e26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakatake T, Yoshida S, Shirasu K.. 2018. Induced cell fate transitions at multiple cell layers configure haustorium development in parasitic plants. Development 145, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood JH, Yoder JI, Timko MP, DePamphilis CW.. 2010. The evolution of parasitism in plants. Trends in Plant Science 15, 227–235. [DOI] [PubMed] [Google Scholar]
- Wickett NJ, Honaas LA, Wafula EK, et al. 2011. Transcriptomes of the parasitic plant family Orobanchaceae reveal surprising conservation of chlorophyll synthesis. Current Biology 21, 2098–2104. [DOI] [PubMed] [Google Scholar]
- Wolf SJ, Timko MP.. 1991. In vitro root culture: a novel approach to study the obligate parasite Striga asiatica (L.) Kuntze. Plant Science 73, 233–242. [Google Scholar]
- Wrobel RL, Yoder JI.. 2001. Differential RNA expression of α-expansin gene family members in the parasitic angiosperm Triphysaria versicolor (Scrophulariaceae). Gene 266, 85–93. [DOI] [PubMed] [Google Scholar]
- Xiao TT, Kirschner GK, Kountche BA, Jamil M, Savina M, Lube V, Mironova V, al Babili S, Blilou I.. 2022. A PLETHORA/PIN-FORMED/auxin network mediates prehaustorium formation in the parasitic plant Striga hermonthica. Plant Physiology 189, 2281–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao TT, Schilderink S, Moling S, Deinum EE, Kondorosi E, Franssen H, Kulikova O, Niebel A, Bisseling T.. 2014. Fate map of Medicago truncatula root nodules. Development 141, 3517–3528. [DOI] [PubMed] [Google Scholar]
- Xiao TT, van Velzen R, Kulikova O, Franken C, Bisseling T.. 2019. Lateral root formation involving cell division in both pericycle, cortex and endodermis is a common and ancestral trait in seed plants. Development 146, dev182592. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wafula EK, Honaas LA, et al. 2015. Comparative transcriptome analyses reveal core parasitism genes and suggest gene duplication and repurposing as sources of structural novelty. Molecular Biology and Evolution 32, 767–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Cui S, Ichihashi Y, Shirasu K.. 2016. The haustorium, a specialized invasive organ in parasitic plants. Annual Review of Plant Biology 67, 643–667. [DOI] [PubMed] [Google Scholar]
- Yoshida S, , KeeYJ. 2021. Large-scale sequencing paves the way for genomic and genetic analyses in parasitic plants. Current Opinion in Biotechnology 70, 248–254. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Kim S, Wafula EK, et al. 2019. Genome sequence of Striga asiatica provides insight into the evolution of plant parasitism. Current Biology 29, 3041–3052. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu Y, Xie J, Zhuang H, Liu H, Shen G, Wu J.. 2021. Parasite dodder enables transfer of bidirectional systemic nitrogen signals between host plants. Plant Physiology 185, 1395–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]


