Abstract
BACKGROUND
Hemodynamic instability and myocardial dysfunction are major factors preventing the transplantation of hearts from organ donors after brain death. Intravenous levothyroxine is widely used in donor care, on the basis of observational data suggesting that more organs may be transplanted from donors who receive hormonal supplementation.
METHODS
In this trial involving 15 organ-procurement organizations in the United States, we randomly assigned hemodynamically unstable potential heart donors within 24 hours after declaration of death according to neurologic criteria to open-label infusion of intravenous levothyroxine (30 μg per hour for a minimum of 12 hours) or saline placebo. The primary outcome was transplantation of the donor heart; graft survival at 30 days after transplantation was a prespecified recipient safety outcome. Secondary outcomes included weaning from vasopressor therapy, donor ejection fraction, and number of organs transplanted per donor.
RESULTS
Of the 852 brain-dead donors who underwent randomization, 838 were included in the primary analysis: 419 in the levothyroxine group and 419 in the saline group. Hearts were transplanted from 230 donors (54.9%) in the levothyroxine group and 223 (53.2%) in the saline group (adjusted risk ratio, 1.01; 95% confidence interval [CI], 0.97 to 1.07; P = 0.57). Graft survival at 30 days occurred in 224 hearts (97.4%) transplanted from donors assigned to receive levothyroxine and 213 hearts (95.5%) transplanted from donors assigned to receive saline (difference, 1.9 percentage points; 95% CI, −2.3 to 6.0; P<0.001 for noninferiority at a margin of 6 percentage points). There were no substantial between-group differences in weaning from vasopressor therapy, ejection fraction on echocardiography, or organs transplanted per donor, but more cases of severe hypertension and tachycardia occurred in the levothyroxine group than in the saline group.
CONCLUSIONS
In hemodynamically unstable brain-dead potential heart donors, intravenous levothyroxine infusion did not result in significantly more hearts being transplanted than saline infusion. (Funded by Mid-America Transplant and others; ClinicalTrials.gov number, NCT04415658.)
THE MAJORITY OF TRANSPLANTED HEARTS come from donors declared dead according to neurologic criteria (termed brain death). Brain death frequently precipitates systemic derangements that negatively affect organ donation, resulting in fewer than half of donor hearts being deemed suitable for transplantation.1,2 A prevalent theory is that neurohormonal insufficiency after brain death, especially thyroid hormone deficiency, leads to myocardial energy depletion and shock.3,4 Several large observational studies have suggested that more hearts and total organs are transplanted from donors who had received thyroid hormone.5,6 These findings have led to widespread adoption of hormonal resuscitation as a cornerstone of deceased donor care, with the majority of organ-procurement organizations (OPOs) administering thyroid hormone, predominantly levothyroxine (synthetic T4), to most or all organ donors, especially in the context of hemodynamic instability.7
The few randomized trials that have evaluated administration of thyroid hormone to donors have focused on intermediate outcomes such as donor hemodynamics and were underpowered to evaluate organ utilization.8–11 Despite little high-quality evidence,12 consensus guidelines continue to recommend thyroid hormone in hemodynamically unstable or heart-eligible donors.13,14 However, treatment has been associated with risks to the donor15; in addition, some studies have suggested higher rates of early allograft failure.16,17 The present clinical trial was designed to evaluate the hypothesis that intravenous levothyroxine would increase the rate of hearts transplanted from hemodynamically unstable brain-dead organ donors being considered for heart donation.
METHODS
TRIAL DESIGN AND OVERSIGHT
We conducted this multicenter, parallel-group, randomized trial at OPOs across the United States. The trial protocol was developed by the principal investigators (the first two authors) and has been published previously18; it is also available with the full text of this article at NEJM.org. The present trial involving deceased organ donors was deemed by the institutional review board at Washington University, St. Louis, to represent research involving nonhuman subjects. A waiver of consent was obtained for data on recipient outcomes; the waiver was based on the finding that the trial interventions posed minimal risk to recipients, because it is a standard treatment given to many but not all organ donors, and on assurances of the protection of recipient privacy (all recipient data were obtained from the Scientific Registry of Transplant Recipients [SRTR] with the use of only donor UNOS [United Network for Organ Sharing] identifiers). Oversight, including interim analysis, was performed by an independent, multidisciplinary data and safety monitoring board (members are listed in the Supplementary Appendix, available at NEJM.org). Data were collected by each OPO, with oversight by the data management team at the coordinating site, and were analyzed by the trial statisticians (the third, fourth, and fifth authors). The manuscript was drafted by the first author, and all the authors critically revised and approved the decision to submit it for publication. There were no restrictions on access to data, and the first two authors vouch for the completeness and accuracy of the data, complete reporting of outcomes and adverse events, and the fidelity of the trial to the protocol.
TRIAL PARTICIPANTS
All deceased patients with authorization for organ donation who received care from the participating OPOs were evaluated for eligibility, which included declaration of death according to neurologic criteria, authorization for research, an age of 14 to 55 years, a weight of 45 kg or more, and hemodynamic instability, defined as receipt of one or more vasopressors or inotropes, after fluid resuscitation (excluding the sole use of vasopressin for management of diabetes insipidus). Donors were excluded if their hearts were not being considered for transplantation because of known heart disease or if they had received thyroid hormone within the past month. (Full exclusion criteria are listed in the Supplementary Appendix.)
RANDOMIZATION AND TRIAL INFUSIONS
Donors who met eligibility criteria underwent randomization within 24 hours after the declaration of death. Randomization was performed by a central randomization site, with 1:1 allocation to levothyroxine or normal saline, with order produced by a random number generator and stratified according to site in blocks of 30. Baseline free T4 levels were obtained before the start of levothyroxine or saline infusion and before organ procurement. Levothyroxine for intravenous use was reconstituted as 500 μg in 500 ml of saline and infused at a rate of 30 ml per hour (i.e., 30 μg per hour). The control group received an equivalent volume of normal saline; the OPO clinicians were aware of the trial-group assignments. The protocol specified that trial infusion should run for 12 hours, although the levothyroxine dose could be decreased or discontinued on the basis of prespecified hemodynamic variables (hypertension, tachycardia, or arrhythmias, defined in the trial protocol). Extension of levothyroxine infusion beyond 12 hours was permitted at the discretion of each OPO, whereas open-label use of levothyroxine was discouraged but permitted in the control group and only after the 12 hours of saline infusion was completed. Reasons for premature weaning or discontinuation and for open-label use in either group were prospectively tracked. All other aspects of donor care followed the policies and practices of each OPO but were not standardized across sites. Enrollment into this trial and trial-group assignments were disclosed to transplantation centers when this information was entered into the Donor Highlights section of DonorNet (the Organ Procurement and Transplantation Network electronic tool).
OUTCOME MEASURES
The primary efficacy outcome was transplantation of the donor heart. The primary safety outcome was graft survival at 30 days after transplantation. Graft survival was defined as patient survival with the originally transplanted heart and without mechanical circulatory support, as reported by all transplantation centers to the SRTR. Secondary outcomes included whether the lungs and other organs were transplanted. The duration of vasopressor support, defined as the time from initiation of levothyroxine or saline infusion to discontinuation of all vasopressors or inotropes (excluding vasopressin at a rate of <1 unit per hour), as well as the proportion of donors who were weaned off vasopressors or inotropes at the end of 12 hours, were compared between the two trial groups.
Vasopressor requirements after 12 hours were compared with the use of the vasopressor–inotrope score. The score was calculated as follows: dopamine dose (in micrograms per kilogram of body weight per minute) + dobutamine dose (in micrograms per kilogram per minute) + (100 × epinephrine dose [in micrograms per kilogram per minute]) + (100 × norepinephrine dose [in micrograms per kilogram per minute]) + (10 × phenylephrine dose [in micrograms per kilogram per minute]) + (10 × milrinone dose [in micrograms per kilogram per minute]) + (10,000 × vasopressin dose [in units per kilogram per minute]).19
An additional prespecified outcome was the time from initiation of levothyroxine or saline infusion to the time that echocardiography was first ordered. In potential heart donors, echocardiography is generally deferred until the donor’s condition is stabilized sufficiently to ensure unbiased assessment of cardiac function. Therefore, time until echocardiography was ordered is a surrogate for when OPO clinicians believed that adequate hemodynamic stabilization had been achieved; we did not use time until echocardiography is performed as an outcome measure because there might have been variation among sites in test or technician availability. The left ventricular ejection fraction (LVEF) was assessed from routine transthoracic echocardiography by either a cardiologist at the donor hospital or a remote expert reader, following the standard practices of each OPO. The readers were not involved in the trial and were not aware of the trial-group assignments. Adverse events were prospectively tracked (for prespecified definitions, see the Supplementary Methods section in the Supplementary Appendix). Serious adverse events included any hemodynamic instability resulting in cardiac arrest or premature donor loss before planned organ recovery.
STATISTICAL ANALYSIS
The percentage of potential heart donors whose hearts could be transplanted was estimated at 30 to 60%, on the basis of our pilot studies and the literature.15,20,21 Proposed effect sizes for thyroid hormone treatment have ranged as high as an absolute increase of 20 percentage points in hearts transplanted.6,22 We tested the primary hypothesis that treatment would result in an increase of 10 percentage points in hearts transplanted. A sample size of 800 donors would provide the trial with at least 80% power to detect this treatment effect, from a baseline value of 35%. The evaluation of an estimated 320 hearts transplanted would provide 78% power to assess noninferiority of graft outcome at a one-sided alpha of 0.025, with application of a noninferiority margin of 6 percentage points (see Supplementary Methods for justification).23
The analysis was conducted according to the prespecified statistical analysis plan, available with the protocol. The primary analyses were performed in the intention-to-treat population, which included all randomly assigned donors, whether or not they received the assigned infusion, except for the few ineligible donors who underwent randomization by mistake and in whom ineligibility was realized immediately and who were never entered into the trial.24 The primary analysis evaluated the proportion of donors with hearts transplanted, with the use of a two-sided alpha of 0.05 to assess superiority of levothyroxine. Treatment effect was modeled with the use of modified Poisson regression, with adjustment for prespecified baseline covariates of donor age and blood type, and clustered according to site with robust standard errors. A post hoc analysis was performed with adjustment for additional covariates that have been implicated in donor heart utilization: sex, history of hypertension, cause of death, troponin level, and Public Health Service–defined increased infectious risk.25 Recipient 30-day graft survival was assessed by estimating the between-group difference in the proportion surviving and 95% confidence intervals to evaluate noninferiority at a margin of 6 percentage points. Analyses of secondary outcomes are described in the Supplementary Methods; the widths of confidence intervals have not been adjusted for multiplicity and may not be used in place of hypothesis testing. Imputation was performed for binary and continuous secondary outcomes with missing data (LVEF, vasopressor–inotrope score, and proportion of donors weaned off vasopressors, as described in the Supplementary Methods).
A prespecified per-protocol analysis was performed, which included eligible donors who underwent randomization and received at least 6 hours of their assigned infusion. A post hoc “as-treated” analysis (see the Supplementary Appendix) was also performed, comparing all the donors who received levothyroxine, including open-label in the saline group, with those who did not. Adjusted modified Poisson regression analyses (clustered according to site with robust standard errors) were performed to examine the consistency of the effect of treatment in two prespecified subgroups: donors who received the trial infusion within 12 hours after the declaration of brain death as compared with those who received the infusion more than 12 hours after the declaration, and donors with an LVEF on first echocardiography equal to or below 50% as compared with those with an LVEF above 50%. Three additional post hoc subgroups were evaluated: donors receiving low-dose as compared with high-dose vasopressors at baseline (threshold at a vasopressor–inotrope score of <10), donors with low free T4 levels (<0.9 ng per deciliter) at baseline as compared with those with normal levels, and donor cause of death. Interim analysis was performed by the data and safety monitoring board, which recommended continuing enrollment (see Supplementary Methods for details). All data analyses were performed with the use of SAS software, version 9.4 (SAS Institute).
RESULTS
TRIAL PARTICIPANTS
A total of 15 OPOs participated in this trial between December 1, 2020, and November 6, 2022. Twelve were using levothyroxine as part of their standard protocols before the trial. All sites gave concurrent glucocorticoids, and 9 cared for donors in organ-recovery facilities. (Details regarding characteristics of the participating OPOs are provided in Table S1 in the Supplementary Appendix.) A total of 3259 brain-dead organ donors were screened, of whom 2685 had provided authorization for research and 1419 were within the target age range and continued to receive vasopressors after fluid resuscitation. After exclusions, 852 donors were enrolled and underwent randomization (Fig. S1).
A total of 13 donors were determined to be ineligible immediately after randomization (reasons are provided in Table S2). The trial protocol was never initiated in these donors, and their data were not collected. Along with 1 donor whose research authorization was withdrawn, these donors who underwent randomization by mistake were excluded from the primary analysis.
Baseline characteristics of the donors were similar in the two groups (Table 1 and Table S3) and were demographically and geographically representative of the target population of hemodynamically unstable potential heart donors (Table S4 and Fig. S2). Free T4 levels were below the normal range in 38% of the donors, and the median vasopressor–inotrope score was approximately 10; 90% had a vasopressor–inotrope score of more than 2.5 and approximately half were receiving vasopressin in each group. The median expected probability that the heart would be transplanted, from the SRTR risk-adjusted models, was 0.63 in the levothyroxine group and 0.59 in the saline group.
Table 1.
Characteristics of the Donors at Baseline.*
| Characteristic | Levothyroxine Group (N = 419) | Saline Group (N = 419) |
|---|---|---|
| Age — yr | 36±11 | 36±10 |
| Female sex — no./total no. (%)† | 127/403 (32) | 153/400 (38) |
| Race or ethnic group — no./total no. (%)† | ||
| White, non-Hispanic | 252/403 (63) | 278/397(70) |
| Black | 75/403 (19) | 49/397 (12) |
| Hispanic or Latino | 60/403 (15) | 63/397 (16) |
| Asian, Pacific Islander, or other | 16/403 (4) | 7/397 (2) |
| Blood group — no. (%) | ||
| O | 224 (53) | 214 (51) |
| A | 134 (32) | 148 (35) |
| B | 50 (12) | 49 (12) |
| AB | 11 (3) | 8 (2) |
| Coexisting conditions — no./total no. (%) | ||
| Hypertension | 98/403 (24) | 104/397 (26) |
| Diabetes | 38/403 (9) | 31/397 (8) |
| Cigarette use | 58/403 (14) | 76/397 (19) |
| Cocaine use | 92/403 (23) | 105/397 (26) |
| Cause of death — no./total no. (%) | ||
| Anoxia | 162/403 (40) | 195/397 (49) |
| Stroke | 87/403 (22) | 79/397 (20) |
| Trauma | 143/403 (35) | 117/397 (29) |
| Other | 11/403 (3) | 6/397 (2) |
| Weight — kg | 86±24 | 85±23 |
| Median creatinine level (IQR) — mg/dl | 1.2 (0.9–1.9) | 1.2 (0.8–2.0) |
| Median troponin I level (IQR) — ng/ml‡ | 0.7 (0.07–10.2) | 0.7 (0.08–7.3) |
| Positivity for hepatitis C virus — no./total no. (%) | 27/403 (7) | 25/397 (6) |
| Median vasopressor–inotrope score (IQR)§ | 10.2 (5–24) | 10.5 (5–22) |
| Median time from declaration of brain death to start of infusion (IQR) — hr | 8.6 (6.3–11.7) | 7.5 (4.9–11.1) |
| Free T4 levels¶ | ||
| Median (IQR) — ng/dl | 1.00 (0.77–1.30) | 1.00 (0.77–1.30) |
| Level below 0.9 ng/dl — no./total no. (%) | 141/370 (38) | 128/346 (37) |
| Median expected heart yield (IQR)‖ | 0.63 (0.15–0.86) | 0.59 (0.15–0.82) |
Plus–minus values are means ±SD. Unless otherwise indicated, the numbers of donors with missing data were as follows: for weight, 38 in the levothyroxine group and 44 in the saline group; for creatinine level, 2 and 10; for troponin level, 65 and 91; for vasopressor–inotrope score, 14 and 32; for time from declaration of brain death to start of infusion, 8 and 35; and for expected heart yield, 16 and 22. Percentages may not total 100 because of rounding. To convert the values for creatinine to micromoles per liter, multiple by 88.4. IQR denotes interquartile range.
Data on sex, race, and ethnic group were obtained from the Scientific Registry of Transplant Recipients (SRTR) donor database, as reported by the patient’s legal representative or extracted from the medical records (more details in Table S4).
The normal range for troponin levels is 0 to 0.04 ng per milliliter.
The vasopressor–inotrope score is calculated as the weighted sum of all vasopressor and inotropic medications: dopamine dose (in micrograms per kilogram of body weight per minute) + dobutamine dose (in micrograms per kilogram per minute) + (100 × epinephrine dose [in micrograms per kilogram per minute]) + (100 × norepinephrine dose [in micrograms per kilogram per minute]) + (10 × milrinone dose [in micrograms per kilogram per minute]) + (10 × phenylephrine dose [in micrograms per kilogram per minute]) + (10,000 × vasopressin dose [in units per kilogram per minute]). We excluded vasopressin from this calculation if infused at less than 1 unit per hour because this dose was used primarily for control of diabetes insipidus and not as a vasopressor.
The normal range for free T4 (levothyroxine) levels is 0.9 to 2.3 ng per deciliter.
Expected heart yield is derived from the SRTR calculator with the use of donor characteristics.
INFUSIONS
The median time from declaration of brain death to infusion of levothyroxine or saline was 8 hours (interquartile range, 5 to 11). Of the donors assigned to receive levothyroxine, only 8 (2%) did not start the infusion (Table 2; reasons are shown in Table S5). Of those starting levothyroxine, 86 (21%) had their infusion weaned or discontinued before 12 hours, primarily because of the development of hypertension, tachycardia, or both (Table S6). Half the donors in the levothyroxine group had their infusion extended beyond the 12-hour period, for a median additional duration of 19 hours (interquartile range, 7 to 36). In the saline group, only 50 donors (12%) received open-label levothyroxine after the 12-hour trial period, for a median duration of 31 hours (interquartile range, 20-43); reasons for open-label use are provided in Table S7. Free T4 levels were higher at the time of organ recovery in the levothyroxine group, with a median increase of 37% (interquartile range, 7 to 96), as compared with no change (interquartile range, −22 to 23%) in the saline group.
Table 2.
Trial Infusions.*
| Variable | Levothyroxine Group (N = 419) | Saline Group (N = 419) |
|---|---|---|
| Assigned infusion started — no. (%)† | 411 (98) | 384 (92) |
| Levothyroxine weaned before 12 hr — no./total no. (%) | 47/411 (11)‡ | NA |
| Levothyroxine discontinued before 12 hr — no./total no. (%) | 58/411 (14)‡ | NA |
| ≥6 Hr of exposure to levothyroxine or saline — no. (%)§ | 351 (84) | 374 (89) |
| Median dose of levothyroxine given within 12 hr (IQR) — μg | 360 (360–360) | 0 (0–0) |
| Echocardiography performed — no. (%) | 350 (84) | 336 (80) |
| Net fluid balance over period of 12 hr — ml | 1181±1563 | 938±1707 |
| Median time from infusion of levothyroxine or saline to organ recovery (IQR) — hr | 42 (25–59) | 44 (24–58) |
| Open-label levothyroxine | ||
| Given beyond 12 hr — no./total no. (%) | 209/411 (50) | 50/395 (12) |
| Median dose given beyond 12 hr — μg¶ | 527 (190–932) | 522 (253–966) |
| Median cumulative dose of levothyroxine for all donors — μg | 360 (360–868) | 0 (0–0) |
| Median free T4 level at time of organ recovery — ng/dl | 1.4 (1.1–2.1) | 1.0 (0.7–1.3) |
Plus–minus values are means ±SD. Unless otherwise indicated, the numbers of donors with missing data were as follows: net fluid balance, 33 in the levothyroxine group and 46 in the saline group; time from infusion to organ recovery, 16 and 42; cumulative dose of T4, 13 and 24; and free T4 level at time of organ recovery, 115 and 131. NA denotes not applicable.
Of the donors in whom the levothyroxine infusion was started, 33 had a starting dose other than 30 μg per hour. Of the donors in whom the saline infusion was started, 23 received the infusion at a rate other than 30 ml per hour. Reasons that the assigned infusion was not started are provided in Table S5.
A total of 86 had the levothyroxine infusion weaned or discontinued (or both).
The per-protocol population comprised the donors who received at least 6 hours of their assigned infusion.
The dose given beyond 12 hours represents the median only in donors who received open-label levothyroxine.
PRIMARY OUTCOMES
Data on primary outcomes were available for all 838 donors (Table 3). Hearts were transplanted from 453 donors, 230 (54.9%) of those assigned to receive levothyroxine and 223 (53.2%) of those assigned to receive saline (absolute difference, 1.7 percentage points; 95% confidence interval [CI], −5.1 to 8.4). The adjusted risk ratio in the primary efficacy analysis was 1.01 (95% CI, 0.97 to 1.07; P = 0.57), an estimate that remained consistent when adjusted for additional covariates (Table S8). Reasons for hearts not being transplanted were qualitatively similar in the two groups (Table S9). Among the 453 hearts transplanted, 30-day graft survival occurred in 224 of 230 (97.4%) transplanted from donors in the levothyroxine group and 213 of 223 (95.5%) transplanted from donors in the saline group (absolute difference, 1.9 percentage points; 95% CI, −2.3 to 6.0; P<0.001 for noninferiority). There were no substantial between-group differences for hearts transplanted in the prespecified subgroups defined according to time from declaration of brain death to infusion (≤12 hours vs. >12 hours) or according to LVEF (≤50% vs. >50%) (Fig. 1). Post hoc analysis showed that the lack of treatment effect was consistent in subgroups defined according to baseline free T4 level, vasopressor dose at baseline, and donor cause of death (Fig. S3). Results were consistent both for hearts transplanted and graft survival in the per-protocol and as-treated analyses (Table S8).
Table 3.
Donor and Recipient Outcomes.*
| Outcome | Levothyroxine Group (N = 419) | Saline Group (N = 419) | Treatment Effect or Difference (95% CI) |
|---|---|---|---|
| Donor outcomes | |||
| Primary outcome: heart transplanted — no. (%) | 230 (54.9) | 223 (53.2) | 1.01 (0.97 to 1.07)† |
| Secondary outcomes‡ | |||
| Median no. of organs transplanted per donor (IQR) | 4 (3 to 5) | 4 (3 to 5) | 1.03 (0.99 to 1.08)§ |
| Lungs transplanted — no./total no. (%) | 163/419 (38.9) | 149/418 (35.6) | 1.09 (0.91 to 1.31)† |
| Liver transplanted — no./total no. (%) | 335/419 (80.0) | 334/418 (79.9) | 1.00 (0.92 to 1.09)† |
| No. of kidneys transplanted per donor (95% CI) | 1.69 (1.61 to 1.77) | 1.64 (1.58 to 1.70) | 1.03 (0.99 to 1.08)§ |
| Weaned off vasopressors at 12 hr — no./total no. (%) | 143/404 (35.4) | 152/388 (39.2) | 0.91 (0.79 to 1.04)† |
| Median time to wean off vasopressors (95% CI) — hr | 22 (19 to 31) | 25 (19 to 38) | 1.07 (0.89 to 1.29)¶ |
| Median time until first echocardiogram was ordered (95% CI) — hr | 12 (11 to 13) | 13 (12 to 14) | 1.09 (0.93 to 1.27)¶1 |
| Left ventricular ejection fraction — % | |||
| First echocardiogram | 59±11 | 58±12 | 1.0 (−0.6 to 2.7)‖ |
| Maximum of all echocardiograms | 60±9 | 60±10 | 0.5 (−0.9 to 2.0)‖ |
| Adverse events** | |||
| Any adverse event — no. of donors (%) | 51 (12.2) | 16 (3.8) | 8.4 (4.7 to 12.0)†† |
| Serious adverse event — no. (%) | 2 (0.5) | 3 (0.7) | −0.2 (−1.3 to 0.8)†† |
| Recipient outcome | |||
| Graft survival at 30 days — no./total no. (%) | 224/230 (97.4) | 213/223 (95.5) | 1.9 (−2.3 to 6.0)†† |
Plus–minus values are means ±SD. Unless otherwise indicated, the numbers of donors with missing data were as follows: number of organs and kidneys transplanted per donor, 1 in the saline group; time to wean off vasopressors, 15 in the levothyroxine group and 34 in the saline group; time until first echocardiogram was ordered, 10 in the levothyroxine group and 34 in the saline group; and left ventricular ejection fraction, 69 in the levothyroxine group and 83 in the saline group. CI denotes confidence interval.
Shown is the adjusted risk ratio from a modified Poisson regression model, with clustering according to site with robust standard errors. The primary outcome (but not secondary outcomes) was also adjusted for age and blood type.
For secondary outcomes, the widths of the confidence intervals have not been adjusted for multiplicity and should not be used in place of hypothesis testing. Treatment-effect estimates for weaned off vasopressors and left ventricular ejection fraction are presented after multiple imputation of missing values.
Shown is the rate ratio from Poisson generalized-estimating-equation regression, with adjustment for the random effect of site.
Shown is the hazard ratio from a Cox regression model. The proportional-hazards assumption was satisfied in both analyses.
Shown is the difference estimated from a liner mixed model, with adjustment for random site effect.
Serious adverse events were defined as cases of cardiac arrest or premature donor loss due to hemodynamic instability; a full list of adverse events is provided in Table S11.
Shown is the difference in percentage points.
Figure 1. Subgroup Analyses of Hearts Transplanted (Primary Outcome).
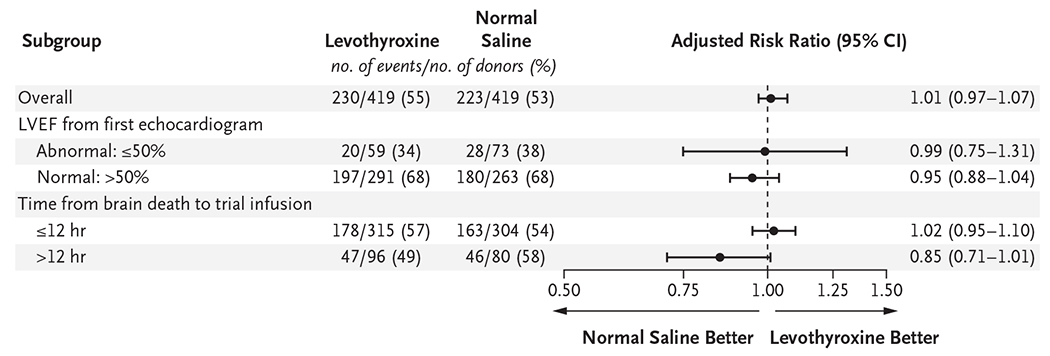
Forest plots show analyses of prespecified subgroups for the primary outcome of transplantation of the donor heart. The trial was not powered and had no prespecified correction for multiple comparisons; the widths of the confidence intervals have not been adjusted for multiplicity and should not be used in place of hypothesis testing. LVEF denotes left ventricular ejection fraction.
SECONDARY OUTCOMES, INCLUDING ADVERSE EVENTS
The median number of organs transplanted per donor was four in both groups. Vasopressor requirements were reduced in both groups (Fig. S4), with no substantial difference in the vasopressor–inotrope score at 12 hours (geometric mean ratio, 1.05; 95% CI, 0.92 to 1.20) or at organ recovery (geometric mean ratio, 0.94; 95% CI, 0.81 to 1.08). The proportion of donors weaned off vasopressor support by 12 hours, the median time to wean off vasopressors, and the median time until echocardiography was ordered were similar in the two groups (Fig. 2 and Fig. S5), as was the LVEF. Results were consistent for analyses of LVEF, vasopressor–inotrope scores, and the proportion of donors weaned off vasopressors in complete-case analyses (Table S10).
Figure 2. Kaplan–Meier Estimates of the Proportion of Donors Continuing to Receive Vasopressors.
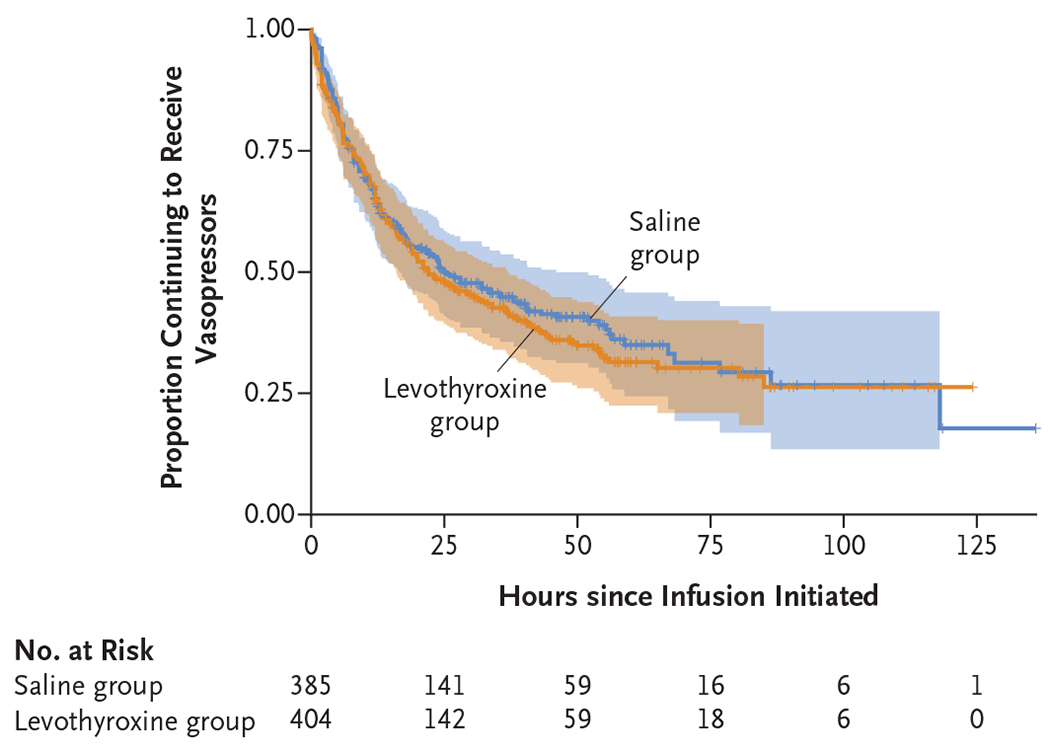
Shown are survival curves in the intention-to-treat population for the time from initiation of levothyroxine or saline infusion until weaning from vasopressors and inotropes (excluding vasopressin at a dose of <1 unit per hour) in the two trial groups. The shaded bands represent 95% confidence intervals. The proportional-hazards assumption was satisfied.
More donors had adverse events in the levothyroxine group than in the saline group (Table 3 and Table S11). Significant between-group differences were observed in the number of cases of severe hypertension (26 in the levothyroxine group vs. 5 in the saline group, P<0.001) and tachycardia (16 vs. 3, P = 0.003). The incidence of serious adverse events was similar in the two groups.
DISCUSSION
In this multicenter, randomized, controlled trial involving hemodynamically unstable potential heart donors, we found no significant benefit of intravenous levothyroxine treatment on improving donor heart utilization. Neither weaning from vasopressor therapy nor ejection fraction on echocardiography was better with levothyroxine than with normal saline, findings that suggest a lack of physiological benefit of levothyroxine on donor cardiovascular function. These results provide evidence that thyroid hormone administration does not improve donor stability or organ-transplantation rates. We did note more adverse events in donors receiving levothyroxine than in those receiving saline, with a greater frequency of hypertension and tachycardia that resolved on weaning or stopping the infusion, without a substantial between-group difference in serious adverse events that could threaten donor loss.
The overarching goal of donor care is to improve the quality and quantity of organs for transplantation.26,27 A key challenge is to counteract the physiological perturbations that occur in the context of brain death and contribute to cardiac dysfunction and hemodynamic instability.28,29 Hormonal resuscitation, including with levothyroxine, is broadly espoused by donor-care guidelines to assist with hemodynamic stabilization and increase the number of organs transplanted, a practice supported only by observational data, which is susceptible to confounding by indication and reverse-causation biases.5,6 A limited number of small randomized studies involving deceased brain-dead donors showed a lack of efficacy of thyroid hormone replacement.8–11,30 Nonetheless, infusion of thyroid hormone (predominantly levothyroxine) remains an integral part of donor protocols, used by 72% of OPOs in one survey and in the majority of hearts transplanted in an international registry.7,16 There remains a lack of high-quality evidence to guide donor interventions that aim to increase organs transplanted31–33; our large multisite trial was able to rigorously evaluate a key aspect of hormonal resuscitation through collaboration among multiple OPOs across the United States. Our patient sample was representative of the target population, and we think these findings may be generalizable to hemodynamically unstable heart donors. Adherence to the trial protocol was high, and no donors or recipients were lost to follow-up.
The pragmatic nature of the trial conferred certain limitations. Blinding of levothyroxine or saline was not feasible across many OPOs, which often care for donors at multiple hospitals. We also felt obligated, given the prevalent belief that thyroid hormone may rescue cardiac function, to inform transplantation centers as to whether a given donor had received levothyroxine or not; similarly, open-label use of levothyroxine was allowed in the control group after 12 hours. However, such open-label use occurred in only 12% of donors and was rarely based on requests from the transplantation center; an as-treated sensitivity analysis did not suggest a benefit even when evaluating all those who received levothyroxine, including open-label. We adopted a dose protocol for levothyroxine in the higher range of that typically used and observed a near 40% increase in free T4 levels after treatment. However, as other studies have suggested, most donors were not profoundly hypothyroid, which limits the rationale for replacement to provide a benefit in this population.34 No benefit was seen even in the subgroup with low free T4 levels; similarly, although some donors in the levothyroxine group had their infusion discontinued early, no benefit was seen in the per-protocol analysis. We did not use the more active form, T3 or triiodothyronine, because a pilot study that we performed suggested no difference in cardiac stabilization between formulations and previous studies showed no clear benefit with T3 treatment.8–10,20 Finally, we did not selectively treat donors with depressed myocardial function, although hemodynamic instability is a reasonable surrogate for cardiac stunning after brain death, and we did not note any benefit in the subgroup with a low LVEF on echocardiography.
In this trial involving hemodynamically unstable brain-dead heart donors, there was no benefit to treatment with intravenous levothyroxine, as compared with normal saline, with respect to the number of hearts transplanted, hemodynamic stability, or donor cardiac function.
Supplementary Material
Acknowledgments
Supported by Mid-America Transplant, which provided support for centralized data collection and data management. Each organ-procurement organization provided internal support for trial coordinator time and the cost of levothyroxine used at their site. Trial data were collected and managed with the use of REDCap electronic data capture tools hosted at Washington University, as supported by the Clinical and Translational Science Award (CTSA) Grant (UL1 TR002345) and Siteman Comprehensive Cancer Center and National Cancer Institute Cancer Support Grant (P30 CA091842). The statistical analysis was supported by the Foundation for Barnes–Jewish Hospital and their generous benefactors and by the Washington University Institute of Clinical and Translational Sciences, which is supported in part by the National Institutes for Health/National Center for Advancing Translational Sciences, CTSA grant (UL1 TR002345).
Footnotes
REFERENCES
- 1.Cooper DK, Novitzky D, Wicomb WN. The pathophysiological effects of brain death on potential donor organs, with particular reference to the heart. Ann R Coll Surg Engl 1989;71:261–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Smith M. Physiologic changes during brain stem death — lessons for management of the organ donor. J Heart Lung Transplant 2004;23:Suppl:S217–S222. [DOI] [PubMed] [Google Scholar]
- 3.Novitzky D, Cooper DK, Morrell D, Isaacs S. Change from aerobic to anaerobic metabolism after brain death, and reversal following triiodothyronine therapy. Transplantation 1988;45:32–6. [DOI] [PubMed] [Google Scholar]
- 4.Novitzky D, Cooper DKC, Rosendale JD, Kauffman HM. Hormonal therapy of the brain-dead organ donor: experimental and clinical studies. Transplantation 2006;82:1396–401. [DOI] [PubMed] [Google Scholar]
- 5.Rosendale JD, Kauffman HM, McBride MA, et al. Hormonal resuscitation yields more transplanted hearts, with improved early function. Transplantation 2003;75:1336–41. [DOI] [PubMed] [Google Scholar]
- 6.Novitzky D, Mi Z, Sun Q, Collins JF, Cooper DKC. Thyroid hormone therapy in the management of 63,593 brain-dead organ donors: a retrospective analysis. Transplantation 2014;98:1119–27. [DOI] [PubMed] [Google Scholar]
- 7.Cooper LB, Milano CA, Williams M, et al. Thyroid hormone use during cardiac transplant organ procurement. Clin Transplant 2016;30:1578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goarin JP, Cohen S, Riou B, et al. The effects of triiodothyronine on hemodynamic status and cardiac function in potential heart donors. Anesth Analg 1996;83:41–7. [DOI] [PubMed] [Google Scholar]
- 9.Pérez-Blanco A, Caturla-Such J, Cánovas-Robles J, Sanchez-Payá J. Efficiency of triiodothyronine treatment on organ donor hemodynamic management and adenine nucleotide concentration. Intensive Care Med 2005;31:943–8. [DOI] [PubMed] [Google Scholar]
- 10.Venkateswaran RV, Steeds RP, Quinn DW, et al. The haemodynamic effects of adjunctive hormone therapy in potential heart donors: a prospective randomized double-blind factorially designed controlled trial. Eur Heart J 2009;30:1771–80. [DOI] [PubMed] [Google Scholar]
- 11.Van Bakel AB, Hino SA, Welker D, et al. Hemodynamic effects of high-dose levothyroxine and methylprednisolone in brain-dead potential organ donors. Transplantation 2022;106:1677–89. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald PS, Aneman A, Bhonagiri D, et al. A systematic review and meta-analysis of clinical trials of thyroid hormone administration to brain dead potential organ donors. Crit Care Med 2012;40:1635–44. [DOI] [PubMed] [Google Scholar]
- 13.Zaroff JG, Rosengard BR, Armstrong WF, et al. Maximizing use of organs recovered from the cadaver donor: cardiac recommendations March 28–29, 2001, Crystal City, Va. Circulation 2002;106:836–41.. [DOI] [PubMed] [Google Scholar]
- 14.Kotloff RM, Blosser S, Fulda GJ, et al. Management of the potential organ donor in the ICU: Society of Critical Care Medicine/American College of Chest Physicians/Association of Organ Procurement Organizations consensus statement. Crit Care Med 2015;43:1291–325. [DOI] [PubMed] [Google Scholar]
- 15.Dhar R, Stahlschmidt E, Marklin G. A randomized trial of intravenous thyroxine for brain-dead organ donors with impaired cardiac function. Prog Transplant 2020;30:48–55. [DOI] [PubMed] [Google Scholar]
- 16.Peled Y, Ram E, Klempfner R, Lavee J, Cherikh WS, Stehlik J. Donor thyroid hormone therapy and heart transplantation outcomes: ISHLT transplant registry analysis. J Heart Lung Transplant 2020;39:1070–8. [DOI] [PubMed] [Google Scholar]
- 17.Peled Y, Lavee J, Kassif Y, et al. Donor thyroid hormone therapy is associated with an increased risk of graft dysfunction after heart transplantation. Clin Transplant 2020;34(7):e13887. [DOI] [PubMed] [Google Scholar]
- 18.Dhar R, Klinkenberg D, Marklin G. A multicenter randomized placebo-controlled trial of intravenous thyroxine for heart-eligible brain-dead organ donors. Trials 2021;22:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belletti A, Lerose CC, Zangrillo A, Landoni G. Vasoactive-inotropic score: evolution, clinical utility, and pitfalls. J Cardiothorac Vasc Anesth 2021;35:3067–77. [DOI] [PubMed] [Google Scholar]
- 20.Dhar R, Stahlschmidt E, Yan Y, Marklin G. A randomized trial comparing triiodothyronine (T3) with thyroxine (T4) for hemodynamically unstable brain-dead organ donors. Clin Transplant 2019;33(3):e13486. [DOI] [PubMed] [Google Scholar]
- 21.Swanson EA, Adams T, Patel MS, et al. Impact of deceased donor management on donor heart use and recipient graft survival. J Am Coll Surg 2020;231(3):351.e5–360.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdelnour T, Rieke S. Relationship of hormonal resuscitation therapy and central venous pressure on increasing organs for transplant. J Heart Lung Transplant 2009;28:480–5. [DOI] [PubMed] [Google Scholar]
- 23.Ardehali A, Esmailian F, Deng M, et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015;385:2577–84. [DOI] [PubMed] [Google Scholar]
- 24.Rehman AM, Ferrand R, Allen E, Simms V, McHugh G, Weiss HA. Exclusion of enrolled participants in randomised controlled trials: what to do with ineligible participants? BMJ Open 2020;10(12):e039546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khush KK, Menza R, Nguyen J, Zaroff JG, Goldstein BA. Donor predictors of allograft use and recipient outcomes after heart transplantation. Circ Heart Fail 2013;6:300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tullius SG, Rabb H. Improving the supply and quality of deceased-donor organs for transplantation. N Engl J Med 2018;379:693–4. [DOI] [PubMed] [Google Scholar]
- 27.Meyfroidt G, Gunst J, Martin-Loeches I, et al. Management of the brain-dead donor in the ICU: general and specific therapy to improve transplantable organ quality. Intensive Care Med 2019;45:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szabó G. Physiologic changes after brain death. J Heart Lung Transplant 2004;23:Suppl:S223–S226. [DOI] [PubMed] [Google Scholar]
- 29.Dujardin KS, McCully RB, Wijdicks EF, et al. Myocardial dysfunction associated with brain death: clinical, echocardiographic, and pathologic features. J Heart Lung Transplant 2001;20:350–7. [DOI] [PubMed] [Google Scholar]
- 30.Frenette AJ, Williamson D, Williams V, Lagacé A-M, Charbonney E, Serri K. A pilot randomized controlled trial comparing levothyroxine to placebo in neurologically deceased donors. Prog Transplant 2019;29:261–8. [DOI] [PubMed] [Google Scholar]
- 31.Schnuelle P, Gottmann U, Hoeger S, et al. Effects of donor pretreatment with dopamine on graft function after kidney transplantation: a randomized controlled trial. JAMA 2009;302:1067–75. [DOI] [PubMed] [Google Scholar]
- 32.Malinoski D, Saunders C, Swain S, et al. Hypothermia or machine perfusion in kidney donors. N Engl J Med 2023;388:418–26. [DOI] [PubMed] [Google Scholar]
- 33.Niemann CU, Feiner J, Swain S, et al. Therapeutic hypothermia in deceased organ donors and kidney-graft function. N Engl J Med 2015;373:405–14. [DOI] [PubMed] [Google Scholar]
- 34.Howlett TA, Keogh AM, Perry L, Touzel R, Rees LH. Anterior and posterior pituitary function in brain-stem-dead donors: a possible role for hormonal replacement therapy. Transplantation 1989;47:828–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


