Abstract
Enzymes directing the biosynthesis of the group A streptococcal hyaluronic acid capsule are encoded in the hasABC gene cluster. Inactivation of hasC, encoding UDP-glucose pyrophosphorylase in the heavily encapsulated group A streptococcal strain 87-282, had no effect on capsule production, indicating that hasC is not required for hyaluronic acid synthesis and that an alternative source of UDP-glucose is available for capsule production. Nucleotide sequence and deletion mutation analysis of the 5.5 kb of DNA upstream of hasA revealed that this region is not required for capsule expression. Many (10 of 23) group A streptococcal strains were found to contain insertion element IS1239′ approximately 50 nucleotides upstream of the −35 site of the hasA promoter. The presence of IS1239′ upstream of hasA did not prevent capsule expression. These results elucidate the molecular architecture of the group A streptococcal chromosomal region upstream of the has operon, indicate that hasABC are the sole components of the capsule gene cluster, and demonstrate that hasAB are sufficient to direct capsule synthesis in group A streptococci.
Group A streptococci (GAS) cause a variety of infections in humans including pharyngitis, invasive infections associated with significant morbidity and mortality, and the unique postinfectious complications of acute rheumatic fever and glomerulonephritis. The GAS hyaluronic acid capsule is a critical virulence factor (20, 22, 33, 37). Three genes, hasA (7, 11), hasB (12), and hasC (4), have been shown to encode enzymes utilized in the synthesis of the polysaccharide. hasA encodes hyaluronan synthase, which adds alternating N-acetyl-d-glucosamine and d-glucuronic acid residues to form the linear hyaluronic acid polymer (7, 11). hasB encodes UDP-glucose dehydrogenase, which forms glucuronic acid from UDP-glucose (12). hasC encodes UDP-glucose pyrophosphorylase, which forms UDP-glucose from UTP and glucose-1-phosphate (4). Although the hasABC genes are contiguous and form an operon (5), complementation experiments with both GAS and heterologous bacteria have suggested that hasC may not be required for capsule synthesis (6).
The small size of the GAS capsule gene region identified to date may reflect the limited genetic requirement for synthesis and export of a linear heteropolymer across the single gram-positive cell membrane. Alternatively, additional genes encoding proteins required for capsule synthesis, regulatory, and export functions may flank the has operon, analogous to the genetic organization in several gram-negative bacterial species (3, 14, 26, 34). Genes immediately downstream of hasC appear unlikely to be involved in either the synthesis or the expression of capsule (2, 8). The purpose of the present study was to define the genes necessary for GAS hyaluronic acid synthesis by determining the requirement for hasC and by characterizing the chromosomal region immediately upstream of the has operon.
hasC is not required for GAS hyaluronic acid expression.
To derive a GAS hasC mutant, initially we amplified by PCR a 630-bp fragment of hasC extending from nucleotide 201 to nucleotide 840 with respect to the hasC initiation codon with the oligonucleotide primers CCCCCCTCTAGACGAGGAAATCCTTGTGGTGAC (forward) and CCCCCCAAGCTTCCAACATCGTAACGATTGCC (reverse) and a chromosomal DNA template from the heavily encapsulated M18 GAS strain 87-282. The forward and reverse primers contained the terminal restriction sites XbaI and HindIII, respectively. We cloned the 630-bp amplicon into the temperature-sensitive shuttle vector pJRS233 (30) to form pJHASC. To inactivate the hasC gene present in pJHASC, we digested the construct with the restriction endonucleases NsiI and SphI, purified the larger fragment present after digestion, and then ligated a 15-bp 5′-phosphorylated oligonucleotide linker (TCCCCCCCCCGGATCCGCATG [forward], CGGATCCGGGGGGGGGATGCA [reverse]) to the pJHASC construct via NsiI and SphI compatible ends present in the linker sequence to generate the plasmid pJHASCΔ. Insertion of the linker introduces a BamHI site and multiple stop codons into the hasC sequence present in pJHASCΔ.
To demonstrate that the interruption present in the mutant hasC allele resulted in loss of UDP-pyrophosphorylase activity, we cloned either the native or mutant hasC allele into the expression vector pET-24a (Novagen, Inc. Madison, Wis.) and assayed UDP-glucose pyrophosphorylase activity in the background of Escherichia coli DEV6 (kindly provided by the E. coli Genetic Stock Center, Yale University, New Haven, Conn.), which is deficient in the enzyme (4). Enzyme activity was detected in DEV6 transformed with the expression vector containing the wild-type hasC allele, but not in DEV6 either having the vector alone or the vector containing the mutant hasC allele, confirming that the mutation in hasC resulted in loss of a functional UDP-glucose pyrophosphorylase.
We replaced the wild-type hasC allele in the 87-282 chromosome with the mutant hasC allele present in pJHASCΔ by using gene replacement mutagenesis, as previously described (21, 28), to derive strain 282hasCΔ. Southern hybridization demonstrated hasC replacement in 282hasCΔ (data not shown). 282hasCΔ had a mucoid colony morphology indistinguishable from that of the parent strain 87-282, suggesting that the two strains produced similar amounts of surface polysaccharide. Measurement of cell-associated hyaluronic acid confirmed that the parent and the hasC mutant strain produced similar amounts of polysaccharide (65 ± 1.0 fg/CFU and 118 ± 1.5 fg/CFU, respectively). These results indicate that hasC is not required for GAS capsule expression and that sufficient UDP-glucose is present in the cells to permit wild-type levels of hyaluronic acid synthesis in the absence of hasC even in a highly encapsulated GAS strain.
Cloning and analysis of 5.5 kb of nucleotide sequence upstream of hasA in the GAS strain 87-282 chromosome.
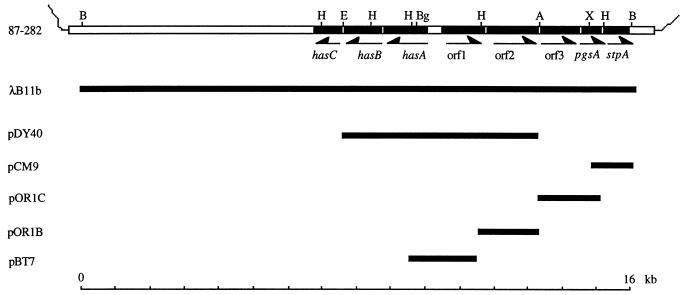
Previously, we described the cloning and purification of λB11b, an EMBL3 bacteriophage clone containing the GAS strain 87-282 capsule gene region within a 16-kb insert (2). We subcloned a series of plasmid constructs (Fig. 1) from λB11b and used the plasmids as templates to determine the nucleotide sequence for the 5.5 kb of DNA upstream of hasA (Fig. 1). Analysis of the predicted amino acid sequence suggested the presence of five complete open frames, all transcribed divergently from the has operon (Fig. 1). The relevant characteristics of these open reading frames, including homologies to sequences in the world database and consensus motifs, are shown in Table 1. None of the first three open reading frames had significant homologies or a consensus motif sufficient to assign a function for the predicted protein. Strong sequence homologies and consensus motifs suggested that orf4 (pgsA) encodes a cytidine-diphosphate-diacylglycerol-glycerol-3-phosphate 3-phosphatidyltransferase (EC 2.7.8.5) (phosphatidylglycerophosphate synthase) (19, 25, 29) and that orf5 (stpA) encodes an ATP binding component of an ATP binding cassette (ABC) transporter (17, 35). Because—with the exception of the most distal gene encoding a component of an ABC transporter—these open reading frames do not have significant homologies to capsule genes in other bacterial species and because ABC transporters are involved in transport of many substrates, it seemed unlikely that the region upstream of hasA was involved in GAS capsule expression.
FIG. 1.
Schematic map of the GAS capsule gene region and subclones. The hasABC genes, the open reading frames upstream of hasA, and the subclones from the capsule gene region are shown in black. Arrows indicate the direction of transcription for the hasABC genes and the upstream open reading frames. Selected restriction endonuclease sites are indicated: B, BamHI; Bg, BglII; H, HindIII; E, EcoRI; A, Asp718; X, XbaI.
TABLE 1.
Characteristics of open reading frames identified upstream of hasA
| Open reading frame | Predicted protein
|
Homology | Consensus motif(s) | |
|---|---|---|---|---|
| Size (kDa) | No. of amino acids | |||
| orf1 | 48.2 | 415 | None | None |
| orf2 | 49.2 | 430 | None | None |
| orf3 | 37.8 | 342 | None | None |
| pgsA | 18.5 | 166 | pgsAa | DGXXARXXXXXXXXGXXXDXXXDb |
| || || | | | | ||||
| DGYLARKWHVVSNFGKFADPLAD | ||||
| stpA | 33.2 | 297 | ABCc | GXXXXGKSd |
| | ||| | ||||
| GHNGSGKS | ||||
| LSGGXXXRVXIAe | ||||
| |||| || || | ||||
| LSGGQKQRVAIA | ||||
Gene encoding phosphatidylglycerophosphate synthase (EC 2.7.8.5). Greatest homology is to Bacillus subtilis pgsA (25).
Consensus motif for phosphatidyltransferases (19, 29). Lower line corresponds to amino acids 52 to 74 of GAS pgsA.
ABC transporters. Greatest homology is to the ATP binding component of a putative peptide transporter in Mycoplasma pneumoniae (18).
Consensus motif for P-loop of nucleotide binding proteins (35). Lower line corresponds to amino acids 57 to 64 of GAS stpA.
Signature motif for ATP binding proteins of ABC transporters (17). Lower line corresponds to amino acids 158 to 169 of GAS stpA.
To confirm that the gene products of the open reading frames immediately upstream of hasA were not required for polysaccharide capsule synthesis and expression, we derived strain 282orf1-2Δ, in which orf1 and orf2 are deleted. To derive 282orf1-2Δ, we used the oligonucleotide primers CCCTCTAGAAAATCCCGACAATTAAGTC (forward) and CCCGGATCCCGATTCTCTTAACACTTCACC (reverse), which contain terminal XbaI and BamHI restriction endonuclease sites, respectively, to amplify a 2,719-bp amplicon from 87-282 chromosomal DNA template. The PCR product was cloned into vector pJRS233Δ to form plasmid pJORF1-2.EcoRV digestion of pJORF1-2, purification of the larger digestion product, and ligation of the purified fragment via the terminal EcoRV sites generated plasmid pJORF1-2Δ. The insert in pJORF1-2Δ was comprised of a 355-bp fragment that included 95 bp of orf1 upstream sequence plus the first 260 nucleotides of orf1 linked to a 342-bp fragment that contained 250 bp of the orf2 3′ terminus plus an additional 92 nucleotides of downstream sequence. In linking these regions in the pJORF1-2Δ construct, 989 bp of orf1 and 1,037 bp of orf2 were deleted. The mutant orf1-orf2 allele present in pJORF1-2Δ was introduced into the 87-282 chromosome by allelic exchange mutagenesis (21, 28) to derive strain 282orf1-2Δ. Southern hybridization analysis confirmed gene replacement in 282orf1-2Δ. Deletion of orf1 and orf2 had no apparent effect on capsule production; both the parent and the mutant strain had a mucoid colony morphology on blood agar medium and similar amounts of cell-associated hyaluronic acid (65 ± 1.0 fg/CFU and 74 ± 2.8 fg/CFU, respectively) (33). These results indicate that the two open reading frames immediately upstream of hasA are not required for GAS capsule expression.
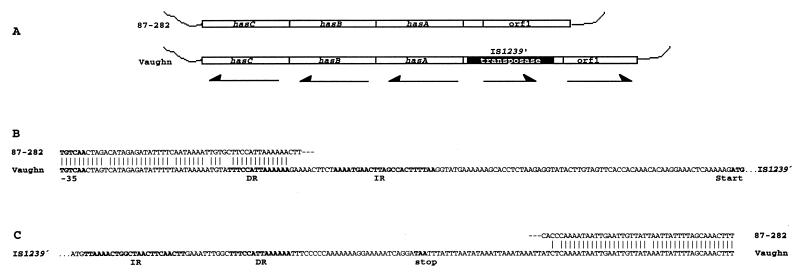
To investigate whether the region upstream of hasA in the mucoid strain 87-282 is conserved in other GAS strains, we used pDY40 to probe a Southern blot of HindIII-digested genomic DNA from 23 GAS strains including a variety of clinical isolates and M protein types. The Southern blot demonstrated that 10 of 23 GAS strains contained an additional 1.1 kb of DNA immediately upstream of hasA (data not shown). We amplified this region from the type 24 GAS strain Vaughn by using PCR and the primers AACGGATAGGTCTGTGCTAAC (forward) and TTATTCAACAACATCGACCTG (reverse). Compared to the 87-282 sequence from this region, the sequence obtained from the strain Vaughn PCR product contained approximately 1.1 kb of additional DNA, of which 969 nucleotides were 99% identical to the sequence of the GAS insertion element IS1239 (23). The only significant difference was an additional 36 nucleotides present in the strain Vaughn element which extended the carboxy terminus of the putative transposase by 12 additional amino acids. Because the insertion element present in strain Vaughn appears to be a slightly larger variant of IS1239, we have designated it IS1239′. Further comparison of the nucleotide sequence between GAS strains 87-282 and Vaughn localized IS1239′ integration to a locus 46 nucleotides upstream of the −35 site of the hasA promoter (Fig. 2). Measurement of cell-associated hyaluronic acid in a sample of 23 GAS strains demonstrated no correlation between the presence of the insertion sequence upstream of hasA and the amount of cell-associated polysaccharide. This observation is consistent with studies showing that full activity of the has operon promoter requires no more than 12 nucleotides of flanking sequence upstream of the −35 site (1) and supports the sequence analysis suggesting that genes upstream of hasA are unlikely to be involved in capsule expression.
FIG. 2.
Schematic representation comparing the 87-282 and Vaughn capsule gene regions and sequence analysis defining the boundaries and insertion site of the insertion element IS1239′. (A) Comparison of the site of IS1239′ insertion in GAS strain Vaughn to the homologous chromosomal region in GAS strain 87-282. The insertion element is shown in black. The genes hasABC, the putative insertion element transposase, and orf1 are identified; arrows indicate the direction of gene transcription. (B) Comparison of the nucleotide sequences beginning 71 nucleotides upstream of the hasA initiation codon in GAS strains 87-282 and Vaughn. Alignment of the homologous sequences is shown; identical nucleotides are indicated by vertical bars. The hasA −35 promoter site is shown in boldface type and indicated. Inverted (IR) and direct (DR) repeat sequences are shown in boldface type. The putative initiation codon for the IS1239′ transposase is indicated (Start). Dashes in the 87-282 sequence indicate continuity with the 87-282 sequence in panel C. Dots in the Vaughn sequence indicate the continuation of the IS1239′ nucleotide sequence, which is not shown. (C) Comparison of the nucleotide sequences approximately 250 bp upstream of the putative initiation codon for orf1 in GAS strains 87-282 and Vaughn. Alignment of the homologous sequences is shown; identical nucleotides are indicated by vertical bars. Dashes in the 87-282 sequence indicate continuity with the 87-282 sequence shown in panel B. Dots in the Vaughn sequence indicate preceding nucleotides present in IS1239′. Inverted (IR) and direct (DR) repeat sequences are shown in boldface type. The putative termination codon for the IS1239′ transposase is indicated (stop).
The results of these studies provide evidence that the GAS capsule gene region is comprised solely of the hasABC genes and that only hasAB are uniquely required for capsule production. Additional proteins must be involved in the biosynthesis of hyaluronic acid—the enzymes involved in the synthesis of UDP–N-acetylglucosamine, for example—but these functions are likely to be shared with other synthetic or metabolic pathways in the cell. The hasC gene product, UDP-glucose pyrophosphorylase, is not required for hyaluronic acid synthesis, indicating that an alternative source of UDP-glucose is available for capsule production. Epimerization of UDP-galactose to UDP-glucose has been reported for Streptococcus pneumoniae (10), but such a pathway seems unlikely in GAS that do not contain galactose as a cell surface component. GAS lipoteichoic acid has been reported to be glucosylated (16). Since glucosylation of lipoteichoic acid requires UDP-glucose in other bacterial systems (36), it is possible that a hasC homolog in GAS is clustered with genes involved in lipoteichoic acid synthesis.
The capsule gene cluster in GAS is quite similar to that of S. pneumoniae type 3, in which the genes uniquely required for capsule production comprise a four-gene cluster that includes three genes analogous to hasABC (9). Both the GAS and S. pneumoniae type 3 capsule gene regions are unusually small compared to most other encapsulated bacteria that contain multiple genes involved in capsule synthesis and surface expression (3, 13, 15, 26, 27 31, 34). The limited genetic requirement for capsule expression in GAS and S. pneumoniae type 3 likely reflects the relative simplicity of the capsular polysaccharide that these organisms produce and supports the prediction that this family of glycosyl transferases exports polysaccharide in the process of polymerization (24, 32).
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study have been submitted to GenBank under accession no. AF082738 and AF082865.
Acknowledgments
We thank Tom DiCesare and Sarah Henderson for expert technical assistance.
This work was supported by Public Health Service grants AI29952 (M.R.W.) and AI01343 (C.D.A.) from the National Institute of Allergy and Infectious Diseases, a Child Health Research grant from the Charles H. Hood Foundation (C.D.A.), and a postdoctoral fellowship from the Fundacion Ramon Areces (S.A.).
REFERENCES
- 1.Albertí S A, Ashbaugh C D, Wessels M R. Structure of the has operon promoter and regulation of hyaluronic acid capsule expression in group A Streptococcus. Mol Microbiol. 1998;28:343–353. doi: 10.1046/j.1365-2958.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 2.Ashbaugh C D, Wessels M R. Cloning, identification, and expression of the group A streptococcal guaB gene encoding inosine monophosphate dehydrogenase. Gene. 1995;165:57–60. doi: 10.1016/0378-1119(95)00422-3. [DOI] [PubMed] [Google Scholar]
- 3.Boulnois G J, Roberts I S, Hodge R, Hardy K R, Jann K B, Timmis K N. Analysis of the K1 capsule biosynthesis genes of Escherichia coli: definition of three functional regions for capsule production. Mol Gen Genet. 1987;208:242–246. doi: 10.1007/BF00330449. [DOI] [PubMed] [Google Scholar]
- 4.Crater D L, Dougherty B A, van de Rijn I. Molecular characterization of hasC from an operon required for hyaluronic acid synthesis in group A streptococci. J Biol Chem. 1995;270:28676–28680. doi: 10.1074/jbc.270.48.28676. [DOI] [PubMed] [Google Scholar]
- 5.Crater D L, van de Rijn I. Hyaluronic acid synthesis operon (has) expression in group A streptococci. J Biol Chem. 1995;270:18542–18548. doi: 10.1074/jbc.270.31.18452. [DOI] [PubMed] [Google Scholar]
- 6.DeAngelis P L, Papaconstantinou J, Weigel P H. Isolation of a Streptococcus pyogenes gene locus that directs hyaluronan biosynthesis in acapsular mutants and in heterologous bacteria. J Biol Chem. 1993;268:14568–14571. [PubMed] [Google Scholar]
- 7.DeAngelis P L, Papaconstantinou J, Weigel P H. Molecular cloning, identification, and sequence of the hyaluronan synthase gene from group A Streptococcus pyogenes. J Biol Chem. 1993;268:19181–19184. [PubMed] [Google Scholar]
- 8.DeAngelis P L, Yang N, Weigel P H. Molecular cloning of the gene encoding RecF, a DNA repair enzyme, from Streptococcus pyogenes. Gene. 1995;156:89–91. doi: 10.1016/0378-1119(95)00069-i. [DOI] [PubMed] [Google Scholar]
- 9.Dillard J P, Vandersea M W, Yother J. Characterization of the cassette containing genes for type 3 capsular polysaccharide biosynthesis in Streptococcus pneumoniae. J Exp Med. 1995;181:973–983. doi: 10.1084/jem.181.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Distler J, Roseman S. Polysaccharide and glycolipid synthesis by cell-free preparations from type XIV pneumococcus. Proc Natl Acad Sci USA. 1964;51:897–905. doi: 10.1073/pnas.51.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dougherty B A, van de Rijn I. Molecular characterization of hasA from an operon required for hyaluronic acid synthesis in group A streptococci. J Biol Chem. 1994;269:169–175. [PubMed] [Google Scholar]
- 12.Dougherty B A, van de Rijn I. Molecular characterization of hasB from an operon required for hyaluronic acid synthesis in group A streptococci. J Biol Chem. 1993;268:7118–7124. [PubMed] [Google Scholar]
- 13.Frosch M, Edwards U, Bousset K, Kraube B, Weisgerber C. Evidence for a common molecular origin of the capsule gene loci in Gram-negative bacteria expressing group II capsular polysaccharides. Mol Microbiol. 1991;5:1251–1263. doi: 10.1111/j.1365-2958.1991.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 14.Frosch M, Weisgerber C, Meyer T F. Molecular characterization and expression in Escherichia coli of the gene complex encoding the polysaccharide capsule of Neisseria meningitidis group B. Proc Natl Acad Sci USA. 1989;86:1169–1673. doi: 10.1073/pnas.86.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidolin A, Morona J K, Morona R, Hansman D, Paton J C. Nucleotide sequence analysis of genes essential for capsular polysaccharide biosynthesis in Streptococcus pneumoniae type 19F. Infect Immun. 1994;62:5384–5396. doi: 10.1128/iai.62.12.5384-5396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamada S, Yamamoto T, Koga T, McGhee J R, Michalek S M, Yamamoto S. Chemical properties and immunobiological activities of streptococcal lipoteichoic acids. Zentbl Bakteriol Mikrobiol Hyg. 1985;259:228–243. doi: 10.1016/s0176-6724(85)80054-7. [DOI] [PubMed] [Google Scholar]
- 17.Higgins C F, Hiles I D, Whalley K, Jamieson D K. Nucleotide binding by membrane components of bacterial periplasmic binding protein-dependent transport systems. EMBO J. 1985;4:1033–1040. doi: 10.1002/j.1460-2075.1985.tb03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Himmelreich R, Plagens H, Herrmann R. Sequence analysis of 56 kb from the genome of the bacterium Mycoplasma pneumoniae comprising the DNAA region, the ATP operon and a cluster of ribosomal protein genes. Nucleic Acids Res. 1996;24:628–639. doi: 10.1093/nar/24.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hjelmstad R H, Bell R M. sn-1,2-diacylglycerol choline- and ethanolaminephosphotransferases in Saccharomyces cerevisiae. Nucleotide sequence of the EPT1 gene and comparison of the CPT1 and EPT1 gene products. J Biol Chem. 1991;266:5094–5103. [PubMed] [Google Scholar]
- 20.Husmann L K, Yung D-L, Hollingshead S K, Scott J R. Role of putative virulence factors of Streptococcus pyogenes in mouse models of long-term throat colonization and pneumonia. Infect Immun. 1997;65:1422–1430. doi: 10.1128/iai.65.4.1422-1430.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji Y, McLandsborough L, Kondagunta A, Cleary P P. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect Immun. 1996;64:503–510. doi: 10.1128/iai.64.2.503-510.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson D R, Stevens D L, Kaplan E L. Epidemiologic analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. J Infect Dis. 1992;166:374–382. doi: 10.1093/infdis/166.2.374. [DOI] [PubMed] [Google Scholar]
- 23.Kapur V, Reda K B, Li L L, Ho L J, Rich R R, Musser J M. Characterization and distribution of insertion sequence IS1239 in Streptococcus pyogenes. Gene. 1994;150:135–140. doi: 10.1016/0378-1119(94)90872-9. [DOI] [PubMed] [Google Scholar]
- 24.Keenleyside W J, Whitfield C. A novel pathway for O-polysaccharide biosynthesis in Salmonella enterica serovar Borreze. J Biol Chem. 1996;271:28581–28592. doi: 10.1074/jbc.271.45.28581. [DOI] [PubMed] [Google Scholar]
- 25.Kontinen V P, Tokuda H. Overexpression of phosphatidylglycerophosphate synthase restores protein translocation in a secG deletion mutant of Escherichia coli at low temperature. FEBS Lett. 1995;364:157–160. doi: 10.1016/0014-5793(95)00378-m. [DOI] [PubMed] [Google Scholar]
- 26.Kroll J S, Zamze S, Loynds B, Moxon E R. Common organization of chromosomal loci for production of different capsular polysaccharides in Haemophilus influenzae. J Bacteriol. 1989;171:3343–3347. doi: 10.1128/jb.171.6.3343-3347.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J C, Xu S, Albus A, Livolsi P J. Genetic analysis of type 5 capsular polysaccharide expression by Staphylococcus aureus. J Bacteriol. 1994;176:4883–4889. doi: 10.1128/jb.176.16.4883-4889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McIver K S, Scott J R. Role of mga in growth phase regulation of virulence genes of group A streptococcus. J Bacteriol. 1997;179:5178–5187. doi: 10.1128/jb.179.16.5178-5187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikawa J, Kodaki T, Yamashita S. Primary structure and disruption of the phosphatidylinositol synthase gene of Saccharomyces cerevisiae. J Biol Chem. 1987;262:4876–4881. [PubMed] [Google Scholar]
- 30.Perez-Casal J, Price J A, Maguin E, Scott J R. An M protein with a single C repeat prevents phagocytosis of Streptococcus pyogenes: use of a temperature-sensitive shuttle vector to deliver homologous sequences to the chromosome of S. pyogenes. Mol Microbiol. 1993;8:809–819. doi: 10.1111/j.1365-2958.1993.tb01628.x. [DOI] [PubMed] [Google Scholar]
- 31.Rubens C R, Haft R F, Wessels M R. Characterization of the capsular polysaccharide genes of group B streptococci. Dev Biol Stand. 1995;85:237–244. [PubMed] [Google Scholar]
- 32.Saxena I M, Brown R M, Jr, Fevre M, Geremia R A, Henrissat B. Multidomain architecture of β-glycosyl transferases: implications for mechanism of action. J Bacteriol. 1995;177:1419–1424. doi: 10.1128/jb.177.6.1419-1424.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schrager H, Wessels M R. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J Clin Investig. 1996;98:1954–1958. doi: 10.1172/JCI118998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vimr E R, Aaronson W, Silver R P. Genetic analysis of chromosomal mutations in the polysialic acid gene cluster of Escherichia coli K1. J Bacteriol. 1989;171:1106–1117. doi: 10.1128/jb.171.2.1106-1117.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward J B. Teichoic and teichuronic acids: biosynthesis, assembly, and location. Microbiol Rev. 1981;45:211–243. doi: 10.1128/mr.45.2.211-243.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wessels M R, Moses A E, Goldberg J B, DiCesare T J. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc Natl Acad Sci USA. 1991;88:8317–8321. doi: 10.1073/pnas.88.19.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]