Abstract
Amyotrophic lateral sclerosis (ALS) risk is linked to environmental exposures. The National Emissions Inventory (NEI) database compiles mandatory reports of levels of airborne contaminants from a variety of stationary and mobile pollution sources across the U.S. The objective of this study was to identify airborne contaminants that may be associated with ALS etiology for future study. We geospatially estimated exposure to airborne contaminants as risk factors for ALS in a nationwide large de-identified medical claims database, the SYMPHONY Integrated Dataverse®. We extracted zip3 of residence at diagnosis of ~26,000 nationally distributed ALS patients and n = 3 non-ALS controls matched per case for age and sex. We individually aggregated the median levels of each of 268 airborne contaminants recorded in the NEI database for 2008 to estimate local residential exposure. We randomly broke the dataset into two independent groups to form independent discovery and validation cohorts. Contaminants associated with increased ALS risk in both the discovery and validation studies included airborne lead (false discovery rate (FDR) = 0.00077), and polychlorinated biphenyls (PCBs), such as heptachlorobiphenyl (FDR = 3.60E-05). Small aircraft were the largest source of airborne lead, while the PCB emissions came from certain power plants burning biomass, and from industrial boilers. Associations with residential history of lead exposure were confirmed in two additional cohorts (10 year top quartile in New Hampshire/Vermont OR 2.46 95% CI 1.46–2.80, and in Ohio OR 1.60 95% CI 1.28–1.98). The results of our geospatial analysis support neurotoxic airborne metals and PCBs as risk factors for ALS.
Keywords: Amyotrophic lateral sclerosis, Risk factors, PCBs, Polychlorinated biphenyls, Lead
Graphical Abstract

1. Introduction
Respiratory failure and death usually occurs over a 3–5 year period after diagnosis with amyotrophic lateral sclerosis (ALS), a devastating disease involving the progressive loss of both upper and lower motor neurons with muscle spasticity and atrophy. Only 10% of ALS cases can be attributed to a familial trait or a particular genetic susceptibility, and environmental factors likely contribute to the development of the majority of ‘sporadic ALS’ cases (Mathis et al., 2019). To date, available drugs for ALS have very modest impacts on the relentless progression of this disease, however these interventions have largely been developed using genetic rather than exposure-driven models. Identifying environmental etiologic factors could help to prevent future ALS cases, and would also enable the development of an environmental exposure model as well as mechanistic insights to find and test new drug interventions to block disease progression.
Several studies suggest geographic variation in ALS rates, and raise the hypothesis that air pollution experienced at an individual’s place of residence could be associated with ALS risk (Mitchell et al., 1998; Noonan et al., 2005; Oliveira et al., 2020; Sabel et al., 2003; Scott et al., 2010; Stallones et al., 1989; Uccelli et al., 2007; Vieira et al., 2018). A recent study in the Netherlands documented substantial geospatial variation in ALS risk and speculated it could be at least partly due to an environmental risk factor (de Jongh et al., 2021). Increased risk of ALS was linked to higher levels of air pollutants, including spatial clusters in areas with agriculture or vehicle traffic (Povedano et al., 2018), fine airborne particulate matter 2.5 μm or less (PM2.5) (Nunez et al., 2021; Seelen et al., 2017), nitrogen oxides (Seelen et al., 2017), or occupational exposure to silica, organic dust, and diesel motor exhaust (Visser et al., 2019), while traffic-related 10 μm particulates showed inconsistent associations with ALS (Filippini et al., 2021). An ALS mortality study in Spain showed higher rates in the Northern provinces and a significant correlation with higher air lead levels (p = 0.001) (Santurtun et al., 2016). A case-control study in Pennsylvania (n = 51 cases diagnosed between 1998 and 2010), examined the relationship between place of residence and levels of 35 pollutants, including metals, aromatic solvents, organic/chlorinated solvents, other hazardous air pollutants, and pesticides. Using air pollution data from 1999 and 2002, they showed increased ALS risk associated with residing in census tracts with exposure to ‘aromatic solvents’, but lacked the statistical power to identify individual chemicals significantly associated with ALS (Malek et al., 2015).
Building on this work, we sought to identify the individual airborne contaminants associated with ALS risk using the power of a recent, large, nationally distributed and de-identified healthcare claims cohort (diagnosis years 2013–2019). The objective of the current study is to assess potential risks of airborne contaminant exposure levels estimated based on location of residence. The National Emissions Inventory (NEI) database compiles mandatory reports collected through implementation of the National Ambient Air Quality Standards, and the EPA’s Air Toxics Program on the levels of hundreds of airborne contaminants from a variety of stationary and mobile pollution sources across the U.S. (USEPA, 2013). Our study used residential location to estimate exposure for 268 contaminants, and a three-phase approach to assess ALS risk in independent, nationwide ‘discovery’ and ‘validation’ cohorts, plus a ‘confirmation’ study.
2. Materials and methods
We used a nationally distributed, large, de-identified healthcare claims dataset from the SYMPHONY Integrated Dataverse® (herein referred to as ‘SYMPHONY’) as the study cohort. This unique resource holds administrative claims for a broad sector of the nationwide U.S. population covering 274 million active patient lives for all payer types, including commercial payers, Medicare/Medicaid, and pharmacy benefit managers/co-pay cards, and the majority of prescription drug data. The dataset has been used in a variety of clinical investigations, such as those investigating prevalence of certain prescription drug uses, e.g. for epilepsy (Faught et al., 2017). Miller et al. recently used SYMPHONY as a data source to estimate nationwide ALS incidence at 1.37/100,000 (Miller et al., 2021). ALS patients diagnosed during 2013–2019 were eligible, and the inclusion criteria were, a) a minimum of two entries into the SYMPHONY database classified as being due to ICD-9/10 codes for ALS at least 3 calendar months apart, b) a minimum of 6 months’ enrollment in the database prior to the first ALS ICD code and, c) age at first ALS ICD code ≥18 yr.
We randomly selected individuals in the overall SYMPHONY network who were similar to the ALS patient cases based on age, sex and length of database history as controls. ALS cases and controls were required to have a minimum of 6 months enrollment in the database. The controls had a similar national distribution to the cases, based on the coverage of the SYMPHONY network. We excluded patients >80 years old due to low coverage in the SYMPHONY network, and those with ICD-9/10 codes for neurodegenerative diseases other than ALS (e.g. Alzheimer’s and Parkinson’s, dementia, or frontotemporal dementia), as they may share etiologic factors. We used the R-package “MatchIt” to perform propensity score matching to select a subset of three controls per case as the comparison group, with the nearest age (range 18–80) and same sex as the ALS cases (Ho et al., 2007).
Airborne exposure estimates
We downloaded data to assess 268 airborne contaminant levels recorded in the U.S. Environmental Protection Agency (EPA)’s National Emissions Inventory (NEI) database for 2008 to estimate potential exposures occurring prior to ALS onset in the cohort. We calculated the median level of the sampled point sources of each contaminant measured within each zip3 region (a polygon encompassing the zipcodes that share the same first three digits). We then used these airborne contaminant level data to estimate potential residential exposure at the zip3 location of the ALS patient at diagnosis, or equivalent timepoint for controls, as this was the only spatiotemporal information available for the SYMPHONY cohort. We also queried the NEI database for the years available (2008, 2011, 2014, and 2017) to assess the continuity of exposure and identify temporal shifts in the industrial sources of the ALS-associated contaminants over time.
Statistical analysis
ALS patients and controls in the SYMPHONY Integrated Dataverse® were randomly broken into two groups based on their zip3 region of residence (863 total regions). One group served as the ‘discovery’ cohort (500 zip3 regions), which we used to identify contaminant exposures estimated from the 2008 NEI database associated with ALS risk. To select contaminants for further study, we used False Discovery Rate (FDR) correction to account for multiple comparisons (268 airborne contaminants), and a significance threshold of <0.2. We then performed a ‘validation’ of these top-hit contaminants in the other independent group (the remaining 363 zip3 regions). Logistic regression analysis assessed the association between the log-transformed level of each contaminant and ALS risk. Odds ratios (OR) reflect the change in ALS risk associated with an increase in the level of a contaminant, using the natural log of 1.0 as the unit. At the validation stage, we used a FDR with a significance threshold of <0.05. We also evaluated combinations of contaminants associated with ALS, testing all combinations with a main effect of FDR <0.2. These analyses were all performed using R: A Language and Environment for Statistical Computing, version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
2.1. Confirmation phase using residential history
Our primary results were generated using a single address. We performed a sensitivity analysis to check that this result is consistent when we use residential history to assess exposure, incorporating exposure changes as people move from location to location. We were able to perform this ‘confirmation’ analysis for lead, which was present in states where we had residential history data (New Hampshire, Vermont, and Ohio). We did not have access to these data for the states with PCBs. For the ‘confirmation’ study, we obtained mortality records attributed to ‘motor neuron disease’ using ICD-10 code G12.2 from the following states, for the years that were available: New Hampshire (2009–2018 n = 337), Vermont (2008–2016 n = 216), and Ohio (2016–2019 n = 799). From among the same catchment counties as the ALS cases, population controls were identified as residents of New Hampshire/Vermont (n = 762), or Ohio (n = 1336) using the U.S. Postal Service Delivery Sequence file licensed to Marketing Systems Group (Horsham, PA). The sampling algorithm was designed to randomly sample individuals in the population based on the expected demographic distribution of the ALS cases, with over-sampling of 50–75-year-olds and males. We obtained the geocodes of addresses held by each confirmation cohort subject over the 15-year period prior to the index date from a commercial financial marketing database query LexisNexis (Dayton, Ohio). The resulting dataset contains multiple rows per subject, with a unique row for each address. We then mapped those addresses into zip3 polygons, showing the residential locations of each subject as they move from place to place over time.
Residential history of lead exposure
Following up on the association between lead and ALS in SYMPHONY, we performed a detailed confirmatory residential history analysis. We constructed raster maps of NEI airborne lead levels modeled for each year prior to diagnosis by performing linear interpolation based on the available 2008, 2011, 2014, and 2017 datapoints.
To estimate the exposure in each year prior to diagnosis, we read the contaminant amount from the NEI raster map for the appropriate year to assess the contaminant level for each case or control subject residence. Our interim dataset contains exposure estimates for each patient’s residence, across his/her multiple residences. We expanded this dataset to include a row for each year of the 15-year pre-diagnostic period. We then calculated the median exposure to lead in epochs representing the period prior to the index year (i.e. for the 5-year epoch of a case diagnosed in 2016, we compiled estimated exposures for residences held from 2010 to 2015). We chose to use the median value rather than the cumulative value to avoid introducing bias due to missing residences for some years. The exposure was calculated as the median across the entire epoch. Because of changes towards stricter regulations on lead in more recent years, we felt that this model would be more conservative, while a lagged model might overestimate exposure.
Statistical analysis
The median residential history based estimate of exposure to lead over the period prior to diagnosis was binned into categories based on the quartile distribution of the contaminant in the controls. Chi-square tests assessed the univariate difference in proportion of cases and controls comparing <50th to the 50th–75th and >75th percentiles, followed by multivariable logistic regression analysis adjusted for age and sex.
3. Results
Table 1 shows that the age- and sex-distribution of the n = 26,199 ALS cases we identified is similar to that of the controls sampled from the SYMPHONY network. The majority of the cases and controls (63%) were 55–75 years of age and approximately 57% were male.
Table 1.
SYMPHONY population characteristics.
| Controls |
ALS patients |
p-Value | ||
|---|---|---|---|---|
| N = 78,597 (%) | N = 26,199 (%) | |||
|
| ||||
| Age | <45 | 5823 (7.4) | 1941 (7.4) | 1 |
| 45–55 | 12,816 (16.3) | 4272 (16.3) | ||
| 55–65 | 24,561 (31.2) | 8187 (31.2) | ||
| 65–75 | 25,269 (32.2) | 8423 (32.2) | ||
| 75 + | 10,128 (12.9) | 3376 (12.9) | ||
| Sex | Female | 33,264 (42.3) | 11,286 (43.1) | 0.033 |
| Male | 45,328 (57.7) | 14,912 (56.9) | ||
Of the 268 airborne contaminants assessed from the 2008 NEI, 49 met the FDR cutoff of <0.2 from our nationwide SYMPHONY database ‘discovery cohort’. Fig. 1 shows a ‘volcano plot’ of those contaminants, showing those with the lowest p-values and largest fold change indicating a positive ALS risk association in the top right.
Fig. 1.

Airborne contaminants associated with ALS risk. Volcano plot depicts airborne contaminants from the 2008 NEI database that met the FDR cutoff <0.2 for association with ALS risk in the ‘discovery cohort’. The y-axis shows the negative logarithm of the p-value, such that the lowest p-values are at the top. The x-axis shows the logarithm of the fold change (Log(FC)), with contaminants associated with decreased ALS risk on the left, and those that increase risk on the right. Contaminants that are most statistically significant (red) and have the largest fold change representing increased ALS risk fall in the top, right-hand side of the figure, including Lead and the PCBs (Hep = Heptachlorobiphenyl; X2_PCB = 2_Chlorobiphenyl_PCB_1; Hex = Hexachlorobiphenyl; Pen = Pentachlorobiphenyl; X244 = 2,4,4_Trichlorobiphenyl_PCB_28).
We then assessed the relationship between each of these 49 airborne contaminants and risk of ALS using the independent ‘validation cohort’. Supplemental Table 1 lists the subset of those contaminants selected from the ‘discovery cohort’ that also met our FDR <0.05 cutoff in the ‘validation cohort’. Those associated with increased risk of ALS were of most interest, namely airborne lead and five highly correlated polychlorinated biphenyls (PCBs) (Table 2).
Table 2.
Airborne contaminants associated with increased ALS risk in both the discovery and validation cohorts.
| Contaminant | Discovery phase |
Validation phase |
||
|---|---|---|---|---|
| p-Value | OR | (95% CI) | FDR | |
|
| ||||
| Lead | 0.0028 | 1.02 | 1.01–1.03 | 0.00077 |
| Heptachlorobiphenyl | 0.0039 | 1.01 | 1.01–1.02 | 3.60E-05 |
| 2, Chlorobiphenyl, PCB 1 | 2.00E-04 | 1.01 | 1.01–1.02 | 3.60E-05 |
| Hexachlorobiphenyl | 0.0039 | 1.01 | 1.01–1.02 | 3.60E-05 |
| Pentachlorobiphenyl | 0.0039 | 1.01 | 1.01–1.02 | 3.60E-05 |
| 2,4,4 Trichlorobiphenyl, PCB 28 | 2.00E-04 | 1.01 | 1.01–1.02 | 3.60E-05 |
We used an independent NH/VT and OH based cohorts to perform a confirmatory analysis of the lead finding using detailed residential history information. Estimates of exposure to lead in 5, 10, and 15-year epochs prior to diagnosis in each region support statistically significant increased risk of ALS associated with lead exposure (e.g. in Table 3 for a 10-year exposure history >75th percentile vs. <50th percentile: NH/VT OR 2.03, 95% CI 1.46–2.80; OH OR 1.60 95% CI 1.28–1.98).
Table 3.
Confirmatory analysis of a residential history of lead exposure and ALS risk.
| New Hampshire/Vermont |
Controls |
Cases |
Univariate |
Multivariablea |
||
|---|---|---|---|---|---|---|
| Lead (tons) | n = 762 (%) | n = 553 (%) | P-value | OR | (95% CI) | |
|
| ||||||
| 5-year | ≤1.37 | 333 (50.1) | 144 (41.1) | 0.001 | 1.0 (ref) | |
| 1.37–26.1 | 166 (25.0) | 125 (35.7) | 1.79 | 1.32–2.43 | ||
| >26.1 | 166 (25.0) | 81 (23.1) | 1.11 | 0.80–1.55 | ||
| 10-year | <1.37 | 333 (50.1) | 109 (31.1) | <0.001 | 1.0 (ref) | |
| 1.37–20.1 | 166 (25.0) | 129 (36.9) | 2.42 | 1.76–3.33 | ||
| >20.1 | 166 (25.0) | 112 (32.0) | 2.03 | 1.46–2.80 | ||
| 15-year | ≤ 1.81 | 333 (50.1) | 126 (36.0) | <0.001 | 1.0 (ref) | |
| 1.81–24.9 | 166 (25.0) | 114 (32.6) | 1.83 | 1.34–2.52 | ||
| >24.9 | 166 (25.0) | 110 (31.4) | 1.73 | 1.26–2.38 | ||
| Ohio | Lead (tons) | Controls |
Cases |
Univariate |
Multivariablea |
|
| n = 1336 (%) | n = 799 (%) | P-value | OR | (95% CI) | ||
|
| ||||||
| 5-year | ≤ 14.7 | 642 (50.0) | 487 (69.7) | <0.001 | 1.0 (ref) | |
| 14.7–50.8 | 321 (25.0) | 115 (16.5) | 0.48 | 0.37–0.61 | ||
| >50.8 | 321 (25.0) | 97 (13.9) | 0.39 | 0.30–0.51 | ||
| 10-year | ≤ 18.5 | 642 (50.0) | 300 (42.9) | <0.001 | 1.0 (ref) | |
| 18.5–61.1 | 321 (25.0) | 158 (22.6) | 1.05 | 0.83–1.33 | ||
| >61.1 | 321 (25.0) | 241 (34.5) | 1.60 | 1.28–1.98 | ||
| 15-year | ≤ 32.3 | 642 (50.0) | 348 (49.8) | 0.458 | 1.0 (ref) | |
| 32.3–87.3 | 321 (25.0) | 161 (23.0) | 0.94 | 0.74–1.18 | ||
| >87.3 | 321 (25.0) | 190 (27.2) | 1.07 | 0.86–1.34 | ||
Adjusted for age and sex.
Stratified analysis shows similar results for residential history of lead exposure in males and females (Supplemental Table 3). In addition, to check that our results were not confounded by socioeconomic status or race, we applied the same address-based geospatial exposure assessment methods to our small questionnaire-based case-control study (Andrew et al., 2021). The elevated risks for the 3rd quartile of airborne lead in that study persist in a model adjusted for family income, race, age and sex (Supplemental Table 4).
Fig. 2a shows individual sites reporting airborne lead emissions, while Fig. 2b depicts the variation in the median levels of airborne lead emissions calculated for each of the zip3 polygons nationwide. According to the NEI database, the highest lead emissions levels were from “Mobile – Aircraft”, and these levels of emissions remained fairly constant over time (nationally 553 tons in 2008, 467 tons in 2017) (Fig. 3) (USEPA, 2008).
Fig.2.
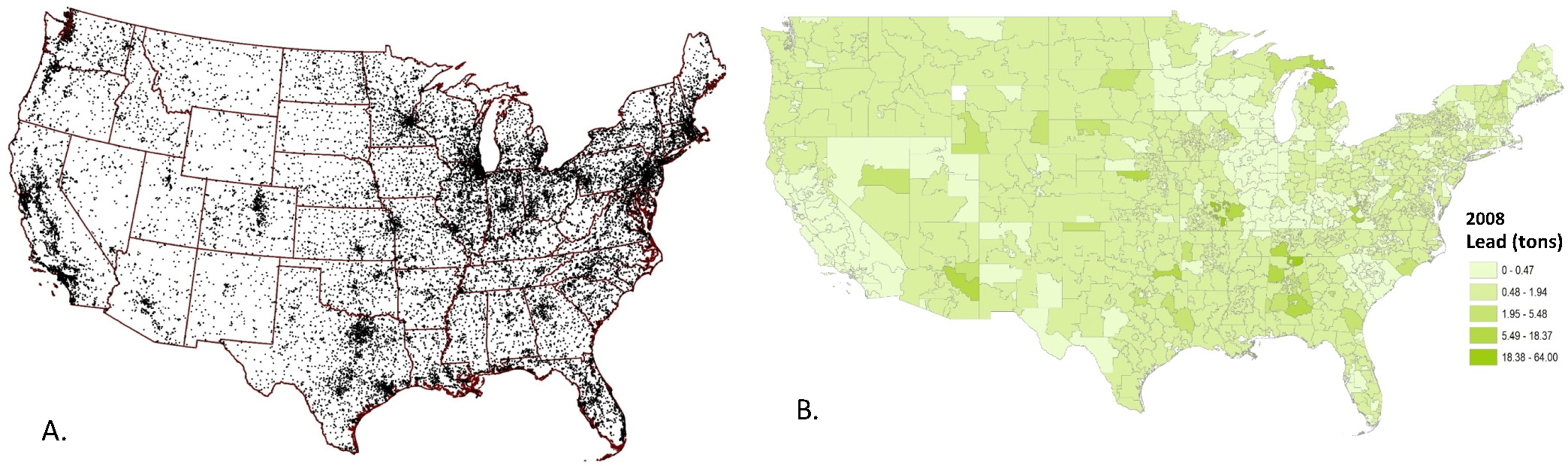
Geospatial distribution of lead emissions. Fig. 2a shows point sources of airborne lead emissions in the NEI 2008 database. Fig. 2b shows these data summarized at the polygon level, using the median level of lead (tons) released in 2008 calculated for each zip3 region.
Fig.3.
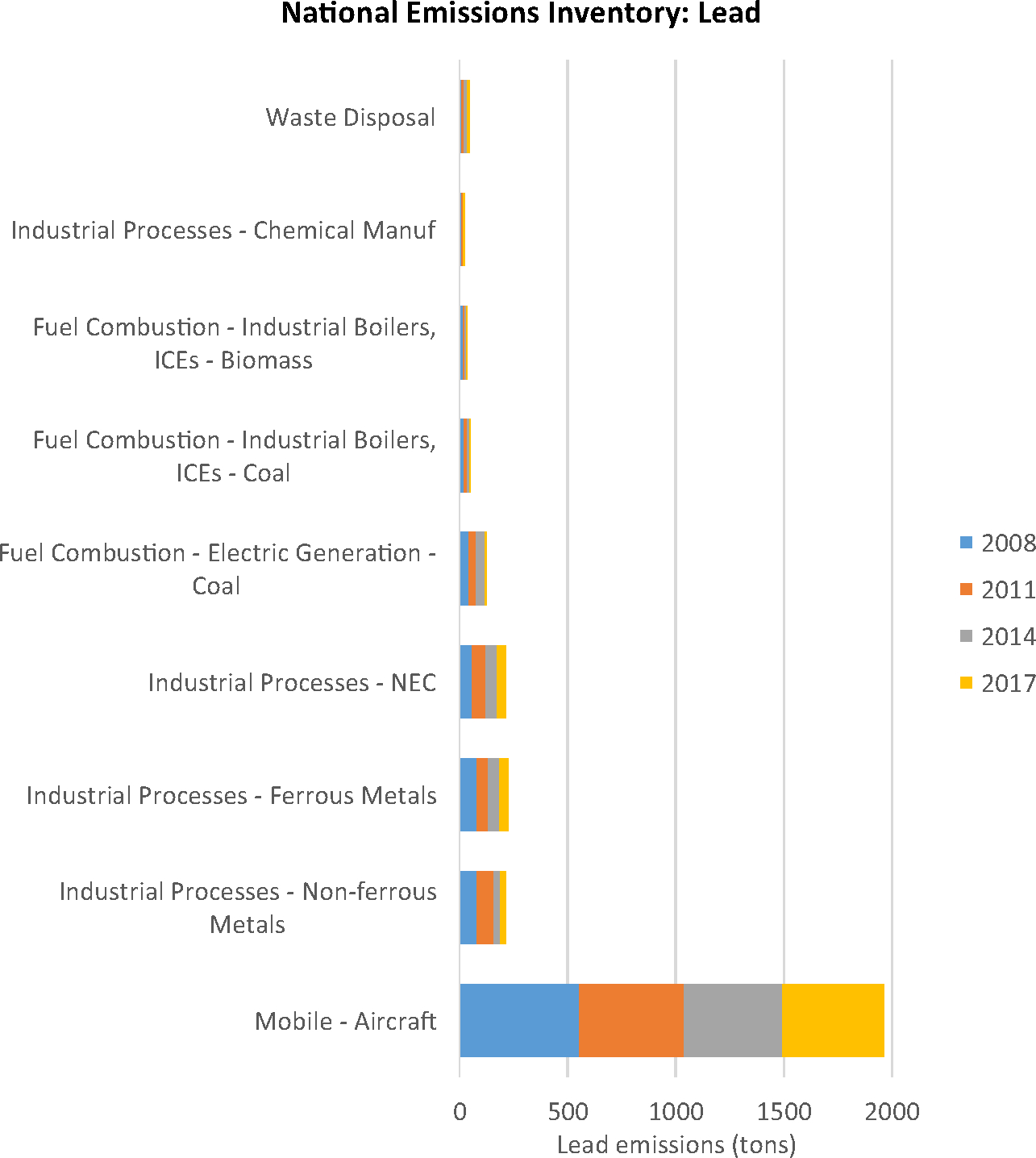
National sources of lead emissions over time. The graph depicts lead emissions levels (tons) by industry reported to NEI in the available years: 2008, 2011, 2014, and 2017.
We found the PCB emissions reported by the NEI were almost exclusively from the Minnesota region (e.g. heptachlorobiphenyl, Fig. 4). The highest PCB emissions were specifically attributed to power plants performing “electricity generation from combustion of biomass” (nationally 0.0009 lbs in 2008, 0.0003 lbs in 2017) (Fig. 3) (USEPA, 2008). Similar amounts are produced by “industrial boilers” (nationally 0.0007 lbs in 2008, 0.0008 lbs in 2017) (Fig. 3) (USEPA, 2008). The relationship between PCB emissions and ALS also appears consistent over time, in a sensitivity analysis, the odds ratios calculated using NEI 2008 airborne emissions data are similar to those calculated for the same PCBs using NEI 2014 data. For example, heptachlorobiphenyl emissions in 2014 were associated with increased ALS risk in the SYMPHONY ‘Discovery cohort’ (p-value = 0.00022), and in the ‘Validation cohort’ (p-value = 1.42E-06, FDR = 4.72E-05). The other PCBs show similar concordance between 2008 and 2014 emissions (Supplemental Tables 1b, 1c).
Fig.4.
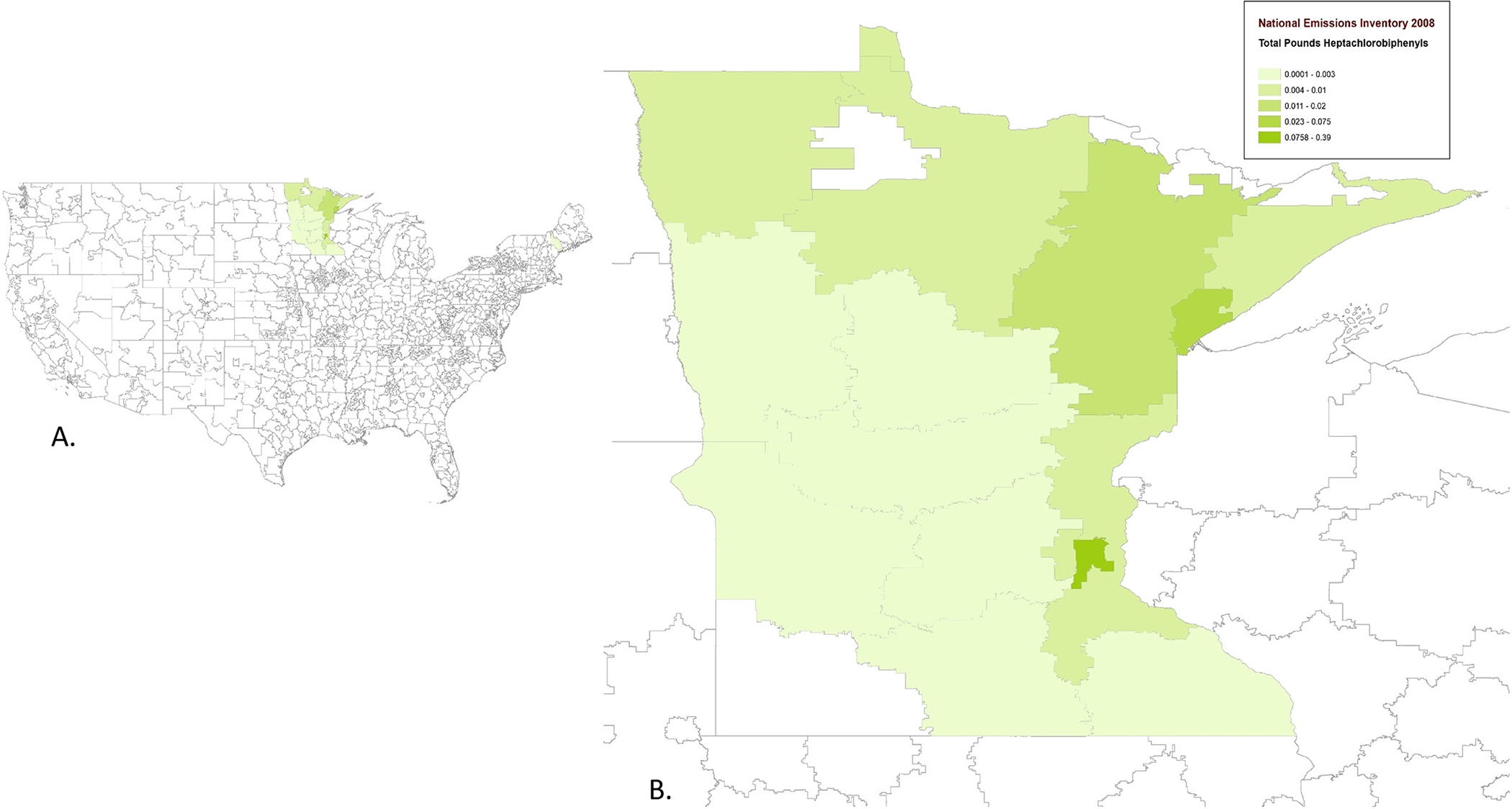
Geospatial distribution of PCB emissions. Fig. 4a depicts nationwide zip3 polygon median emission levels of the PCB heptochlorobiphenyl (lbs) for 2008. Fig. 4b shows a magnified depiction of the heptochlorobiphenyl (lbs) for 2008 in the Minnesota area.
We then evaluated the ALS risks following exposure to combinations of contaminants in the SYMPHONY dataset using a pairwise interaction-effects model by testing all paired combinations of the 49 contaminants that met the FDR cutoff of <0.2. Supplemental Table 2 shows the results for the contaminant pairs with a positive interaction coefficient that met our FDR <0.05 threshold in both the ‘discovery’ and ‘validation’ cohorts and are ranked by the largest contaminant 1 ∗ contaminant 2 interaction coefficient in the ‘validation’ cohort. We tried to identify combinations of contaminants that may have greater-than-additive (e.g. synergistic) effects. We identified a single potentially interacting pair (Benzo(j)fluoranthene and Sulfur_Dioxide, FDR = 0.022). A related polycyclic aromatic hydrocarbon, benzo(a)pyrene, was associated with increased risk in the ‘discovery’, but not the ‘validation’ phase of our main effects analysis (Supplemental Table 1).
4. Discussion
Sporadic ALS etiology remains largely unexplained and airborne exposures to neurotoxicants may be a risk factor for disease. The objective of our study was to use the National Emissions Inventory (NEI) to estimate potential exposure at residential locations for a large, nationally distributed and de-identified healthcare claims dataset. Uniquely, across two large sub-cohorts, we observed consistent positive associations for the airborne emissions of lead, and of PCBs. Detailed residential history data further confirmed the lead association.
Lead is one of the few exposures with ‘convincing evidence’ of a causal link to ALS based on biomarkers, questionnaires, and occupational exposures, and our geospatial study of airborne lead adds support to that evidence (Belbasis et al., 2016;Wang et al., 2017). Our geospatial approach has limitations and misclassification in our estimation of exposure may explain the non-linear dose-response relationship and the attenuated association we observe for 15 years, compared to the 10-year epoch. Nonetheless, biomarkers of exposure provide substantiating evidence of the lead exposure to ALS link. Doubling blood lead levels was associated with increased risk of ALS in U.S. veterans (1.9-fold increase) (95% CI 1.3–2.7) (Fang et al., 2010). Analysis of cerebrospinal fluid (CSF) also shows elevated lead levels in ALS patients, compared to controls (Roos et al., 2013; Vinceti et al., 2017a, 2017b). Likewise, in a New England cohort, doubling the lead levels detected in the tibia bone using X-ray fluorescence was associated with a 2.3–3.6 fold increase in ALS risk (Kamel et al., 2005). In our prior questionnaire-based case-control study, participating in hobbies involving lead was also associated with ALS risk (adjusted OR 2.92 95% CI 1.45–5.41), particularly for exposures occurring 20 or more years prior to diagnosis, when use of lead was more common (Andrew et al., 2021). In Denmark, occupational lead exposure 10-years prior to diagnosis also increased ALS risk (OR 1.33, 95% CI 1.03–1.72) (Dickerson et al., 2019), and similar links between occupational metal exposure and ALS are observed in Australia and Italy (Filippini et al., 2020;Pamphlett, 2012).
Our study supports evidence that airborne lead is an ALS risk factor. An Australian study linked legacy petrol lead air emissions to motor neuron disease death rates both temporally and spatially, with decreasing disease rates correlating with phasing lead out of automobile fuel (Zahran et al., 2017). Tetraethyl lead is added to fuel improve control over the ignition of the fuel in the combustion chamber, such that the pistons in the engine are not damaged. Due to environmental and health concerns, bans on adding tetraethyl lead to automobile gas in the U.S. were enforced by 1986 (Price, 2021). Surprisingly, piston engine aircraft in the U.S. still run on leaded fuel. These planes are typically used for business purposes, typically flying <6 passengers at low altitudes out of small general aviation airports (Miranda and Farivar, 2021;NBAA, 2021). Most of the aviation gasoline used in the U.S. piston engine aircraft is 100 motor octane number, ‘AVGAS 100LL’, which contains 0.027–0.053% tetraethyl lead (NBAA, 2021; Shell, 2020). In contrast, fuel for modern commercial jet aircraft does not contain lead. A study of children found elevated blood lead levels near airports that use leaded AVGAS compared to other children, with the lead levels monotonically decreasing out to 1 km (Miranda et al., 2011). The Federal Aviation Administration is sponsoring efforts to develop a safe unleaded fuel for the ~167,000 piston engine aircraft operating in the U.S. (Miranda and Farivar, 2021; Price, 2021). A 2021 congressionally mandated study by the “Committee on Lead Emissions From Piston-Powered General Aviation Aircraft” cites a need for concerted efforts across multiple government agencies, pilots, airport managers, fuel suppliers, and aircraft manufacturers to address the need to reduce lead use and emissions (Robinson, 2021).
We also identified increased ALS risk associated with airborne polychlorinated biphenyl (PCB) emissions. PCBs are halogenated hydrocarbons that were used in electrical equipment because of their insulating capacity. The U.S. halted the manufacture of PCBs in 1977 because of emerging data on harmful health effects (ATSDR, 2014). PCBs remain persistent in locations where industrial wastes and consumer products were improperly handled, because they do not break down easily. Capacitor plants and other sources release PCBs into air. They are deposited as residue at measurable levels in the surrounding soil (Chavhan et al., 2012). As we observed in the NEI dataset, incinerators release PCBs into the air, as PCBs may be formed and released when burning a source of carbon together with chlorine, which can occur during waste incineration (ATSDR, 2014).
The link between PCBs and ALS is supported by a tragic accident and by occupational studies. In 1979, a group of Taiwanese accidentally ingested rice oil contaminated with high levels of PCBs. This exposure induced dose related slowing of their motor neuron conduction velocities, suggesting that PCBs have neurotoxic potential in adults, however the mechanism remains unclear (Chen et al., 1985). An occupational cohort of ~17,000 electrical capacitor plant workers from the 1940s–1970s had serum PCB levels 10 times those of community controls, and significant excess ALS mortality for the women (standardized mortality ratio (SMR) 2.6 95% CI 1.08–4.15, based on 10 deaths) (Prince et al., 2006; Steenland et al., 2006). Similarly, an analysis of long-term (3+ month) employees across capacitor plants in Massachusetts, Indiana, and New York found an increased ALS standardized mortality rate (SMR) 1.90 95% CI 1.09–3.09 for women, but not among men SMR 0.44 95% CI 0.12–1.12 (Ruder et al., 2014). The basis for the noted sex difference could be attributed to work assignment differences, or to differences in response to the endocrine disrupting effects of PCBs (Ruder et al., 2014). A study in Michigan (n = 101 cases and 110 controls) found significantly increased odds of ALS for blood levels of PCB-175 (OR 1.81) and PCB-202 (OR 2.11) (Su et al., 2016). A study of cerebrospinal fluid from n = 38 cases and n = 38 controls in Italy (1994–2013) found a non-significant increased risk of ALS in older males associated with higher PCB-28 levels (Vinceti et al., 2017a, 2017b), which is one of the PCBs associated with ALS in our SYMPHONY analysis (2,4,4 Trichlorobiphenyl PCB 28). Thus, the literature supports a link between PCB exposure and ALS risk.
The mechanisms underlying the neurotoxicity of PCBs and lead are still not clear. Animal studies demonstrate the transport of lead ions across the blood-brain barrier (Neal and Guilarte, 2013). In prior work, we injected lead into the triceps surae muscle of a rat and observed retrograde transport into the spinal cord within 9 days via the sciatic nerve (Baruah et al., 1981). Lead induces free radical formation, peroxidative damage to cell walls, accumulation of insoluble TDP-43, and neuronal cell death (Ash et al., 2019; Basha et al., 2005). PCBs also induce formation of reactive oxygen species in neurons and induce neuronal cell death (Fonnum et al., 2006). A recent study found that lead also disrupts TDP-43 in cultured neurons, forming nuclear granules and accumulation of insoluble TDP-43 in the cortex of exposed mice (Ash et al., 2019). PCBs led to a 3-fold accumulation of the ALS-associated protein TDP-43 in human motor neuron-derived induced pluripotent stem cells and in mouse brains (Ash et al., 2017).
We additionally observed some negative relationships between exposures and ALS risk, such as for hexane, however we did not anticipate this effect a priori and it could be a chance finding. The polycyclic aromatic hydrocarbon (PAH) benzo(j)fluoroanthrene was only emitted in a few (n = 12) of the zip3 regions, thus the interaction observed with sulfur dioxide is based on small numbers. The unstable behavior of the statistical model, and the lack of neurotoxicity literature on benzo(j)fluoroanthrene suggest caution in the interpretation of this finding. We did not see reproducible associations for a related polycyclic aromatic hydrocarbon, benzo.a.pyrene, which led to locomotor impairment and development of neurodegenerative phenotypes affecting the dopaminergic system in zebrafish (Das et al., 2020).
Advantages of our study include a large de-identified database with over 26,000 ALS patients occurring in the nationally distributed SYMPHONY healthcare network-based population. The higher proportion of males among cases was expected based on ALS literature (McCombe and Henderson, 2010). The primary analysis using the SYMPHONY data and included independent ‘discovery’ and ‘validation’ cohorts and the geospatial data was limited to a single zip3 location at diagnosis, we lack the ability to assess residential history more accurately. Thus, we performed a sensitivity analysis for lead using detailed individual-level spatiotemporal migration history datasets we had in three states where we had run case-control studies, but we did not have access to these data in other states. We did reconfirm the airborne lead finding using this detailed exposure assessment based on residential history from localized independent cohorts, however we could not assess PCBs using residential history as they were not present in the NH/VT or OH areas. Additional sensitivity analyses showed that the ALS association for residential history of lead persisted in a model adjusted for family income, race, age and sex and is thus unlikely due to confounding by these factors. The NEI airborne contaminant data are collected in intervals every few years. While continuous temporal variation data are not available, we observed consistency in the levels of the ALS-related contaminants over time. We based our exposure estimates on the zip3 of the residence, so exposures for patients spending substantial time outside that area would be misclassified, but this would tend to bias findings towards the null.
In conclusion, we identified airborne contaminants in the area of residence associated with risk of ALS in a large healthcare claims network. Our study particularly implicates airborne lead exposure as an ALS risk factor and highlights an urgency for transition to unleaded fuel in piston engine aircraft, as this appears to be the major remaining source of airborne lead emissions in the U.S. The observed link between ALS risk and PCB emissions highlights the potential harms from release of PCBs during incineration, suggesting that practices could be re-evaluated for exposure mitigation.
Supplementary Material
HIGHLIGHTS.
We assessed risk factors for ALS in a large de-identified medical claims database with 26,000 nationally distributed ALS patients.
Small aircraft were the largest source of airborne lead, which was geospatially associated with increased ALS risk.
The coherence of results implicate lead as a risk factor and suggest a need for public health intervention to reduce exposure.
PCB emissions came from power plants burning biomass, and from industrial boilers.
Acknowledgments
This nationwide analysis was inspired by our regional work with CDC/ATSDR grant R01TS000288.
Funding
Funded by MTPA. AH is an employee of MTPA.
Abbreviations:
- OR
odds ratio
- CI
confidence interval
- ALS
amyotrophic lateral sclerosis
- NEI
National Emissions Inventory
- FDR
False Discovery Rate
- PCBs
polychlorinated biphenyls
- avgas
aviation gasoline
- SMR
standardized mortality ratio
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Angeline Andrew: Conceptualization, Writing - original draft; Jie Zhou: Formal analysis; Jiang Gui: Supervision; Antoinette Harrison: Conceptualization; Xun Shi: Conceptualization; Meifang Li: Data curation; Bart Guetti: Data curation; Ramaa Nathan: Supervision; Maeve Tischbein: Project administration, Erik Pioro: Data collection; Elijah Stommel: Writing - review & editing, Walter Bradley: Conceptualization, Funding acquisition.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2022.153096.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of Mitsubishi Tanabe Pharma America (MTPA).
References
- Andrew AS, Bradley WG, Peipert D, Butt T, Amoako K, Pioro EP, Tandan R, Novak J, et al. , 2021. Risk factors for amyotrophic lateral sclerosis: a regional United States case-control study. Muscle Nerve 63, 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash PEA, Stanford EA, Al Abdulatif A, Ramirez-Cardenas A, Ballance HI, Boudeau S, Jeh A, Murithi JM, et al. , 2017. Dioxins and related environmental contaminants increase TDP-43 levels. Mol. Neurodegener. 12, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash PEA, Dhawan U, Boudeau S, Lei S, Carlomagno Y, Knobel M, Al Mohanna LFA, Boomhower SR, et al. , 2019. Heavy metal neurotoxicants induce ALS-linked TDP-43 pathology. Toxicol. Sci. 167, 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR, 2014. In: Registry AfTSaD (Ed.), ToxFAQs for Polychlorinated Biphenyls (PCBs). [Google Scholar]
- Baruah JK, Rasool CG, Bradley WG, Munsat TL, 1981. Retrograde axonal transport of lead in rat sciatic nerve. Neurology 31, 612–616. [DOI] [PubMed] [Google Scholar]
- Basha MR, Murali M, Siddiqi HK, Ghosal K, Siddiqi OK, Lashuel HA, Ge YW, Lahiri DK, et al. , 2005. Lead (Pb) exposure and its effect on APP proteolysis and Abeta aggregation. FASEB J. 19, 2083–2084. [DOI] [PubMed] [Google Scholar]
- Belbasis L, Bellou V, Evangelou E, 2016. Environmental risk factors and amyotrophic lateral sclerosis: an umbrella review and critical assessment of current evidence from systematic reviews and meta-analyses of observational studies. Neuroepidemiology 46, 96–105. [DOI] [PubMed] [Google Scholar]
- Chavhan C, Sheikh J, Algiwale T, Thokchom B, Thacker N, 2012. Releases of dioxin-like PCBs in water, soil and residue produced from high thermal processes and waste incinerators. Bull. Environ. Contam. Toxicol. 89, 537–541. [DOI] [PubMed] [Google Scholar]
- Chen RC, Tang SY, Miyata H, Kashimoto T, Chang YC, Chang KJ, Tung TC, 1985. Polychlorinated biphenyl poisoning: correlation of sensory and motor nerve conduction, neurologic symptoms, and blood levels of polychlorinated biphenyls, quaterphenyls, and dibenzofurans. Environ. Res. 37, 340–348. [DOI] [PubMed] [Google Scholar]
- Das SK, Aparna S, Patri M, 2020. Chronic waterborne exposure to benzo[a]pyrene induces locomotor dysfunction and development of neurodegenerative phenotypes in zebrafish. Neurosci. Lett. 716, 134646. [DOI] [PubMed] [Google Scholar]
- de Jongh AD, van Eijk RPA, Peters SM, van Es MA, Horemans AMC, van der Kooi AJ, Voermans NC, Vermeulen RCH, et al. , 2021. Incidence, prevalence and geographical clustering of motor neuron disease in the Netherlands. Neurology 96 (8), e1227–e1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson AS, Hansen J, Specht AJ, Gredal O, Weisskopf MG, 2019. Population-based study of amyotrophic lateral sclerosis and occupational lead exposure in Denmark. Occup. Environ. Med. 76, 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, Kwee LC, Allen KD, Umbach DM, Ye W, Watson M, Keller J, Oddone EZ, et al. , 2010. Association between blood lead and the risk of amyotrophic lateral sclerosis. Am. J. Epidemiol. 171, 1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faught E, Laliberte F, Wang Z, Barghout V, Haider B, Lejeune D, Germain G, Choi J, et al. , 2017. Health care resource utilization before and after perampanel initiation among patients with epilepsy in the United States. Epilepsia 58, 1742–1748. [DOI] [PubMed] [Google Scholar]
- Filippini T, Tesauro M, Fiore M, Malagoli C, Consonni M, Violi F, Iacuzio L, Arcolin E, et al. , 2020. Environmental and occupational risk factors of amyotrophic lateral sclerosis: a population-based case-control study. Int. J. Environ. Res. Publ. Health 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini T, Mandrioli J, Malagoli C, Costanzini S, Cherubini A, Maffeis G, Vinceti M, 2021. Risk of amyotrophic lateral sclerosis and exposure to particulate matter from vehicular traffic: a case-control study. Int. J. Environ. Res. Publ. Health 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F, Mariussen E, Reistad T, 2006. Molecular mechanisms involved in the toxic effects of polychlorinated biphenyls (PCBs) and brominated flame retardants (BFRs). J. Toxicol. Environ. Health A 69, 21–35. [DOI] [PubMed] [Google Scholar]
- Ho D, Imai K, King G, Stuart E, 2007. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit. Anal. 15, 199–236. [Google Scholar]
- Kamel F, Umbach DM, Hu H, Munsat TL, Shefner JM, Taylor JA, Sandler DP, 2005. Lead exposure as a risk factor for amyotrophic lateral sclerosis. Neurodegener. Dis. 2, 195–201. [DOI] [PubMed] [Google Scholar]
- Malek AM, Barchowsky A, Bowser R, Heiman-Patterson T, Lacomis D, Rana S, Ada Y, Talbott EO, 2015. Exposure to hazardous air pollutants and the risk of amyotrophic lateral sclerosis. Environ. Pollut. 197, 181–186. 10.1016/j.envpol.2014.1012.1010 (Epub 2014 Dec 1023). [DOI] [PubMed] [Google Scholar]
- Mathis S, Goizet C, Soulages A, Vallat JM, Masson GL, 2019. Genetics of amyotrophic lateral sclerosis: a review. J. Neurol. Sci. 399, 217–226. [DOI] [PubMed] [Google Scholar]
- McCombe PA, Henderson RD, 2010. Effects of gender in amyotrophic lateral sclerosis. Gend. Med. 7, 557–570. [DOI] [PubMed] [Google Scholar]
- Miller C, Apple S, Paige JS, Grabowsky T, Shukla O, Agnese W, Merrill C, 2021. Current and future projections of amyotrophic lateral sclerosis in the United States using administrative claims data. Neuroepidemiology 55, 275–285. [DOI] [PubMed] [Google Scholar]
- Miranda L, Farivar C, April 22, 2021. Leaded gas was phased out 25 years ago. Why are these planes still using toxic fuel? NBC Business News. https://www.nbcnews.com/business/business-news/leaded-gas-was-phased-out-25-years-ago-why-are-n1264970. [Google Scholar]
- Miranda ML, Anthopolos R, Hastings D, 2011. A geospatial analysis of the effects of aviation gasoline on childhood blood lead levels. Environ. Health Perspect. 119, 1513–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JD, Gatrell AC, Al-Hamad A, Davies RB, Batterby G, 1998. Geographical epidemiology of residence of patients with motor neuron disease in Lancashire and south Cumbria. J. Neurol. Neurosurg. Psychiatry 65, 842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NBAA, National Business Aviation Association, 2021. Piston Engine Aircraft. NBAA; Washington, DC. [Google Scholar]
- Neal AP, Guilarte TR, 2013. Mechanisms of lead and manganese neurotoxicity. Toxicol. Res. (Camb.) 2, 99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan CW, White MC, Thurman D, Wong LY, 2005. Temporal and geographic variation in United States motor neuron disease mortality, 1969–1998. Neurology 64, 1215–1221. [DOI] [PubMed] [Google Scholar]
- Nunez Y, Boehme AK, Weisskopf MG, Re DB, Navas-Acien A, van Donkelaar A, Martin RV, Kioumourtzoglou MA, 2021. Fine particle exposure and clinical aggravation in neurodegenerative diseases in New York state. Environ. Health Perspect. 129, 27003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira M, Padrao A, Ramalho A, Lobo M, Teodoro AC, Goncalves H, Freitas A, 2020. Geospatial analysis of environmental atmospheric risk factors in neurodegenerative diseases: a systematic review. Int. J. Environ. Res. Publ. Health 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamphlett R, 2012. Exposure to environmental toxins and the risk of sporadic motor neuron disease: an expanded Australian case-control study. Eur. J. Neurol. 19, 1343–1348. [DOI] [PubMed] [Google Scholar]
- Povedano M, Saez M, Martinez-Matos JA, Barcelo MA, 2018. Spatial assessment of the association between long-term exposure to environmental factors and the occurrence of amyotrophic lateral sclerosis in Catalonia, Spain: a population-based nested case-control study. Neuroepidemiology 51, 33–49. [DOI] [PubMed] [Google Scholar]
- Price HJ, 2021. Fact Sheet – Leaded Aviation Fuel and the Environment. U.S. Department of Transportation, Federal Aviation Administration, Washington, DC. [Google Scholar]
- Prince MM, Hein MJ, Ruder AM, Waters MA, Laber PA, Whelan EA, 2006. Update: cohort mortality study of workers highly exposed to polychlorinated biphenyls (PCBs) during the manufacture of electrical capacitors, 1940–1998. Environ. Health 5, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A, 2021. Eliminating Lead Emissions From Small Aircraft Will Require Concerted Efforts Across the Aviation Sector, Says New Report. National Academy of Sciences. [Google Scholar]
- Roos PM, Vesterberg O, Syversen T, Flaten TP, Nordberg M, 2013. Metal concentrations in cerebrospinal fluid and blood plasma from patients with amyotrophic lateral sclerosis. Biol. Trace Elem. Res. 151, 159–170. [DOI] [PubMed] [Google Scholar]
- Ruder AM, Hein MJ, Hopf NB, Waters MA, 2014. Mortality among 24,865 workers exposed to polychlorinated biphenyls (PCBs) in three electrical capacitor manufacturing plants: a ten-year update. Int. J. Hyg. Environ. Health 217, 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabel CE, Boyle PJ, Loytonen M, Gatrell AC, Jokelainen M, Flowerdew R, Maasilta P, 2003. Spatial clustering of amyotrophic lateral sclerosis in Finland at place of birth and place of death. Am. J. Epidemiol. 157, 898–905. [DOI] [PubMed] [Google Scholar]
- Santurtun A, Villar A, Delgado-Alvarado M, Riancho J, 2016. Trends inmotor neuron disease: association with latitude and air lead levels in Spain. Neurol. Sci. 37, 1271–1275. [DOI] [PubMed] [Google Scholar]
- Scott KM, Abhinav K, Wijesekera L, Ganesalingam J, Goldstein LH, Janssen A, Dougherty A, Willey E, et al. , 2010. The association between ALS and population density: a population based study. Amyotroph. Lateral Scler. 11, 435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelen M, Toro Campos RA, Veldink JH, Visser AE, Hoek G, Brunekreef B, van der Kooi AJ, de Visser M, et al. , 2017. Long-term air pollution exposure and amyotrophic lateral sclerosis in Netherlands: a population-based case-control study. Environ. Health Perspect. 125, 097023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shell, 2020. Safety Data Sheet: AVGAS 100LL. Houston, TX. [Google Scholar]
- Stallones L, Kasarskis EJ, Stipanowich C, Snider G, 1989. Secular trends in mortality rates from motor neuron disease in Kentucky 1964–1984. Neuroepidemiology 8, 68–78. [DOI] [PubMed] [Google Scholar]
- Steenland K, Hein MJ, Cassinelli RT., Prince MM., Nilsen NB., Whelan EA., Waters MA., Ruder AM., et al. , 2006. Polychlorinated biphenyls and neurodegenerative disease mortality in an occupational cohort. Epidemiology 17, 8–13. [DOI] [PubMed] [Google Scholar]
- Su FC, Goutman SA, Chernyak S, Mukherjee B, Callaghan BC, Batterman S, Feldman EL, 2016. Association of environmental toxins with amyotrophic lateral sclerosis. JAMA Neurol. 73, 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli R, Binazzi A, Altavista P, Belli S, Comba P, Mastrantonio M, Vanacore N, 2007. Geographic distribution of amyotrophic lateral sclerosis through motor neuron disease mortality data. Eur. J. Epidemiol. 22, 781–790. [DOI] [PubMed] [Google Scholar]
- USEPA, 2008. National Emissions Inventory Data. [Google Scholar]
- USEPA, 2013. In: U.S. Environmental Protection Agency OoAQPaS, Air Quality Assessment Division, Emissions Inventory and Analysis Group (Eds.), 2008 National Emissions Inventory, Version 3, Technical Support Document. USEPA, Research; Triangle Park, NC. [Google Scholar]
- Vieira VM, Hansen J, Gredal O, Weisskopf MG, 2018. Spatial analyses of ALS incidence in Denmark over three decades. Amyotroph Lateral Scler Frontotemporal Degener 19, 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M, Filippini T, Mandrioli J, Violi F, Bargellini A, Weuve J, Fini N, Grill P, et al. , 2017a. Lead, cadmium and mercury in cerebrospinal fluid and risk of amyotrophic lateral sclerosis: a case-control study. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 43, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M, Violi F, Tzatzarakis M, Mandrioli J, Malagoli C, Hatch EE, Fini N, Fasano A, et al. , 2017b. Pesticides, polychlorinated biphenyls and polycyclic aromatic hydrocarbons in cerebrospinal fluid of amyotrophic lateral sclerosis patients: a case-control study. Environ. Res. 155, 261–267. [DOI] [PubMed] [Google Scholar]
- Visser AE, D’Ovidio F, Peters S, Vermeulen RC, Beghi E, Chio A, Veldink JH, Logroscino G, et al. , 2019. Multicentre, population-based, case-control study of particulates, combustion products and amyotrophic lateral sclerosis risk. J. Neurol. Neurosurg. Psychiatry 90, 854–860. [DOI] [PubMed] [Google Scholar]
- Wang MD, Little J, Gomes J, Cashman NR, Krewski D, 2017. Identification of risk factors associated with onset and progression of amyotrophic lateral sclerosis using systematic review and meta-analysis. Neurotoxicology 61, 101–130. [DOI] [PubMed] [Google Scholar]
- Zahran S, Laidlaw MA, Rowe DB, Ball AS, Mielke HW, 2017. Motor neuron disease mortality and lifetime petrol lead exposure: evidence from national age-specific and state-level age-standardized death rates in Australia. Environ. Res. 153, 181–190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


