Abstract
Background:
The mouse hind limb model represents a powerful research tool in vascularized composite tissue allotransplantation, but its applicability is limited due to poor graft survival (62%–83%). Vascular thrombosis and massive hemorrhage are the major causes for these drop-outs. We hypothesize that because of better anticoagulation effect and lower risk of thrombocytopenia, application of low molecular weight heparin (LMWH) will minimize vascular complications and enhance graft and animal survival.
Methods:
Fifty allogeneic hind limb transplantations were performed (C57BL/6 to DBA/2 mice) using five different anticoagulation protocols. Bleeding and thromboembolic events were recorded macroscopically by postoperative hemorrhage and livid discoloration of the graft, respectively. Graft perfusion and survival were monitored daily by capillary-refill-time of graft toes within 2–3 seconds. Vascular congestion and tissue necrosis were examined by histological evaluation of hematoxylin-eosin-stained tissue sections.
Results:
All transplantations were technically successful. Increase in thromboembolic events and a concomitant decrease in bleeding events were observed with the decreasing concentration of heparin in the perfusion solution. Although treatment of donor and recipient with low dose of LMWH could not reduce thromboembolic events, moderate dose effectively reduced these events. Compared with the poor outcome of graft perfusion with heparin alone, additional treatment of donor and recipient with low dose of LMWH improved graft and animal survival by 18%. Interestingly, animals treated with moderate dose of LMWH demonstrated 100% graft and animal survival.
Conclusions:
Treatment of donor and recipient mice with a moderate dose of LMWH prevents vascular complications and improves the outcome of murine hind limb transplants.
Takeaways
Question: How to avoid graft and animal loss due to vascular complications in a complex VCA transplant model.
Findings: Implementation of a feasible perioperative anticoagulation protocol including LMWH significantly improves overall success rates.
Meaning: Refinement of a murine hind limb transplant model by introducing a novel anticoagulation protocol to avoid vascular complications.
INTRODUCTION
Conceptional and procedural achievements in vascularized composite tissue allotransplantation have transformed this field from a very experimental approach to a clinical reality, with more than 200 published cases to date.1–5 Animal-based research played a pivotal role in these advancements in vascularized composite tissue allotransplantation. However, many fundamental questions regarding acute and chronic rejection, nerve regeneration, and the improvement of functional outcomes are still unanswered.6–9 Besides, there is a critical need to investigate promising data obtained from in vitro experiments in in vivo small and large animal models.10–12
Traditionally, microsurgical transplant models in the rat are favored over mice because the critical anatomical structures are approximately 10 times larger and, thus, render relatively less surgical complications. The mouse model, on the other hand, is superior due to its closer genetic resemblance to human and the vast array of genetic tools and resources available for detailed mechanistic and translational studies.5–11 However, surgery performed on mice is highly sophisticated and requires a very high level of microsurgical expertise.13,14 In particular, the femoral artery and vein are very fragile and narrow at the site of vascular anastomosis. Thus, proper handling and well-controlled anticoagulation therapy is required to avoid any potential vascular complications. Current anticoagulation protocols rely mainly on unfractionated heparin, which does not suffice the anticoagulation need in such a complicated model and often results in higher frequency of vascular complications.15,16 For instance, Sucher and colleagues identified that vascular thrombosis due to insufficient anticoagulation was the major cause of graft failure in the mouse model of hind limb transplantation.13 Thus, there is a critical need of optimizing anticoagulation protocols for this model.
In the clinical setting, low molecular weight heparin (LMWH) has demonstrated several advantages over unfractionated heparin, including more a precise anticoagulation effect, longer half-life, and lower risk of heparin-induced thrombocytopenia (HIT).17–20 Therefore, we hypothesize that adding LMWH to the anticoagulation protocol will reduce vascular complications associated with inefficient coagulation and will thus improve graft and animal outcomes in this complex microsurgical model.
MATERIAL AND METHODS
Animals
Female mice that were 2–3 months old, weighing 25 ± 1.2 g, were used in this study. Fifty mice of DBA/2J and C57BL/6J backgrounds each served as recipient and donor, respectively. All animals were purchased from Charles River Laboratories (Charles River Research Models and Services, Germany GmbH, Sulzfeld, Germany) and housed at the animal facility (Forschungseinrichtung für Experimentelle Medizin, Campus Virchow-Klinikum, Berlin) with unlimited supply of autoclaved food and water. The project was approved by the state office of health and social affairs in Berlin, Germany (Landesamt für Gesundheit und Soziales, Berlin, G 0300/17).
Experimental Design
Five different anticoagulation protocols were used with varying concentrations and combinations of heparin (Heparin-Natrium-Braun, B Braun, Hessen, Germany) and LMWH (Fraxiparine, Mylan Healthcare GmbH, Hessen, Germany), as described in Table 1 and Figure 1. In all groups, grafts were flushed with 2 mL of ice cold heparin-saline solutions in specified concentrations shortly before vessel anastomosis. The grafts in the high-heparin-flush group (group I), moderate-heparin-flush group (group II), and low-heparin-flush group (group III) received 100 IU, 75 IU and 50 IU of heparin, respectively. In addition to perfusion of the grafts with low dose of heparin (50 IU/mL), donors of the low-LMWH-low-flush group (group IV) and the moderate-LMWH-low-flush group (group V) received subcutaneous injections of 9 IU and 13.5 IU of LMWH, respectively, 15 minutes before organ procurement. Besides that, 15 minutes before transplantation, the recipient mice in group IV and group V were subcutaneously treated with 2.25 IU and 4.5 IU of LMWH, respectively. The low-heparin-flush group (group III) served as control.
Table 1.
Overview of All Anticoagulation Regimens Subdivided into Five Different Groups
| High-heparin-flush Group (Group I) | Moderate-heparin-flush Group (Group II) | Low-heparin-flush Group (Group III) | Low-LMWH-low-flush Group (Group IV) | Moderate-LMWH-low-flush Group (Group V) | |
|---|---|---|---|---|---|
| (n = 10) | (n = 9) | (n = 8) | (n = 11) | (n = 12) | |
| Heparin-saline for flush | 100 IU/mL | 75 IU/mL | 50 IU/mL | 50 IU/mL | 50 IU/mL |
| LMWH donors | — | — | — | 9 IU | 13.5 IU |
| LMWH recipients | — | — | — | 2.25 IU | 4.5 IU |
Fig. 1.
Experimental design of the study. Fifty hind limb transplantations were performed by following five anticoagulation protocols. After transplantation, bleeding and thromboembolic events were macroscopically recorded. Successful graft and animal survival was defined thorough perfusion of the graft until POD 6.
Surgical Procedure
Orthotopic hind limb transplantations were performed under inhalation anesthesia with isoflurane (2%), as previously described by Sucher et al.13 In brief, for donor operation, the animals were placed on a warm plate in a supine position and the skin incision was made at the level of the inguinal ligament. The femoral artery and vein were identified and dissected over an adequate length for vessel anastomosis. The femur osteotomy was performed with a sharp scalpel and a 25-gauge needle was placed as an intramedullary rod. The “backtable preparation” started with perfusion of the graft via femoral artery with 2 mL of ice cold histidine-tryptophan-ketoglutarate (HTK) solution (Cardiolink, Barcelona, Spain). The success of the graft perfusion was marked by a clear outflow of the HTK solution from the femoral vein. Polyamid cuffs were placed over the femoral vessels and fixed with a 10-0 silk suture (Ethilon, Ethicon Deutschland, Johnson & Johnson Medical GmbH, Schleswig-Holstein), followed by cold storage of the graft in ice cold HTK solution until transplantation.
To prepare the recipient for implantation, hind limb amputation was performed similar to donor surgery, but vessels were isolated and transected proximal to the epigastric branches for anastomosis. The graft was placed in an orthotopic position and connected via osteosynthesis, followed by the re-connection of thigh muscle groups. Vascular anastomosis was performed with a nonsuture cuff technique using a 10-0 nylon suture.13,14 Nerve coaptation was not performed, as functional examination was not part of the study protocol. For postoperative analgesia, animals received 0.05 mg per kg BW of buprenorphine (Temgesic, Indivior Europe Limited, Dublin, Ireland) and 5 mg per kg BW of carprofen (Rimadyl, Pfizer, Berlin, Germany). No immunosuppressive medications were used. All surgical procedures were performed by a single qualified microsurgeon. Animal deaths due to technical failure were excluded from the study.
Analysis of Bleeding and Thromboembolic Events
Massive bleeding from the osteotomy and vascular thromboembolism at anastomosis site were the two most frequently observed complications after successful hind limb transplantations in the mouse model. Bleeding from the osteotomy was marked by unstoppable and continuous bleeding at the site of osteosynthesis, leading to death of the animal either shortly after transplantation or within 12 hours. Thromboembolic events were daily recorded by macroscopic evaluation of the grafts for livid discoloration until the observation period of postoperative day (POD) 6.
Assessment of Graft Perfusion and Survival
The perfusion of grafts was monitored daily via capillary refill test of toes within 2–3 seconds, and the macroscopic appearance was documented via photographs. Technical success was defined as survival of the transplanted limb without vascular complications, and overall success was defined as survival of both, the transplanted limb and the mouse itself until the study endpoint (POD 6).
Histopathological Analysis
At the study endpoint (POD 6), skin and muscle biopsies were taken. The tissue samples were fixed in 4% paraformaldehyde, followed by processing and paraffin embedding following standard procedures. Paraffin sections of 5-µm thickness were cut and stained with hematoxylin-eosin (H&E) dye to analyze infiltration and graft morphology. Results were analyzed by a blinded and independent pathologist (E.M.), who did not receive any information regarding sample or the treatment groups.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (Prism 8 for Macintosh Version 8.4.3, GraphPad Software, La Jolla, Calif.). The D’Agostino and Pearson omnibus normality test was used to test normal data distribution. For the comparison of complications and thromboembolic events, contingency tables were used, and statistical differences were calculated by Fisher exact test or chi-square test, respectively. Graft survival was analyzed by the Kaplan-Meier method followed by the log-rank test to evaluate statistical differences between groups. A two-tailed P value less than 0.05 was considered statistically significant.
RESULTS
The Procedure of Mouse Hind Limb Transplantation Was Technically Successful
A total of 50 technically successful mouse hind limb transplantations were performed in a randomized manner. The surgical procedure of the donor lasted for 49 ± 4 minutes with an additional backtable preparation time of 26 ± 3 minutes, where the graft was flushed with HTK and cuffs were mounted on both vessels. Recipient surgery was performed in 84 ± 10 minutes. The variation of operation time is attributed to the technical complexity of the microsurgical procedure. Upon reperfusion, both femoral artery and vein showed an adequate blood flow and pulse with no signs of congestion or stenosis. [See figure, Supplemental Digital Content 1, which displays the surgical procedure of mouse hind limb transplantation. Representative images taken during (A, B) and shortly after transplantation (C). Image A and B confirm reperfusion of the graft soon after removing micro clamps, which were placed for vessel anastomosis. (C) Color of transplanted limb (left) was comparable to the native limb (right) with no signs of bleeding through the femur (representative image of moderate-LMWH-low-flush group). http://links.lww.com/PRSGO/C757.] [See Video 1 (online), which displays the successful vessel anastomosis via nonsuture cuff technique. After clamp removal, an adequate reperfusion of femoral artery and vein has been observed.]
Video 1. displays the successful vessel anastomosis via non-suture cuff technique. After clamp removal, an adequate reperfusion of femoral artery and vein has been observed.
Pink coloration of digits confirmed blood circulation in the smaller capillaries of the graft. Importantly, as shown in the figure, the pedis plant and digits of the transplanted foot showed thorough reperfusion of the graft, like that of a native limb. [See figure, Supplemental Digital Content 2, which displays the macroscopic comparison of a failed and successful mouse hind limb transplant. Visual comparison of (A) a thrombotic hind limb graft with livid discoloration (low-LMWH-low-flush group) that was considered a failed graft compared with (B) a pink, well-perfused graft (moderate-LMWH-low-flush group). Representative pictures taken at POD 2 are shown. http://links.lww.com/PRSGO/C758.] [See Video 2 (online), which displays the proper blood perfusion of the graft that has been proven evident by reperfusion of toes.]
Video 2. displays the proper blood perfusion of the graft that has been proven evident by reperfusion of toes.
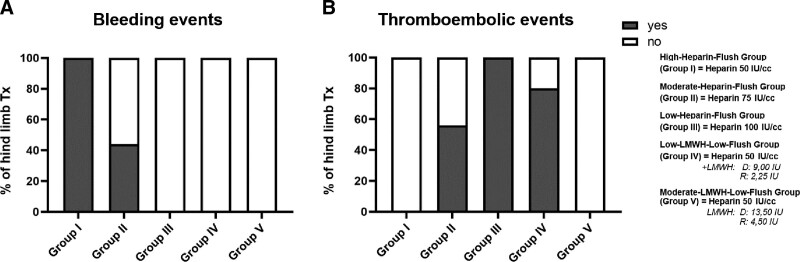
Bleeding and/or Thromboembolic Events Were Observed in All Groups Except the Moderate-LMWH-low-flush Group
Recipients of the high-heparin-flush grafts (group I) demonstrated the highest number of bleeding events compared with recipients in the other groups (Fig. 2A). Importantly, the number of bleeding events decreased with the decreasing concentration of heparin in the flush solution. In contrast, the number of thromboembolic events increased, with the decreasing concentration of heparin, reaching 100% at the lowest heparin concentration (50 IU; group III) (Fig. 2B). The number of thromboembolic events decreased slightly (18%) in the low-LMWH-low-flush group (group IV), wherein the grafts were flushed with a low dose of heparin (50 IU), combined with treatment of donor and recipients with low doses of LMWH. This indicates that the low dose of LMWH was insufficient to prevent vascular thrombosis, though bleeding events were controlled owing to low heparin dosing. Interestingly, treatment of donor and recipients with moderate doses of LMWH, in addition to graft perfusion with a low dose of heparin (group V), almost completely abolished posttransplant bleeding and thromboembolic complications (Fig. 2).
Fig. 2.
Incidence of bleeding and thromboembolic events after hind limb transplantation in the mouse model. The number of bleeding events at osteotomy (A) and thromboembolic events (B) were recorded macroscopically and presented as percentages of the animals showing said events (yes). “No” denotes absence of the event. (n = 8–12).
The Moderate-LMWH-low-flush Group Demonstrated Highest Graft Survival
Recipients of the grafts flushed with higher concentration of heparin (group I) demonstrated the poorest survival rates. All animals died either shortly after transplantation or within 12 hours due to massive hemorrhage from the osteotomy (Fig. 3). In total, 44% (4/12) of the recipients died of uncontrollable hemorrhage shortly after transplantation in the moderate-heparin-flush group (group II); the remaining 55.6% (5/9) had to be euthanized at the latest by POD 3, as the graft became necrotic. Likewise, the grafts flushed with a low concentration of heparin (group III) demonstrated a median graft survival of 2 days, resulting from sever vascular thrombosis and tissue necrosis due to insufficient anticoagulation. The treatment of donor and recipient with low doses of LMWH (group IV) improved the graft survival, with 18% (2/11) of the grafts surviving until the study endpoint (POD 6). Interestingly, the graft and the recipient treatment with moderate doses of LMWH (group V) resulted in the overall graft and animal survival of 100% (P < 0.0001).
Fig. 3.
Effect of anticoagulation protocols on overall graft and animal survival in the mouse model of hind limb transplantation. Best overall survival was observed in the moderate-LMWH-low-flush group with significant differences to all remaining groups (P < 0.0001) (n = 8–12). The survival data were analyzed by log-rank test and are presented as Kaplan-Meier survival curve.
Macroscopic and Histological Evaluation of Grafts Confirmed Adequate Blood Perfusion in the Moderate-LMWH-low-flush Group
Next, we sought to determine if higher graft and animal survival in the moderate-LMWH-low-flush group correlates with the better blood reperfusion in these grafts. Severe congestion of the transplanted limb with livid discoloration was evident in the low-LMWH-low-flush group animals at POD 2 (Supplemetal Digital Content 2A, http://links.lww.com/PRSGO/C758). In contrast, grafts of moderate-LMWH-low-flush group recipients demonstrated a well-perfused limb with sufficient blood flow [Supplemental Digital Content 2B (http://links.lww.com/PRSGO/C758) and Video 2].
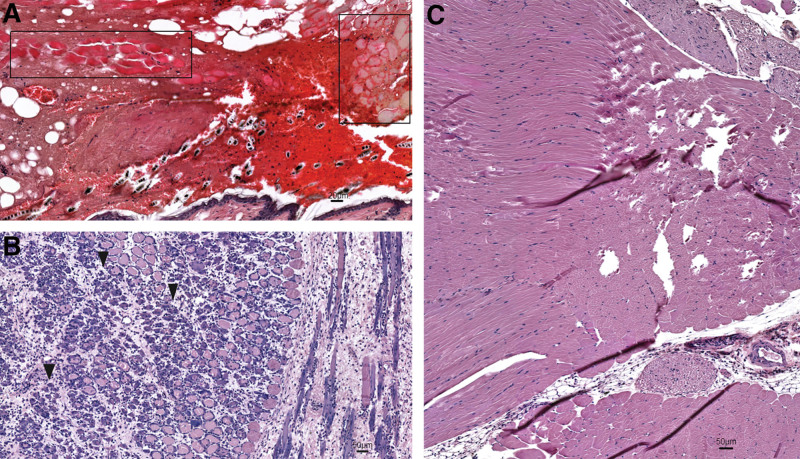
Histological examination of grafts at POD 6 showed severe tissue necrosis with 0% graft survival in the low-heparin-flush group (group III; Fig. 4A). Tissue congestions with massive infiltration, but relatively less damage to the smooth muscle fibers was detected in the grafts of the low-LMWH-low-flush group (group IV) (Fig. 4B). Grafts of the moderate-LMWH-low-flush group demonstrated intact morphology, with vital muscle fibers and skin tissue (Fig. 4C).
Fig. 4.
Effect of LMWH on the histopathology of mouse hind limb transplants. Representative images of the H&E-stained sections of mouse hind limb transplants at POD 6. A, Necrotic debris of the epidermis in a nonvital limb indicative of a failed graft in low-heparin-flush group; necrotic muscle fibers are framed in boxes. B, Brisk inflammatory infiltrate associated–damage to smooth muscle fibers (arrowheads) in the grafts of the low-LMWH-low-flush group. C, A well-perfused graft with no vascular complications (moderate-LMWH-low-flush group).
DISCUSSION
Hind limb transplantation in the mouse serves as an outstanding model for preclinical research in the field of vascularized composite tissue allotransplantation (VCA). Mouse models in general offer a better translational relevance and broad availability of genetically defined inbred and knockout strains to answer causalities of critical mechanisms when compared with other rodents such as the rat.11,21–26
Nevertheless, the performance of the murine hind limb transplant model remains demanding due to the intricate nature of the technical procedure, as it requires precise surgical skills to ensure proper osteosynthesis, muscle approximation, and connection of vessels. The smaller size of the femoral vessels, with extremely thin vessel walls and a diameter of only 0.2–0.4 mm, and their fragility pose additional challenges and, thus, require advanced technical expertise.13,27 The microsurgeon (BK) has more than 12 years of experience in experimental microsurgery and performed the transplantation with technical success of 100%. Success of the transplantation was marked by adequate reperfusion of the graft soon after opening the clamp, equal size of the vessel diameter proximal and distal to the anastomosis site, and no instant bleeding, as shown in Supplemental Digital Content 1 (http://links.lww.com/PRSGO/C757). Several vascular complications following VCA in the mouse model have been reported, including stenosis, bleeding, etc., accounting for lower rates of graft survival (62%–83%).13,15,16,24 Unfractionated heparin has been used as an anticoagulant agent in these studies at varying concentrations and treatment protocols. Heparin is probably the most frequently used anticoagulant in the clinical settings, which acts by enhancing the activity of antithrombin III, leading to inhibition of multiple clotting factors, including thrombin (factor IIa) and factor Xa.17–19 However, it acts rapidly but has a relatively short duration of action. An imprecise dosing of the heparin may result in several complications such as excessive bleeding, HIT, increased risk of blood clot formation, and thromboembolic events. In our study, we also observed massive bleeding events through the osteotomy when grafts were flushed with a relatively higher dose of heparin-saline solution (100 IU/mL) (Fig. 2A), consequently all animals died in the postoperative course (Fig. 3). Contrarily, Foster et al reported a graft loss of 27% due to venous and arterial thrombosis with the same dose of heparin (100 IU/mL).24 It should be noted that Swiss-Webster mice were used in the study by Foster et al, which are twice as big as other mouse strains. In contrast, Zhang et al repeatedly flushed stumps of both donor and recipient vessels with heparinized saline. They reported a graft survival of 83%, but technical success was defined by patent vessel anastomosis. Importantly, the drop-outs due to hypovolemic shock within the first 24–48 hours postoperatively were not included in their study.16
All animals where grafts were flushed with 50 IU/mL heparin solution did not reach the study end point of POD 6 (Fig. 3), because of graft loss due to vascular thrombosis (Fig. 2B). Sucher et al, who published the first report on the orthotopic mouse hind limb transplantation model via nonsuture super-microsurgery, demonstrated an overall success rate of only 62%. They reported vascular thrombosis being the main reason for graft loss. The anticoagulation protocol of this study included heparinization of the donor animal only with 50 IU at the time of skin incision, whereas the recipient had not received anticoagulation.13
Furthermore, all transplants flushed with a heparin-saline solution of 75 IU/mL failed, either due to vascular thrombosis, followed by graft loss or due to massive bleeding, followed by animal death in the posttransplant course (Figs. 2 and 3). With both complications occurring in the same group, which appears contradictory on a first glance, we assume that the single use of heparinized saline represents an unsteady anticoagulation protocol in this microsurgical mouse model.
The administration of LMWH changed the outcomes in this study drastically, resulting in 18% and 100% graft survival in low-LMWH-low-flush group and moderate-LMWH-low-flush group, respectively. LMWH consists of smaller fragments of heparin and differs slightly in its mechanism of action from heparin. LMWH primarily inhibits factor Xa with less effect on thrombin. Compared with heparin, it has a longer half-life and more selective and predictable anticoagulant effects. LMWH has proven superior in the prophylaxis and therapy of venous thromboembolism and arterial thrombosis in humans.20 In the clinical setting, LMWH has replaced unfractionated heparin in many cases because of its pharmacokinetic advantages, which provide a safe and effective application without hospital admission or the risk of dangerous complications such as HIT.19 We suggest that a well-balanced anticoagulation by the addition of moderate level of LMWH is the basis for preventing vascular complications and achieving a successful graft and animal survival.
One limitation of this study is the absence of an actual control group with no anticoagulation protocol. Such a control, however, is unnecessary because without any anticoagulation the transplants would fail anyway due to higher incidence of vascular thrombosis. To date, published data are conflicting with respect to the posttransplant complications as well as animal and graft outcomes. Further studies with comparable anticoagulation protocols are needed to confirm our findings. This report will, therefore, not only help advance research in reconstructive transplantation through an effective experimental model but will also help to reduce the number of animals used in experimental research studies.
CONCLUSIONS
In comparison with previous reports, we successfully performed the mouse hind limb transplant model with 100% graft survival using a novel LMWH anticoagulation protocol. The protocol successfully balanced the risk between thromboembolic events and hemorrhage in a transplant model requiring an open osteotomy.
DISCLOSURES
Barbara Kern is participant in the BIH – Charité Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health. Stefan Tullius is an Einstein BIH Visiting Fellow. This study was funded by Stiftung Charité in cooperation with Einstein Foundation Berlin. All the other authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
Drs. Witzel and Sauer contributed equally to this work.
REFERENCES
- 1.Shores JT, Brandacher G, Lee WPA. Hand and upper extremity transplantation: an update of outcomes in the worldwide experience. Plast Reconstr Surg. 2015;135:351e–360e. [DOI] [PubMed] [Google Scholar]
- 2.Ng ZY, Lellouch AG, Rosales IA, et al. Graft vasculopathy of vascularized composite allografts in humans: a literature review and retrospective study. Transpl Int. 2019;32:831–838. [DOI] [PubMed] [Google Scholar]
- 3.Petit F, Minns AB, Dubernard JM, et al. Composite tissue allotransplantation and reconstructive surgery: first clinical applications. Ann Surg. 2003;237:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swearingen B, Ravindra K, Xu H, et al. Science of composite tissue allotransplantation. Transplantation. 2008;86:627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandacher G, Khalifian S, Lee WPA. Reconstructive transplantation: from scientific dream to clinical reality. In: Brandacher G, ed. The Science of Reconstructive Transplantation. New York, NY: Springer New York, 2015:3–11. [Google Scholar]
- 6.Kollar B, Kamat P, Klein HJ, et al. The significance of vascular alterations in acute and chronic rejection for vascularized composite allotransplantation. J Vasc Res. 2019;56:163–180. [DOI] [PubMed] [Google Scholar]
- 7.Vyas KS, Mohan AT, Morrison SD, et al. Cell-based therapies in vascularized composite allotransplantation. J Reconstr Microsurg. 2018;34:642–650. [DOI] [PubMed] [Google Scholar]
- 8.Morelon E, Petruzzo P, Kanitakis J. Chronic rejection in vascularized composite allotransplantation. Curr Opin Organ Transplant. 2018;23:582–591. [DOI] [PubMed] [Google Scholar]
- 9.Fischer S, Lian CG, Kueckelhaus M, et al. Acute rejection in vascularized composite allotransplantation. Curr Opin Organ Transplant. 2014;19:531–544. [DOI] [PubMed] [Google Scholar]
- 10.Kern B, Sucher R. Small Animal Models for Reconstructive Transplantation. In: Brandacher G, ed. The Science of Reconstructive Transplantation. New York, NY: Springer New York, 2015:53–61. [Google Scholar]
- 11.Brandacher G, Grahammer J, Sucher R, et al. Animal models for basic and translational research in reconstructive transplantation. Birth Defects Res C Embryo Today. 2012;96:39–50. [DOI] [PubMed] [Google Scholar]
- 12.Matar AJ, Crepeau RL, Mundinger GS, et al. Large animal models of vascularized composite allotransplantation: a review of immune strategies to improve allograft outcomes. Front Immunol. 2021;12:664577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sucher R, Lin CH, Zanoun R, et al. Mouse hind limb transplantation: a new composite tissue allotransplantation model using nonsuture supermicrosurgery. Transplantation. 2010;90:1374–1380. [DOI] [PubMed] [Google Scholar]
- 14.Furtmuller GJ, Oh B, Grahammer J, et al. Orthotopic hind limb transplantation in the mouse. J Vis Exp. 2016;108:53483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vernon R, Wang J, Song M, et al. Vascularized composite allotransplantation: a functional hind limb model in mice. J Surg Res. 2020;250:119–124. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F, Shi DY, Kryger Z, et al. Development of a mouse limb transplantation model. Microsurgery. 1999;19:209–213. [DOI] [PubMed] [Google Scholar]
- 17.Siragusa S, Cosmi B, Piovella F, et al. Low-molecular-weight heparins and unfractionated heparin in the treatment of patients with acute venous thromboembolism: results of a meta-analysis. Am J Med. 1996;100:269–277. [DOI] [PubMed] [Google Scholar]
- 18.Zee AA, van Lieshout K, van der Heide M, et al. Low molecular weight heparin for prevention of venous thromboembolism in patients with lower-limb immobilization. Cochrane Database Syst Rev. 2017;8:CD006681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weitz JI. Low-molecular-weight heparins. N Engl J Med. 1997;337:688–698. [DOI] [PubMed] [Google Scholar]
- 20.Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119:64S–94S. [DOI] [PubMed] [Google Scholar]
- 21.Klysik J. Mice and humans: chromosome engineering and its application to functional genomics [published online ahead of print Nov 8, 2002]. Acta Biochim Pol. 2002;49:553–569. [PubMed] [Google Scholar]
- 22.Shapiro RI, Cerra FB. A model for reimplantation and transplantation of a complex organ: the rat hind limb. J Surg Res. 1978;24:501–506. [DOI] [PubMed] [Google Scholar]
- 23.van der Weyden L, Bradley A. Mouse chromosome engineering for modeling human disease. Annu Rev Genomics Hum Genet. 2006;7:247–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster RD, Liu T. Orthotopic hindlimb transplantation in the mouse. J Reconstr Microsurg. 2003;19:49–52; discussion 53–54. [DOI] [PubMed] [Google Scholar]
- 25.Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47–50. [PubMed] [Google Scholar]
- 26.Zimmermann FA, Butcher GW, Davies HS, et al. Techniques for orthotopic liver transplantation in the rat and some studies of the immunologic responses to fully allogeneic liver grafts. Transplant Proc. 1979;11:571–577. [PubMed] [Google Scholar]
- 27.Cooley BC, Daley R. Free flap transplantation in mice. Microsurgery. 1998;18:320–323. [DOI] [PubMed] [Google Scholar]