Summary:
Our team recently described targeted nipple reinnervation (TNR) during female-to-male gender-affirming mastectomy with free nipple grafting using either direct nerve coaptation or nerve allograft. The goals of TNR are to improve sensation (including erogenous sensation) and prevent numbness, paresthesias, chronic pain, and phantom sensation. Here, we describe our modified technique, which has evolved to use autologous intercostal nerve branches as donor nerves for reinnervation if direct nerve coaptation cannot be achieved. During TNR, the T3-T5 sensory branches are preserved and coapted to the repositioned nipple–areolar complex (NAC). In patients with donor nerves that were not adequate in length to allow for direct coaptation, autologous intercostal nerve branches were not used for coaptation (branches present along the chest wall that would otherwise be lost) or one of the T3-T5 branches were harvested. An end-to-end nerve repair between the autograft and donor nerves was done, and the donor nerve/autograft complex was coapted to the NAC. Targeted muscle reinnervation was performed after autograft harvest to prevent neuroma formation. TNR with intercostal nerve autograft is technically feasible in female-to-male gender-affirming mastectomy with free nipple grafting when direct coaptation is not possible. Chest reinnervation using autologous intercostal nerve branches as donor nerves is another option for reinnervation when the nerves are too short for direct coaptation. Because the collection of long-term data is ongoing, the effectiveness of NAC reinnervation using our technique will be described in a future publication.
Takeaways
Question: Is nipple reinnervation during gender-affirming mastectomy feasible using autologous intercostal nerve grafting?
Findings: Chest reinnervation using autologous intercostal nerve branches as donor nerves is technically feasible and adds another option for reinnervation when the nerves are too short for direct coaptation.
Meaning: Chest reinnervation with targeted nipple reinnervation can be performed via direct coaptation by use of an allograft, and we now describe a technique for use of intercostal nerve autograft.
INTRODUCTION
Gender-affirming mastectomy is the most common plastic surgery procedure performed for female-to-male (FTM) transgender individuals.1–3 Among the participants of the 2015 US Transgender Survey Report, 21% of FTM respondents have had chest surgery (reduction or reconstruction), and 52% of respondents want future chest surgery.4
During a traditional mastectomy, the intercostal nerves that innervate the nipple–areola complex (NAC) and breast skin are removed, resulting in loss of sensation and increased risk of chronic neuropathic pain, and phantom sensation.5–8 Studies of cancer-related mastectomies clearly demonstrate the morbidity associated with sensation loss and the importance of breast sensation.9,10
Therefore, restoration of breast sensation has become an important goal in reconstruction after breast cancer mastectomy. Techniques for peripheral nerve reconstruction after mastectomy include nerve transfer and reconstruction with nerve allograft.11 Several studies have demonstrated promising results for sensory return after breast reinnervation for autologous and implant-based reconstruction after cancer mastectomy.12–14
Only recently, chest reinnervation in transgender patients undergoing gender-affirming mastectomies has become an area of clinical interest.15 Rochlin et al used lateral intercostal nerves for direct coaptation to nerve stumps at the base of the NAC. The study demonstrated that patients treated with this method achieved improved sensation at the nipple, areola, and peripheral breast skin when compared with control patients.15 Our team was the first to describe targeted nipple reinnervation (TNR) using either direct nerve coaptation or nerve allograft in patients undergoing FTM gender-affirming mastectomy with free nipple grafting.16 TNR adheres to peripheral nerve surgery principles and has several goals, which include (1) inclusion of the maximum number of axons by preservation of the third to fifth lateral cutaneous nerves for coaptation, (2) individualized approach based on patient anatomy, (3) fascicle split of the distal donor nerve or allo-/autograft to increase the reinnervation zone and avoid focal hypersensitivity, and (4) coaptation to the dermatosensory peripheral nerve elements of the dermis.
Using TNR, direct coaptation of the donor nerves to the new NAC is feasible if enough donor nerve length is preserved to perform a tension-free coaptation. In instances where a tension-free repair is not possible due to anatomic limitations, use of a nerve graft is required. To date, there are no clinical trials that directly compare the use of allograft versus autograft in breast reinnervation. Few studies directly compare the clinical effectiveness of autografts with that of allografts in any setting.17 However, there is basic scientific evidence to suggest that autografts provide an immunogenically inert scaffold that contains a viable source of Schwann cells and may enhance axonal regeneration.18
TNR has only been described for direct nerve coaptation and nerve allograft.16 This article describes a novel TNR technique using autologous intercostal nerve grafts instead when the donor nerve stumps are too short for direct coaptation.
METHODS
Surgical Technique
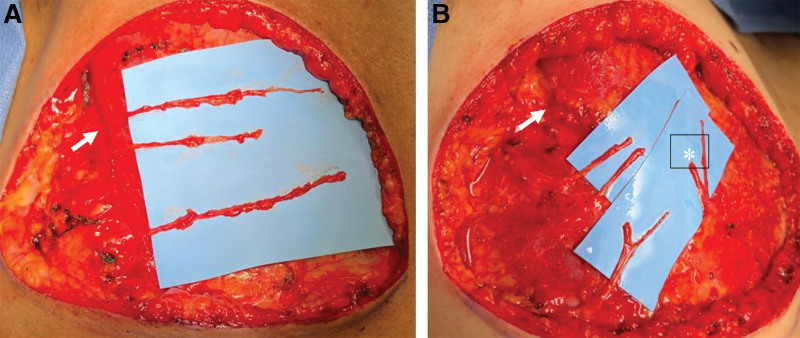
This study was approved by the Weill Cornell Medicine institutional review board. Gender-affirming mastectomy with free nipple grafting was performed via standard techniques, as described in our prior publication.16 First, the NAC is removed and preserved as a graft in moist saline sponges. Then, incisions are made approximately 1 cm above the inframammary crease—as well as superior to the NAC—and carried down to the pectoralis fascia medially. Laterally, at the level of the anterior axillary line, the incision is carried down to the breast capsule. Importantly, the incision is not extended to the pectoralis fascia at this time, as nerves that traverse this area may be inadvertently transected. The mastectomy flaps are created superiorly and inferiorly without going past the anterior axillary line. Then, the breast tissue is separated from the pectoralis muscle fascia from medial to lateral. This dissection is performed under loupe magnification. It is important to identify the sensory branches of the intercostal nerves starting medially, as there may be large branches that can be used as autografts. At the lateral border of the pectoralis muscle, the sensory branches of the third to fifth intercostal nerves become apparent at the edge of the muscle and up to 2 cm lateral from the muscle border; however, anatomy is highly variable (Fig. 1). These branches are preserved as donor nerves. In some patients, there may only be two sensory branches that can be identified. The T3-T5 sensory branches are dissected as far distally as possible for maximum length, and the blood supply to the nerves is preserved without stripping the vessels running with the nerve from the nervous tissue. If there is bleeding, the vessel can be clipped toward the end of the nerve with a micro clip and separated from the vessel the shortest distance required to place the clip.
Fig. 1.
Intercostal nerve dissection. A, The T3-T5 nerve branches have been identified at the lateral border of the pectoralis muscle (white arrow). B, In addition, medial branches (white *) can be identified and preserved.
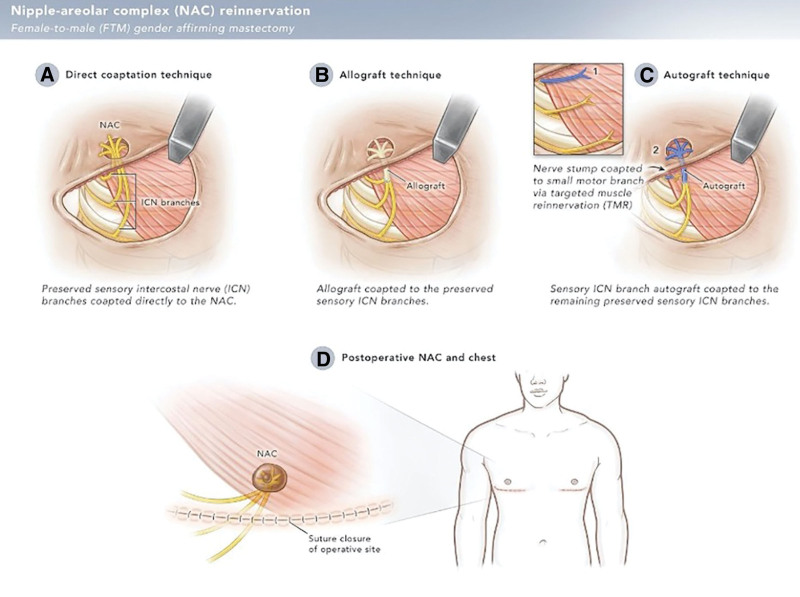
Preservation of the T3-T5 nerves allows for chest reinnervation using three approaches: (1) direct coaptation to the NAC, (2) use of allograft nerve repair, and (3) use of autograft nerve repair (Fig. 2). We have described direct coaptation and use of allograft repair in a previous publication.16 Here, we describe autograft nerve repair.
Fig. 2.
Illustration depicting different techniques used for nipple-areolar complex (NAC) reinnervation in FTM gender-affirming mastectomy with TNR. A, If tension-free repair is possible, the branches of T3-T5 are directly coapted to the dermatosensory elements of the newly positioned NAC. If the donor nerves cannot reach the NAC without tension, either an allograft (B) or autograft (C) is used for nerve repair. B, The donor intercostal nerves are coapted to the allograft (B) or autograft (C), which is then passed through the NAC. Importantly, the distal nerves are split into fascicles to avoid focal hypersensitivity and increase the reinnervation zone. (D) The nipple graft is applied directly over the nerve graft and sutured in place.
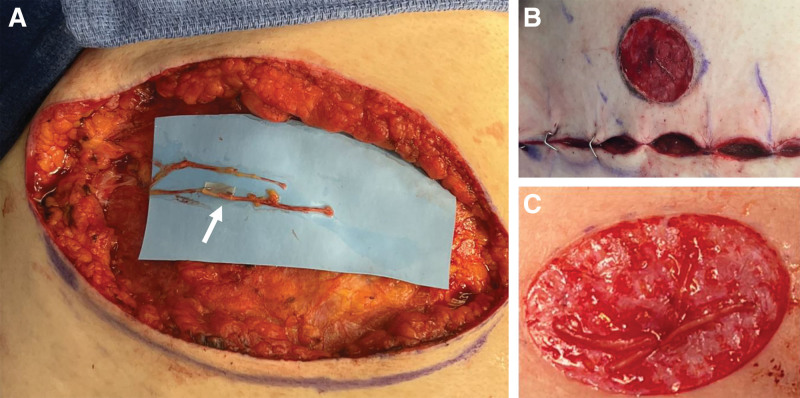
When one or more of the T3-T5 nerves are not adequate in length for direct coaptation, an autograft from the preserved medial sensory branches or one of the T3-T5 nerve branches can be used for coaptation (Fig. 3). The autograft is coapted in an end-to-end fashion to the lateral cutaneous branches, using a standard epineurial nerve repair technique with 9-0 nylon suture under loupe magnification.
Fig. 3.
Nerve autograft technique. A, An autograft is harvested from a previously preserved T3–T5 nerve branch or anterior nerve branch and coapted to the shorter lateral cutaneous intercostal nerve in an end-to-end fashion (white arrow). B, The distal nerve ends are passed through a subcutaneous tunnel in the NAC and (C) split into fascicles to prevent hypersensitivity and increase the reinnervation zone.
After the mastectomy is complete and the new position for NAC decided, the skin at the site of the NAC is deepithelialized, and a small full-thickness tunnel is created through the mid NAC. The distal ends of the nerves are passed through this tunnel. The distal autograft is divided into fascicles and spread out to cover more surface area, thereby increasing the reinnervation zone and preventing focal hypersensitivity. The NAC graft is then applied over the nerve grafts and sutured into place.
It is important to address the residual nerve stump that remains after autograft harvest to prevent chronic pain and phantom pain. The senior authors’ preferred technique is targeted muscle reinnervation to a motor branch of either the pectoralis major or serratus muscle (depending on the location of autograft harvest).19 However, other published techniques such as muscle burial or regenerative peripheral nerve interface can also be used.20 (See Video [online], which provides further description of this technique.)
Video 1. This video displays the donor nerves are harvested and coapted to recipient nerves using the nerve autograft technique described in this manuscript to ultimately increase sensation of the NAC.
DISCUSSION
During traditional and gender-affirming mastectomy alike, intercostal nerves that innervate the breast and NAC are removed, causing loss of sensation.5,9 Chest reinnervation to restore sensation to the free nipple grafting has the potential to benefit patients by restoring sensation and decreasing the odds of chronic pain and phantom sensation/pain. In addition to direct coaptation of the donor nerves to the new NAC and allograft reconstruction, this study describes a reinnervation approach that uses intercostal nerve autograft for coaptation to the new NAC. This option can be considered when the donor nerve stump is short and does not allow for direct coaptation, but the patient has an additional medial cutaneous branch or several lateral cutaneous branches that can be lost. The use of intercostal nerve autografts avoids the donor site morbidity associated with nerve harvest from other sites (eg, sural nerve autograft). Although there is a learning curve, the dissection and harvest of the medial and/or lateral cutaneous intercostal nerve branches as an autograft is facile, and has no significant impact on operative time for an experienced nerve surgeon. These nerves reside within the same operative field and are optimally approached during the mastectomy dissection. For less-experienced surgeons, the dissection of additional nerve branches may be time consuming and should be considered when planning this operation. Although there are no clinical trials comparing allograft versus autograft in breast reinnervation surgery, there is some literature to suggest that autografts provide an immunogenically inert scaffold that is superior to allografts.18 Moreover, the use of nerve autograft is financially less burdensome than the use of allograft.21 Limitations of the autograft TNR technique are that this technique is ultimately dependent on the patient’s anatomy and the availability/viability of donor nerves. The medial branches can be very small and not suited for autograft harvest. Further, not all lateral branches may have adequate caliber to be used as donor nerves or autograft.
Long-term follow-up data are needed to assess the sensory outcome and patient satisfaction after autograft nerve repair. In addition, it will be important to better understand the postoperative time to sensory return and compare the effectiveness of direct coaptation versus autograft and allografts for TNR.
CONCLUSIONS
Chest reinnervation using autologous intercostal nerve branches as donor nerves is technically feasible and adds another option for reinnervation when the nerves are too short for direct coaptation. Because the collection of long-term data is ongoing, the effectiveness of NAC reinnervation using our technique will be described in a future publication.
DISCLOSURES
Lisa Gfrerer is a consultant for Sientra and Cytrellis. Ian Valerio is a consultant for Axogen, Checkpoint, and Integra. All the other authors have no financial interest to declare in relation to the content of this article. This work was supported by the American Association of Plastic Surgeons Academic Scholar Program to Lisa Gfrerer.
Footnotes
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health. 2022;23:S1–S259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammari T, Sluiter EC, Gast K, et al. Female-to-male gender-affirming chest reconstruction surgery. Aesthet Surg J. 2018;39:150–163. [DOI] [PubMed] [Google Scholar]
- 3.Kailas M, Lu HMS, Rothman EF, et al. Prevalence and types of gender-affirming surgery among a sample of transgender endocrinology patients prior to state expansion of insurance coverage. Endocr Pract. 2017;23:780–786. [DOI] [PubMed] [Google Scholar]
- 4.James SE, Herman JL, Rankin S, et al. The Report of the 2015 U.S. Transgender Survey. Washington, DC.: National Center for Transgender Equality; 2016. [Google Scholar]
- 5.Khan A, Zhang J, Sollazzo V, et al. Sensory change of the reconstructed breast envelope after skin-sparing mastectomy. Eur J Surg Oncol. 2016;42:973–979. [DOI] [PubMed] [Google Scholar]
- 6.Mustonen L, Vollert J, Rice ASC, et al. Sensory profiles in women with neuropathic pain after breast cancer surgery. Breast Cancer Res Treat. 2020;182:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krøner K, Krebs B, Skov J, et al. Immediate and long-term phantom breast syndrome after mastectomy: incidence, clinical characteristics and relationship to pre-mastectomy breast pain. Pain. 1989;36:327–334. [DOI] [PubMed] [Google Scholar]
- 8.Rothemund Y, Grüsser SM, Liebeskind U, et al. Phantom phenomena in mastectomized patients and their relation to chronic and acute pre-mastectomy pain. Pain. 2004;107:140–146. [DOI] [PubMed] [Google Scholar]
- 9.Koçan S, Gürsoy A. Body image of women with breast cancer after mastectomy: a qualitative research. J Breast Health. 2016;12:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misery L, Talagas M. Innervation of the male breast: psychological and physiological consequences. J Mammary Gland Biol Neoplasia. 2017;22:109–115. [DOI] [PubMed] [Google Scholar]
- 11.Gfrerer L, Sager JE, Ford OA, et al. Targeted nipple areola complex reinnervation: technical considerations and surgical efficiency in implant-based breast reconstruction. Plast Reconstr Surg Glob Open. 2022;10:e4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peled AW, Peled ZM. Nerve preservation and allografting for sensory innervation following immediate implant breast reconstruction. Plast Reconstr Surg Glob Open. 2019;7:e2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Djohan R, Knackstedt R, Scomacao I, et al. A novel approach to sensory re-innervation to the nipple areolar complex after mastectomy with implant-based reconstruction: anatomic and technical considerations. J Plast Reconstr Aesthet Surg. 2020;73:983–1007. [DOI] [PubMed] [Google Scholar]
- 14.Tevlin R, Brazio P, Tran N, et al. Immediate targeted nipple-areolar complex re-innervation: improving outcomes in immediate autologous breast reconstruction. J Plast Reconstr Aesthet Surg. 2021;74:1503–1507. [DOI] [PubMed] [Google Scholar]
- 15.Rochlin DH, Brazio P, Wapnir I, et al. Immediate targeted nipple-areolar complex reinnervation: improving outcomes in gender-affirming mastectomy. Plast Reconstr Surg Glob Open. 2020;8:e2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gfrerer L, Winograd JM, Austen WG, Jr, et al. Targeted nipple areola complex reinnervation in gender-affirming double incision mastectomy with free nipple grafting. Plast Reconstr Surg Glob Open. 2022;10:e4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauch JT, Bae A, Shubinets V, et al. A systematic review of sensory outcomes of digital nerve gap reconstruction with autograft, allograft, and conduit. Ann Plast Surg. 2019;82(4S Suppl 3):S247–S255. [DOI] [PubMed] [Google Scholar]
- 18.Boyd KU, Nimigan AS, Mackinnon SE. Nerve reconstruction in the hand and upper extremity. Clin Plast Surg. 2011;38:643–660. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien AL, Kraft CT, Valerio IL, et al. Targeted muscle reinnervation following breast surgery: a novel technique. Plast Reconstr Surg Glob Open. 2020;8:e2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo SL, Kung TA, Brown DL, et al. Regenerative peripheral nerve interfaces for the treatment of postamputation neuroma pain: a pilot study. Plast Reconstr Surg Glob Open. 2016;4:e1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brattain K. Analysis of the peripheral nerve repair market in the United States. Minneapolis: Magellan Med Technol Consult Inc; 2014. Available at http://content.stockpr.com/axogeninc/files/docs/Magellan_Study_-_Analysis_Of_The_Peripheral_Nerve_Repair_Market_In_The_United_States.pdf. Accessed December 1, 2022. [Google Scholar]