Abstract
Background:
This study aimed to evaluate the ultrasonic manifestations of diversified corium fillers in the temporal region and to provide clinicians with suggestions for diagnosis and treatment.
Methods:
The facial ultrasound images of 116 patients, including 110 women and six men, 20–61years of age, were analyzed at the Chinese Academy Medical Sciences and Peking Union Medical College from November 2014 to November 2021.
Results:
We identified 48 cases of polyacrylamide, 31 cases of fat, 27 cases of hyaluronic acid, seven cases of hydroxyapatite, two cases of silicone oil, and one case of prosthesis. Polyacrylamide mainly showed irregular flocculent hypoechoic or fine punctate hypoechoic in ultrasound; it could be aggregated as a cystic hypoechoic area or diffused in the tissue space, and the probe could be pressurized to see the echo floating or dislocation flow. Fat mainly showed lobulated hypoechoic deposition with some hyperechoic linear intervals. Hyaluronic acid mainly showed an anechoic structure with a clear boundary, uniform internal echo, and no obvious blood flow signal. If diffused in the surrounding tissues, it was mainly characterized by anechoic or low-echo areas in the stratified tissues. Hydroxyapatite mainly showed strongly hyperechoic patch areas with posterior acoustic shadowing artifacts. Silicone oil was mostly found under the epidermis, showing a high echo in the form of clouds and causing unclear displays of deep tissue. Prosthesis showed hypoechoic prosthesis structure.
Conclusion:
High-frequency ultrasound had a certain significance in the identification of the fillers of the temporal region.
Takeaways
Question: What are the ultrasound characteristics of different temporal fillers?
Findings: We believe that our study makes a significant contribution to the literature because it not only reveals the specific ultrasonographic manifestations of polyacrylamide, fat, hyaluronic acid, calcium hydroxyapatite, silicone oil, and prosthesis, but it also provides evidence for the use of ultrasound as a safe, noninvasive, high definition detection of blood flow.
Meaning: High frequency ultrasound had a certain significance in the identification of the fillers of the temporal region.
INTRODUCTION
The content and structure of human bones, muscles, and fat can reflect the appearance of a person. As people grow older, the loss of temporal fat capacity, ligaments, musculofascial system support, and bone all lead to the ptosis of volume tissue, thus causing the hollow of temporal appearance. The hollow of the temporal region affects people’s appearances and mental outlooks, manifested as facial depression and weight loss, which often causes a displeased, aged facial expression and induces a psychological burden, negatively affecting patients’ work and personal lives. With the rapid development of medical cosmetology and biomaterial science, the technique of injecting degradable and nondegradable materials to fill the temporal region has been favored by many plastic surgeons and beauty enthusiasts for its advantages of less trauma and rapid recovery.1 Common fillers for facial injections include hyaluronic acid (HA), ometine, and autologous fat. In addition, bone meal, microcrystalline porcelain, and silicone oil have been expressly prohibited by the Ministry of Health and the Food and Drug Administration for use as fillers. These illegal fillers can stay in the body for a long time and may erode tissues, causing potential complications.2 Some of our patients need to be refilled after removing the temporal filling material, and they are not clear about what the previous filling material was. Therefore, developing an effective method to identify fillers and avoid potential complications is of paramount importance. Different fillers have different manifestations under ultrasound. This article discusses the application value of high-frequency ultrasound in the identification and evaluation of temporal fillers.
MATERIALS AND METHODS
Patients who underwent the facial ultrasound examination from November 2014 to November 2021 were selected as the research participants. The inclusion criteria were as follows: ultrasound results showing that the temporal region was filled with injection material or prosthesis. Exclusion criteria were as follows: a history of temporal filling injection with a variety of fillers; a history of filler injection, but the injection material was completely absorbed or removed without positive imaging findings; and patients who received plastic and cosmetic interventions other than filler injections or prosthesis in the temporal region. Applying the inclusion and exclusion criteria, 116 patients were finally included in the study, including six male and 110 female patients. The average age of the patients was 40 ± 10.68 years (range, 20–61 y). The study was approved by the ethics committee.
Ultrasound images were obtained by LogicE9 (GE Healthcare, Chicago, Ill.) with a probe frequency of 15 MHz. Experienced ultrasound doctors combined each patient’s medical history and the description of the filler removal surgeon to analyze the ultrasound imaging manifestations and to determine the type of fillers.
RESULTS
The ultrasound manifestations of the injection materials were evaluated, including 48 cases of polyacrylamide (PAM), 31 cases of fat, 27 cases of HA, seven cases of calcium hydroxyapatite (CaHA), two cases of silicone oil, and one case of prosthesis.
Polyacrylamide
The ultrasonographic manifestations of PAM can be divided into the aggregation type (28 cases), diffusion type (19 cases), and complicated with infection (one case). The ultrasonography of the aggregation-wrapped type showed a subcutaneous cystic mass with an enhanced echo and a fine punctured echo in the posterior wall. There was a sense of dislocation flow when squeezed. The ultrasound of the aggregation-unwrapped type showed a subcutaneous area of hypoechoic agglomeration with fine punctured echoes, extrusion with a sense of dislocation flow, no obvious envelope, and a trend of spreading to surrounding tissues. The ultrasound of the diffusion type showed a slightly higher or lower echogenic area in the subcutaneous cavity and diffusely distributed fine punctate echogenic areas. In the aggregation and diffusion modes, cystic masses with fine punctate echoes were observed, and fine punctate echoes were also observed in the space of surrounding tissues. Under ultrasound, the tissue echo of the filling site where infection occurred decreased overall, while the blood flow signal increased, and the fine dot echo was observed (Fig. 1).
Fig. 1.
The ultrasonic manifestations of polyacrylamide. A, Aggregation type of PAM. B, Diffusion type of PAM. C, PAM associated with infection.
Fat
The ultrasonographic manifestations of fat included the evenly distributed low-echo areas in the fat layer with some hyperechoic linear intervals. Intermediate liquefaction necrosis may occur in fat, which was manifested as an anechoic zone in the hypoechoic zone within the fat layer (Fig. 2).
Fig. 2.
Ultrasonic manifestations of fat. A, Fat distributed diffusely between tissues. B, Fat liquefaction necrosis.
Hyaluronic Acid
According to the ultrasonic manifestations of HA, patients were divided into the aggregation type (19 cases) and diffusion type (eight cases). The aggregation type showed a clear boundary of the non-echo area, and the internal echo seemed to have an envelope. Sometimes multiple anechoic zones were connected piled upon each other. The ultrasound of the diffusion type showed a loose subcutaneous tissue structure and multiple thin strips of hypoechoic and anechoic areas scattered in the hypoechoic space, showing a stratified distribution. When infection occurred in the HA filling site due to tissue edema, the tissue hierarchy was unclear, the structure was disordered, the echo reduced overall, and the blood flow signal increased (Fig. 3).
Fig. 3.
The ultrasonic manifestations of hyaluronic acid. A, Aggregation type of HA. B, Diffusion type of HA.
Calcium Hydroxyapatite
The ultrasonography manifestation of CaHA was a patchy strong echo area with an obvious sound shadow in the post (Fig. 4).
Fig. 4.
Ultrasonic manifestations of CaHA. A, Aggregation type of CaHA. B, Diffusion type of CaHA.
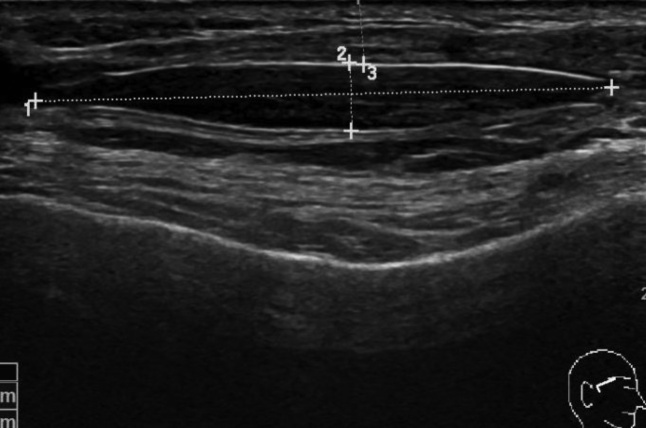
Silicone Oil
The ultrasonography manifestation of silicone oil was a cloud-like echo area, resulting in unclear deep tissue (Fig. 5).
Fig. 5.
Ultrasonic manifestations of silicon oil.
Prosthesis
In the single case of silicone prosthesis, the ultrasound manifestations included hypoechoic prosthesis structure, boundary echo enhancement, and internal echo uniformity (Fig. 6).
Fig. 6.
Ultrasonic manifestations of temporal silicone prosthesis.
DISCUSSION
The temporal region is the most complex structural level in the maxillofacial region. The upper margin is the superior temporal line, the lower margin is the superior margin of zygomatic arch, the medial margin is the outer orbital margin, and the lateral part is the temporal hairline. Most scholars have divided the temporal tissue into 10 levels from shallow to deep, as follows: skin, subcutaneous fat, superficial temporal fascia, middle temporal fascia, superficial temporal fascia, superficial temporal fat pad, deep temporal fascia, deep temporal fat pad, temporalis muscle, and periosteum. The volume composition of the whole temporal region is mainly composed of the subcutaneous fat layer, superficial temporal fat pad, deep temporal fat pad, and temporalis muscle. The deep temporal fat pad, which is the extension of the buccal fat pad in the temporal region and is communicated with the buccal fat pad, is located on the medial side of the zygomatic arch and is small in size. The temporalis muscle is located in the temporal fossa, relatively thin above and thicker below, near the zygomatic arch. The temporalis muscle does not completely fill the temporal fossa, but it lies near the fornix of the skull and does not exceed 50% of the total depth of the temporal fossa.3 With aging, changes in the temporal region can be found at all levels from the bone to the subcutaneous tissue, with the absorption of the temporal bone, atrophy of the concomitant temporal muscle, and reduced temporal fat pads contributing to the formation of the temporal hollow.4
Temporal fillers include permanent materials and temporary materials. In this article, we analyzed the ultrasonic images of the patients with temporal fillers who underwent facial ultrasound examination in our hospital from November 2014 to November 2021, and we summarized the material classification and image characteristics of temporal fillers. We found that PAM was the most used temporal filler in the study. PAM has been popular during the last 9 years in China. Due to the occurrence and in-depth study of complications such as displacement, ectopic, sclerosis, infection, and potential neurotoxicity, the production and clinical application of PAM have been completely prohibited in China5; PAM was then followed by fat and HA filling. The scope of losing tissue in the temporal area was often larger than that in other facial regions, and the filling demand is large. Autologous fat has a wide range of sources and is convenient to obtain. HA is the most commonly used temporary filling material for facial fillers; it is naturally present in the extracellular matrix of the dermis, and it is both safe and reliable.6 In addition, small amounts of CaHA, silicone oil, and silicone implants were seen in patients with temporal implants. Bone meal and microcrystalline porcelain are filled with CaHA as the main component. They are poisonless, not easy to shift, and of high histocompatibility, which stimulate the proliferation of fibroblasts and collagen formation, improve skin quality, and have stable and lasting effects.7 However, due to the presence of nondegradable materials and the simplicity of wrapping around the normal tissue, it is difficult to remove when the filling is not satisfied. Silicone oil is an industrial material and is prohibited to be used as fillers by most countries in the world. Because of the low price of the material, some illegal personnel use silicone oil, claiming the material to be HA or other fillers.8 Silicone prostheses are also sometimes used to fill severe hollows in the temporal region.
When complications occur with fillers or facial morphology is not satisfied, the patient often seeks examination and treatment to remove the padding. This study found that high-frequency ultrasound equips certain advantages regarding the identification and detection of fillers, compared with computed tomography and magnetic resonance imaging (MRI). MRI showed clear positioning for soft tissue, but most of the facial fillers have high water content and appear similar in MRI. Computed tomography is good for manifesting high density calcification, but it is difficult to identify soft tissue fillers. At present, many scholars have reported the use of high-frequency ultrasound for the detection of facial fillings and for the follow-up and treatment of complications.8–12 Ultrasound is a noninvasively operated instrument that displays the anatomy of skin and deep tissues in real time.13 The high-frequency ultrasound probe has a high resolution and is suitable for examining the small, shallow focus of facial fillers. Ultrasound advantages include safety, noninvasion, high definition, and real-time detection of blood flow.14 Not only can facial fillers be clearly located with ultrasound but also, different image features are displayed to help doctors further distinguish the filler material. However, the ultrasound results are subjective and require experienced ultrasound doctors to conduct testing and judgment. This technology is only a reference and cannot 100% determine the effectiveness of the filling materials. The diagnosis of injection fillers requires a combination of medical history, ultrasound features, and pathological diagnosis of the extracted material. High-frequency ultrasound examination can be used not only for the assessment of temporal fillers but also for the fillers in other facial parts. More comprehensive research needs to be completed in the future.
CONCLUSIONS
High-frequency ultrasound examination has significance in distinguishing different temporal fillers, and it can determine the residual site when removing fillers during surgery.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
ACKNOWLEDGMENTS
We are grateful that this work was supported by key projects of medical school development of Shijingshan District. This study was approved by the Ethics Committee of the Plastic Surgery Hospital of the CAMS. For this type of study informed consent is not required.
Footnotes
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Stefura T, Kacprzyk A, Droś J, et al. Tissue fillers for the nasolabial fold area: a systematic review and meta-analysis of randomized clinical trials. Aesthet Plast Surg. 2021;45:2300–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu MH, Beynet DP, Gharavi NM. Overview of deep dermal fillers. Facial Plast Surg. 2019;35:224–229. [DOI] [PubMed] [Google Scholar]
- 3.Hicks DL, Watson D. Soft tissue reconstruction of the forehead and temple. Facial Plast Surg Clin North Am. 2005;13:243–251, vi. [DOI] [PubMed] [Google Scholar]
- 4.Rose AE, Day D. Esthetic rejuvenation of the temple. Clin Plast Surg. 2013;40:77–89. [DOI] [PubMed] [Google Scholar]
- 5.Cheng NX, Xu SL, Deng H, et al. Migration of implants: a problem with injectable polyacrylamide gel in aesthetic plastic surgery. Aesthet Plast Surg. 2006;30:215–225. [DOI] [PubMed] [Google Scholar]
- 6.Henius L. Hyaluronic acid fillers: a comprehensive review. Facial Plast Surg FPS. 2009;25:86. [DOI] [PubMed] [Google Scholar]
- 7.Kadouch JA. Calcium hydroxylapatite: a review on safety and complications. J Cosmet Dermatol. 2017;16:152–161. [DOI] [PubMed] [Google Scholar]
- 8.Grippaudo FR, Mattei M. High-frequency sonography of temporary and permanent dermal fillers. Skin Res Technol. 2010;16:265–269. [DOI] [PubMed] [Google Scholar]
- 9.Wortsman X, Wortsman J, Orlandi C, et al. Ultrasound detection and identification of cosmetic fillers in the skin. J Eur Acad Dermatol Venereol. 2012;26:292–301. [DOI] [PubMed] [Google Scholar]
- 10.Schelke LW, Van Den Elzen HJVD, Erkamp PPM, et al. Use of ultrasound to provide overall information on facial fillers and surrounding tissue. Dermatol Surg. 2010;36(s3suppl 3):1843–1851. [DOI] [PubMed] [Google Scholar]
- 11.Skrzypek E, Górnicka B, Skrzypek DM, et al. Granuloma as a complication of polycaprolactone-based dermal filler injection: ultrasound and histopathology studies. J Cosmet Laser Ther. 2018;21:65–68. [DOI] [PubMed] [Google Scholar]
- 12.Grippaudo FR, Mattei M. The utility of high-frequency ultrasound in dermal filler evaluation. Ann Plast Surg. 2011;67:469–473. [DOI] [PubMed] [Google Scholar]
- 13.Wortsman X, Alfageme F, Roustan G, et al. Guidelines for performing dermatologic ultrasound examinations by the DERMUS group. J Ultrasound Med. 2016;35:577–580. [DOI] [PubMed] [Google Scholar]
- 14.Wortsman X. Practical applications of ultrasound in dermatology. Clin Dermatol. 2021;39:605–623. [DOI] [PubMed] [Google Scholar]