Abstract
PURPOSE
Lung cancer is the leading cause of cancer-related deaths in the United States. This study aims to analyze lung cancer incidence, mortality, and related statistics from 1990 to 2019, focusing on national- and state-level trends and exploring potential disparities between sexes.
METHODS
The Global Burden of Disease database was used to extract tracheal, bronchus, and lung cancer mortality data from 1990 to 2019 for both males and females and across all states of the United States. Age-standardized incidence rates, age-standardized mortality rates, disability-adjusted life years (DALYs), and mortality-to-incidence indices (MIIs) were studied to assess for gender-based, geographic, and temporal disparities. Joinpoint regression analysis was performed to further evaluate trends.
RESULTS
The incidence of these cancers in the United States decreased between 1990 and 2019 by 23.35%, with a more significant decline in males (37.73%) than females (1.41%). Similarly, for mortality, a decrease was observed for both sexes combined (26.83%), but much more significantly for males (40.23%) than females (6.01%). The MIIs decreased overall, but there were variations across states. DALYs decreased for both sexes combined, with males experiencing a larger reduction, but an increase was noted in some states for females.
CONCLUSION
This analysis reveals diverse trends pertaining to the incidence, mortality, and disability burden associated with lung cancer by sex and states in the United States, emphasizing the need for targeted interventions to reduce disparities. These findings contribute to our understanding of the current landscape of lung cancer and can inform future strategies for prevention, early detection, and management.
Over 30 years, lung cancer incidence (–23.4%) and mortality (–26.8%) dropped. However, since 2017, there's been a male incidence increase and a worrisome rise in female mortality rates.
INTRODUCTION
Lung cancer is the second most common cancer in the United States and remains the leading cause of cancer-associated mortality in the United States, with 139,682 deaths in the year 2019.1 Five-year survival after a diagnosis of lung cancer remained low at 20% between 2010 and 2016 because of advanced-stage diagnosis.2
CONTEXT
Key Objective
Are there gender disparities in the national- and state-level trends of lung cancer incidence, mortality, and related statistics from 1990 to 2019?
Knowledge Generated
Using the Global Burden of Disease database, the incidence and mortality of tracheal, bronchus, and lung cancers in the United States were found to be decreasing much more significantly in males than females. In addition, while the disability-adjusted life years decreased in males, some states noted an increase for females.
Relevance
By analyzing trends rather than absolute annual mortality rates, our study helps appreciate the population-level landscape of lung cancer over an extended observation period, which can inspire plans of action for prevention, early detection, and management, notably to reduce the observed gender disparities.
In 2011, the National Lung Screening Trial and NELSON trial reported a 20% and 24% mortality risk reduction, respectively, for annual low-dose computed tomography scan (LDCT) implementation.3,4 In 2013, the US Preventive Services Task Force (USPTF) recommended annual screening for lung cancer with LDCT in adults age 50-80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years.5 Operational needs and resource allocation post substantial barriers to implementation of a lung cancer screening program. Uptake of LDCT screening for lung cancer has been slow, and in 2018, only approximately 12% of eligible individuals received lung cancer screening, with significant state-to-state variability.6 We hypothesize that there has been no improvement in lung cancer–related statistics at the national level in the United States since the USPTF recommendations for annual lung cancer screening in high-risk individuals were released.
The first objective of this study is to analyze the rate and trends of incidence, mortality, mortality-to-incidence indices (MIIs), and disability-adjusted life years (DALYs) attributed to cancers of the trachea, bronchus, and lung in the United States between 1990 and 2019. The second objective is to report the state-level statistics from all 51 states. The final objective is to compare the differential trends between males and females to identify gender disparities. We used data from the Global Burden of Disease (GBD) database for this analysis.
METHODS
Characteristics of the Data Source
This observational analysis of tracheal, bronchus, and lung cancers in 51 US states was performed using data extracted from the GBD database. This WHO-commissioned database is an amalgamation of 127 countries’ data sets and registries that provides epidemiologic characteristics (incidence, prevalence, mortality, DALYs, years of life lost, etc) for some of the world’s most important health concerns.
Data sets used by the GBD researchers include insurance data, admission and outpatient encounter data, and systematic reviews, among others. For data, the GBD maps all mortality and incidence data related to the International Classification of Diseases (ICD) codes (codes C33-C34, D02.1-D02.2, D38.1, 162-162.9, 231.1, 231.2, 231.8, 235.7 from ICD10 and B101 from ICD9). These data are combined by Bayesian meta-regression with the DisMod-MR 2.19 (Institute of Health Metrics and Evaluation, University of Washington, Seattle, WA) tool that analyzes and adjusts for bias and produces disease estimates with CIs.7,8 GBD has different mappings of ICD codes on the basis of incidence or mortality. In brief, generally, for incidence, GBD excludes the most benign codes. At the same time, for mortality, they include many benign codes (assuming that if a tumor was assigned a benign code but led to death, it was likely misclassified). Mortality data are collected from vital registration sources, verbal autopsy reports, and surveillance data and entered into the GBD cause-of-death database. The quality of mortality data from each country is then evaluated by the GBD methodology on the basis of a five-star rating system for each location-year to assist in the reader’s comprehension of the reliability of the cause-of-death data.
Handling of the GBD Data
We extracted age-standardized incidence rates (ASIRs), age-standardized mortality rates (ASMRs), and DALYs for tracheal, bronchus, and lung cancers from 51 US states between 1990 and 2019 using the dedicated GBD Results tool.9 Age-standardized rates were used to account for the variations in age structures for each state. The method used by the GBD involves calculating a standard population from the United Nations Population Division’s World Population Prospects (2012 revision). We have previously performed similar studies to assess mortality trends for lung cancer, kidney cancer, and intracerebral hemorrhage.10-12
We calculated absolute and relative changes in ASIRs, ASMRs, and DALYs between 1990 and 2019 for each sex in each state. The MIIs were calculated by dividing ASMR by ASIR for each year (1990 and 2019) for both sexes in all states. MIIs allow for the comparison of disease burden by normalizing mortality to incidence. A DALY incorporates morbidity and mortality figures to calculate the years lived with and lost from a disability. The WHO uses it to indicate the overall disease burden on a health system. These measures facilitate our understanding of the varying temporal impact of tracheal, bronchus, and lung cancers. Trends for the entire United States are reported as well.
Statistical Analysis
Joinpoint Command Line Version 4.5.0.1 (Division of Cancer Control and Population Sciences, NCI, Rockville, MD) was used to apply a Joinpoint regression analysis to the incidence, mortality, and DALY data. This software is provided for free by the US National Cancer Institute Surveillance Research Program.13 It analyzes trends in the data over the period studied and connects these trends with the simplest model possible on a logarithmic scale. The simplest model has no joinpoints and represents a straight line. As more joinpoints are added, the significance of each is tested using a Monte Carlo permutation method. An estimated annual percentage change (EAPC; with 95% CIs) for each Joinpoint line segment is also computed using the Joinpoint software and tested for significance. The result of the analyses is a series of statistically significant joinpoints for each state, with each trend (either positive or negative) represented by a potentially significant EAPC. This allows for a thorough assessment of temporal trends and for intracountry comparability.
RESULTS
Trends in Tracheal, Bronchus, and Lung Cancer Incidence
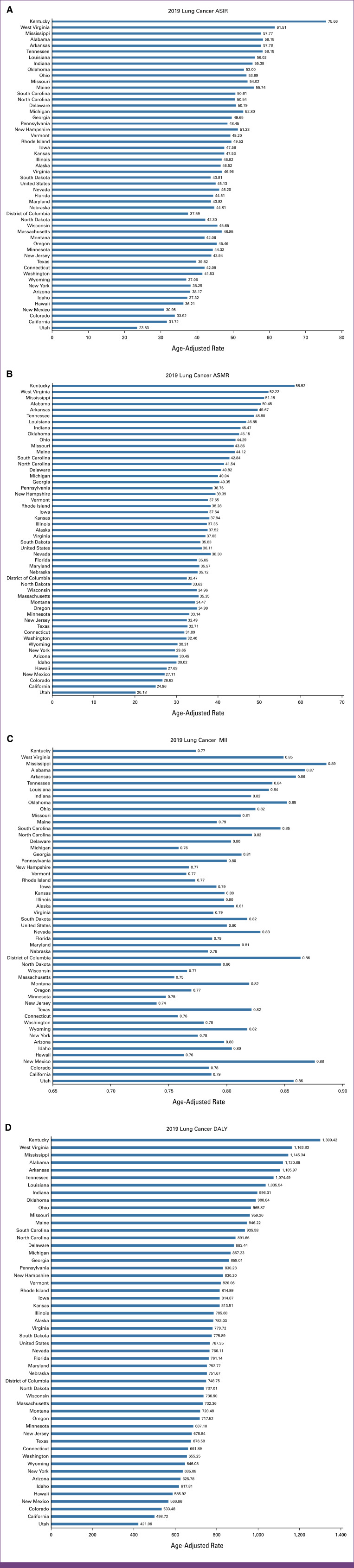
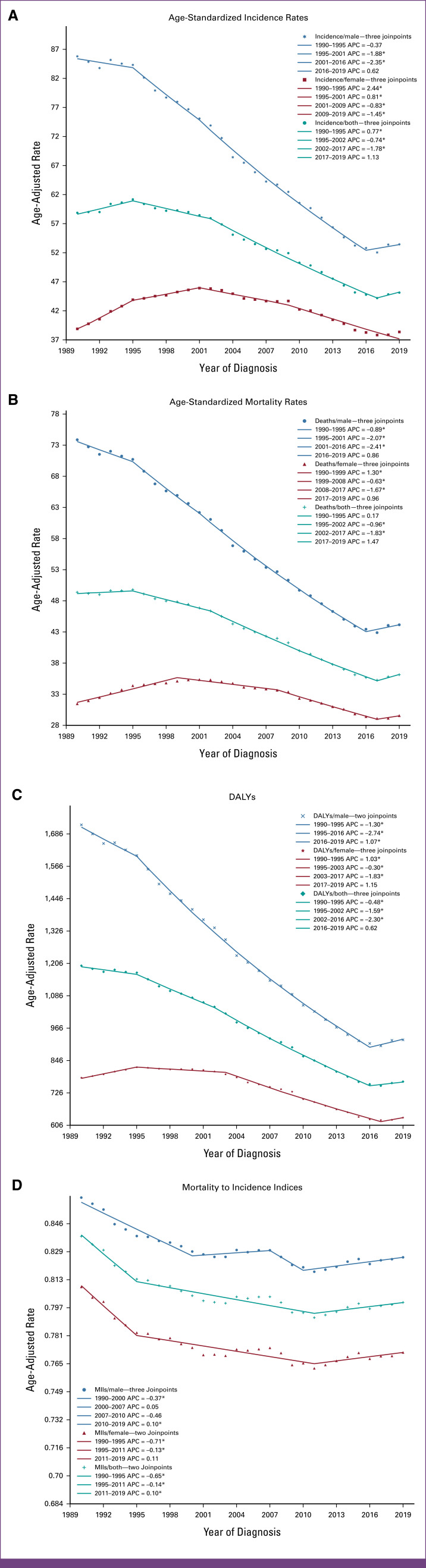
Between 1990 and 2019, the ASIR in the United States decreased by 23.35% for both sexes combined. However, the decrease was more substantial among males (37.73%) compared with a modest 1.41% decline for females. In 2019, the ASIR for the entire United States was 45.13 per 100,000, with rates of 53.44 per 100,000 for males and 38.35 per 100,000 for females (Fig 1A). Among the 51 states, which include the District of Columbia (DC), the highest ASIR was observed in Kentucky (75.66 per 10,000). By contrast, the lowest ASIR was observed in Utah (23.53 per 100,000). Nationally and across all 51 states in the United States, the ASIR showed a decrease for both sexes combined and males. However, in the case of females, ASIR was observed to be increasing in 34 of 51 states (66.7% of the total states). On performing a joinpoint trend analysis for the entire United States (Fig 2A), three joinpoints were identified for males, females, and both sexes combined. As for the overall trend for both sexes, ASIR demonstrated an increase until 1995, followed by a decrease until 2017, with a more rapid decline since 2002 (EAPC, –1.78). However, an increase in ASIR with an EAPC of 1.13 has been seen since 2017. Similar trends were observed for males, with a relative increase since 2016 (EAPC, 0.62). In the case of females, however, ASIR was found to be increasing until 2001, with a subsequent decline.
FIG 1.
(A) ASIRs, (B) ASMRs, (C) MII, and (D) DALYs for both sexes combined in 2019. All indices are per 100,000 population. ASIR, age-standardized incidence rate; ASMR, age-standardized mortality rate; DALY, disability-adjusted life year; MII, mortality-to-incidence index.
FIG 2.
Trends in (A) ASIRs, (B) ASMRs, (C) ASDR, and (D) age-standardized MIIs per 100,000 for tracheal, bronchus, and lung cancers in states of the United States between 1990 and 2019. *P < .05. APC, annual percentage change; ASDRs, age-standardized DALY rates; ASIRs, age-standardized incidence rates; ASMRs, age-standardized mortality rate; DALY, disability-adjusted life year; MIIs, mortality-to-incidence indices.
Trends in Tracheal, Bronchus, and Lung Cancer Mortality
In the United States, the ASMR decreased by 26.83% for both sexes combined. At the same time, it decreased by 40.23% for males but only by 6.01% for females. In 2019, for the entire United States, the ASMR was 36.11 per 100,000, with 44.15 per 100,000 for males and 29.58 per 100,000 for females. Among 51 states, including DC, similar to ASIR, the highest ASMR was also observed in Kentucky (58.52 per 100,000), whereas the lowest was in Utah (20.18 per 100,000). For both sexes combined and males, ASMR showed a decrease both nationwide and for all 51 states in the United States. However, for females, ASMR showed an increasing trend in 29 of 51 states (56.9%). On performing a joinpoint trend analysis for the entire United States, three joinpoints were identified for males, females, and both sexes combined. ASMR was observed to be increasing in recent years, for males (since 2016), females (since 2017), and both sexes combined (since 2017), as shown in Figure 2B.
Trends in Tracheal, Bronchus, and Lung Cancer Mortality-to-Incidence Indices
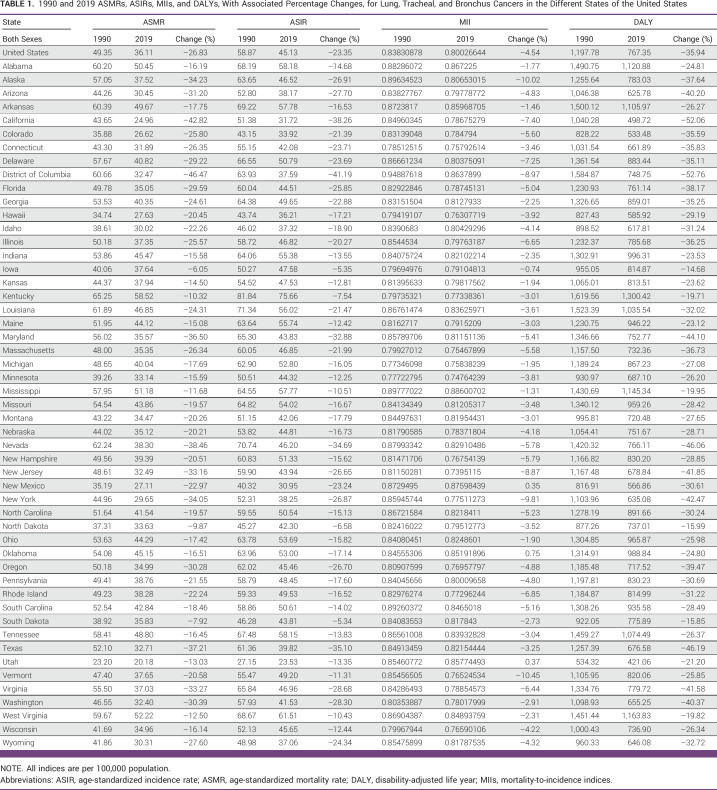
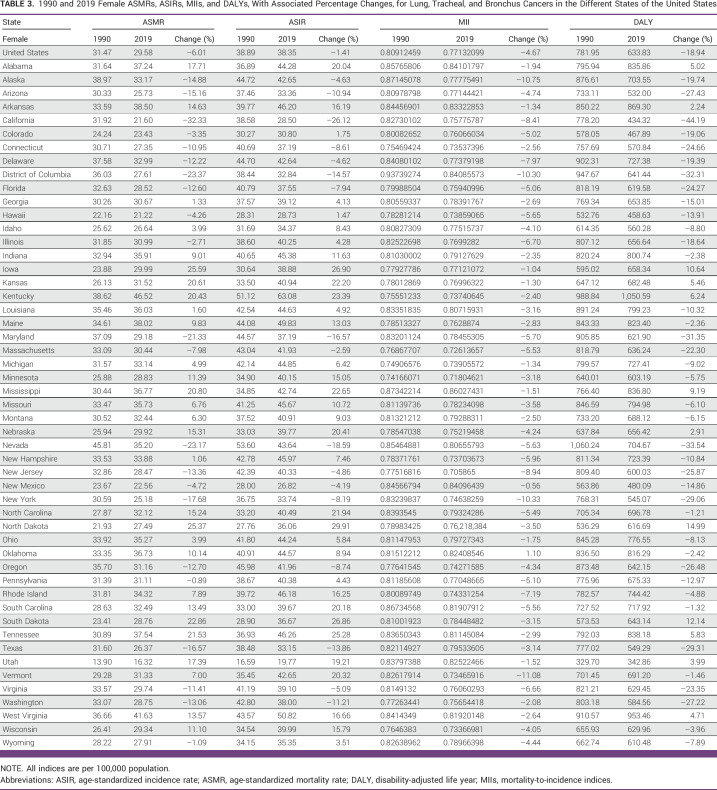
For both sexes combined, MIIs decreased by 4.54% from 1990 to 2019 for the entire United States, specifically decreasing by 4.02% for males and by 4.67% for females. Across all 51 states, MIIs decreased when observed for both sexes combined, except New Mexico, Oklahoma, and Utah. Similarly, for males, MIIs decreased in all states except in New Mexico, Oklahoma, and Utah. Although both ASMRs and ASIRs showed an increase in more than 50% of the states for females, only Oklahoma experienced an increase in MII for females (Tables 1–3).
TABLE 1.
1990 and 2019 ASMRs, ASIRs, MIIs, and DALYs, With Associated Percentage Changes, for Lung, Tracheal, and Bronchus Cancers in the Different States of the United States
TABLE 3.
1990 and 2019 Female ASMRs, ASIRs, MIIs, and DALYs, With Associated Percentage Changes, for Lung, Tracheal, and Bronchus Cancers in the Different States of the United States
TABLE 2.
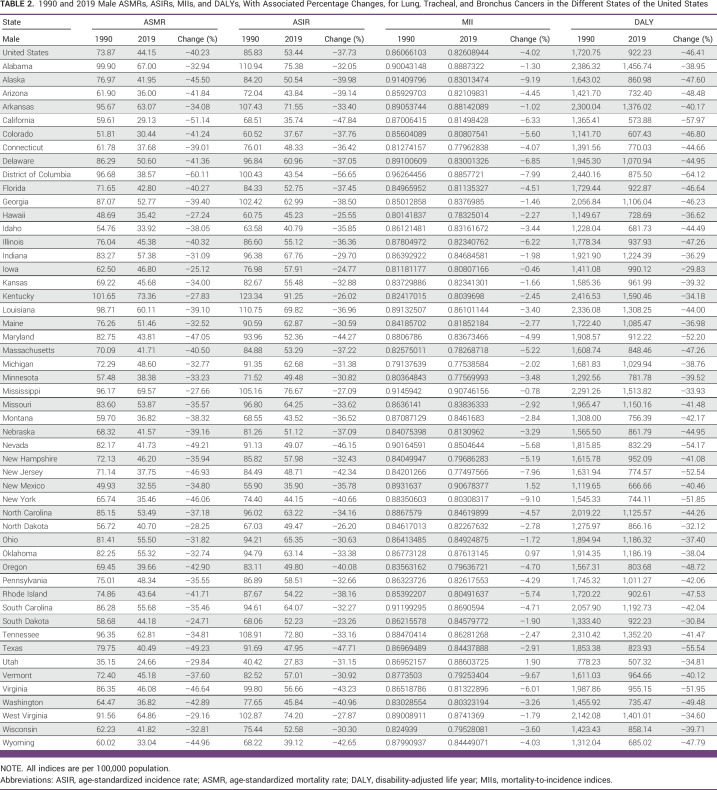
1990 and 2019 Male ASMRs, ASIRs, MIIs, and DALYs, With Associated Percentage Changes, for Lung, Tracheal, and Bronchus Cancers in the Different States of the United States
Trends in Tracheal, Bronchus, and Lung Cancer Disability-Adjusted Life Years
In the United States, DALYs decreased by 35.94% for both sexes combined. The decrease was more pronounced for males at 46.41%, but relatively lower for females at 18.94%. In 2019, the age-standardized DALY for the entire United States was 767.35 per 100,000, with rates of 922.23 per 100,000 for males and 633.83 per 100,000 for females. Among the 51 states, including DC, Kentucky had the highest DALY (1,300.42 per 10,000), whereas Utah had the lowest (421.06 per 100,000). For both sexes combined and males, DALY showed a decreasing trend nationally and across all 51 states. However, for females, DALY was found to be increasing in 12 of 51 states (23.5%). On performing a joinpoint trend analysis for the entire United States, three joinpoints were identified for males, females, and both sexes combined. Recent trends showed DALY to be increasing for males, females, and both sexes combined, as shown in Figure 2D.
DISCUSSION
In this study, we assessed the trends in incidence, mortality, MIIs, and DALYs pertaining to tracheal, bronchus, and lung cancers in the United States. For both sexes combined and for males, the incidence, mortality, and DALY rates showed a decrease, both nationally and for all 51 US states. However, these rates were observed to be increasing in most states for females.
We observed a persistent gender-based disparity in lung cancer incidence and mortality, as ASIRs and ASMRs were higher in males than females. This was concordant with our previous findings from the trends we extracted from the CDC Wonder database.14 However, the magnitude of the gap in male-female lung cancer incidence and mortality is decreasing over time. This is probably due to a relatively higher decrease in these rates in males, as opposed to an increase in females, although the latter has been described in previous studies, especially in young White and Hispanic women.15,16 Recent cancer statistics suggest an accelerating decrease in lung cancer incidence, with an absolute number of new lung cancer cases in 2022 projected to be higher in females than males for the first time in the United States.17 Some authors attributed this increasing incidence and mortality in females to their higher susceptibility to tobacco carcinogens than males.18-20 However, another study concluded that despite this higher susceptibility to tobacco carcinogens, females still demonstrate a lower mortality rate from lung cancer compared with males, suggesting that if lung cancer is more commonly curable in women, then the need to screen women at a lower threshold than men is warranted.21 Thus, the increasing incidence and mortality in females seem to be multifactorial and cannot be fully explained by smoking behaviors itself.16 A study on lung cancer screening in the Health Information National Trends Survey revealed that females were 32% less likely to be informed about lung cancer screening than males.22 At the same time, many trials described a higher benefit of the screening and early detection of lung cancer in females than in males.23-25 Thus, further work is needed to increase awareness of female lung cancer screening, and future studies are needed to evaluate possible causes, likely genetics including molecular targets (epidermal growth factor receptor [EGFR]), for increasing trends in females.
The decreasing MIIs of lung cancer can be attributed to the advancements in treatment depending on the histologic subtype. At the same time, the tobacco epidemic remains closely tied to the incidence and mortality of lung cancer, ranking as the topmost risk factor for this disease.26 Lung cancer mortality rates have shown a pattern mirroring the smoking epidemic but with an approximate 8-year lag.27,28 Notably, despite a decline in the number of smokers, there has been an increase in lung cancer cases among nonsmokers.29,30 This increase is particularly pronounced in cases of adenocarcinoma within non–small-cell lung cancer (NSCLC).29 Analysis of lung cancer histology in the United States through 2010 reveals that rates of squamous, large-cell, and small-cell carcinomas have continued to decline across all sexes and racial groups. By contrast, rates of adenocarcinoma have remained relatively steady in males and have been increasing in females.31 Furthermore, among all racial and ethnic groups, young females have exhibited higher rates of adenocarcinoma than their male counterparts.32 Overall, these trends indicate a shifting paradigm within NSCLC accompanied by a reduction in smoking habits.
Therefore, the need for biomarkers identifying the underlying genetic risk factors in nonsmokers has become a focal point of current research.33,34 The mortality from NSCLC particularly decreased substantially after the routine testing for molecular alterations in EGFR and anaplastic lymphoma kinase and the approval of targeted therapy in the United States, such as the PD-1–PD-L1 inhibitors.35,36 Despite the limited advancements in treating SCLC, the decreasing mortality relative to this histologic subtype can be attributed to a decrease in the incidence itself.31,35 As the incidence of SCLC is highly correlated with smoking, most of the decrease in its incidence is largely attributable to the significant reduction in smoking rates in the United States since the 1960s.28,37-40
The study’s strength lies in the analysis of trends rather than absolute annual mortality rates. This allows for the assessment of population-level trends over an extended observation period using the annual mortality data collected from the GBD. However, the GBD database has some limitations that the GBD study collaborators have previously elaborated on.10 The first limitation is the alteration in the data coding system and country-specific practices over the study period, particularly the transition from ICD9 to ICD10. However, the GBD authors address this by mapping mortalities to the cause-of-death lists for coding system adjustments. The second limitation would be the variable reliability of death certification, with global error rates ranging from 39% to 61%.41-43 Nonetheless, the United States was ranked among the best regions with higher quality civil registration and vital statistics.44 Furthermore, the GBD uses garbage code distribution algorithms and corrections to label deaths resulting from poorly defined diagnoses or those that cannot scientifically be the sole underlying cause of death.45,46 The third limitation is the inability to subcategorize the individual histologic subtypes of lung cancer from the GBD study result tool. This should be considered when interpreting the results as the histopathologic subtypes and stages of lung cancer result in varying clinical significance and management. Finally, our study is observational; therefore, we could not conclude causal inferences, and we could not account for certain potential confounders despite using gender-specific and age-standardized incidence and mortality rates.
In conclusion, over 30 years in the United States, the incidence, mortality, and DALYs decreased nationally and in all states in both sexes combined and males. However, the numbers were increasing in most states for females, which warrants further attention. For both sexes, MIIs decreased in all states, probably because of a decrease in incidence and advancements in treatment.
Samuel Kareff
Honoraria: Academy for Continued Healthcare Education, Integrity Continuing Education, HealthCourse, Inc, Precisca, Research to Practice
Speakers' Bureau: i3 Health
Travel, Accommodations, Expenses: FLASCO, IASLC
Carey C. Thomson
Employment: Fulcrum Therapeutics
Leadership: Fulcrum Therapeutics
Stock and Other Ownership Interests: Fulcrum Therapeutics
Consulting or Advisory Role: Median Technologies
Patents, Royalties, Other Intellectual Property: UpToDate royalties for asthma card, Springer—book lung cancer screening
Vamsidhar Velcheti
Honoraria: ITeos Therapeutics
Consulting or Advisory Role: Bristol Myers Squibb, Merck, AstraZeneca/MedImmune, GlaxoSmithKline, Amgen, Elevation Oncology, Merus, Taiho Oncology
Research Funding: Genentech (Inst), Trovagene (Inst), Eisai (Inst), OncoPlex Diagnostics (Inst), Alkermes (Inst), NantWorks (Inst), Genoptix (Inst), Altor BioScience (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Atreca (Inst), Heat Biologics (Inst), Leap Therapeutics (Inst), RSIP Vision (Inst), GlaxoSmithKline (Inst)
No other potential conflicts of interest were reported.
Footnotes
C.T.J. and H.S. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Chinmay T. Jani, Harpreet Singh, Shreya Arora, Justin Salciccioli, Vamsidhar Velcheti
Financial support: Vamsidhar Velcheti
Administrative support: Vamsidhar Velcheti
Provision of study materials or patients: Harpreet Singh, Vamsidhar Velcheti
Collection and assembly of data: Chinmay T. Jani, Shreya Arora, Justin Salciccioli, Vamsidhar Velcheti
Data analysis and interpretation: Chinmay T. Jani, Nour Abdallah, Christian Mouchati, Shreya Arora, Samuel Kareff, Justin Salciccioli, Carey C. Thomson, Vamsidhar Velcheti
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Samuel Kareff
Honoraria: Academy for Continued Healthcare Education, Integrity Continuing Education, HealthCourse, Inc, Precisca, Research to Practice
Speakers' Bureau: i3 Health
Travel, Accommodations, Expenses: FLASCO, IASLC
Carey C. Thomson
Employment: Fulcrum Therapeutics
Leadership: Fulcrum Therapeutics
Stock and Other Ownership Interests: Fulcrum Therapeutics
Consulting or Advisory Role: Median Technologies
Patents, Royalties, Other Intellectual Property: UpToDate royalties for asthma card, Springer—book lung cancer screening
Vamsidhar Velcheti
Honoraria: ITeos Therapeutics
Consulting or Advisory Role: Bristol Myers Squibb, Merck, AstraZeneca/MedImmune, GlaxoSmithKline, Amgen, Elevation Oncology, Merus, Taiho Oncology
Research Funding: Genentech (Inst), Trovagene (Inst), Eisai (Inst), OncoPlex Diagnostics (Inst), Alkermes (Inst), NantWorks (Inst), Genoptix (Inst), Altor BioScience (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Atreca (Inst), Heat Biologics (Inst), Leap Therapeutics (Inst), RSIP Vision (Inst), GlaxoSmithKline (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.CDC : Lung Cancer Statistics. 2022. https://www.cdc.gov/cancer/lung/statistics/index.htm [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. : SEER Cancer Statistics Review, 1975-2017. Bethesda, MD, National Cancer Institute, 2020. https://seer.cancer.gov/csr/1975_2017/ [Google Scholar]
- 3.National Lung Screening Trial Research Team : Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365:395-409, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ru Zhao Y, Xie X, de Koning HJ, et al. : NELSON lung cancer screening study. Cancer Imaging 11:S79-S84, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. CDC: Lung Cancer Screening, US Preventive Services Task Force. https://www.cdc.gov/cancer/lung/statistics/index.htm.
- 6.Fedewa SA, Kazerooni EA, Studts JL, et al. : State variation in low-dose computed tomography scanning for lung cancer screening in the United States. J Natl Cancer Inst 113:1044-1052, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Global Burden of Disease Collaborative Network : Global Burden of Disease Study 2019 (GBD 2019) Results. Seattle, WA, Institute for Health Metrics and Evaluation (IHME), 2020. https://vizhub.healthdata.org/gbd-results/ [Google Scholar]
- 8.Global Burden of Disease Collaborative Network : Global Burden of Disease Study 2017 (GBD 2017) Causes of Death and Nonfatal Causes Mapped to ICD Codes. Seattle, Washington, Institute for Health Metrics and Evaluation, 2018. https://ghdx.healthdata.org/record/ihme-data/gbd-2017-cause-icd-code-mappings [Google Scholar]
- 9. Global Burden of Disease Results. https://vizhub.healthdata.org/gbd-results/
- 10.Jani C, Marshall DC, Singh H, et al. : Lung cancer mortality in Europe and the USA between 2000 and 2017: An observational analysis. ERJ Open Res 7(4), 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLago AJ Jr, Singh H, Jani C, et al. : An observational epidemiological study to analyze intracerebral hemorrhage across the United States: Incidence and mortality trends from 1990 to 2017. J Stroke Cerebrovasc Dis 31:106216, 2022 [DOI] [PubMed] [Google Scholar]
- 12.Jani C, Abdallah N, Mouchati C, et al. : Trends of kidney cancer burden from 1990 to 2019 in European Union 15 + countries and World Health Organization regions. Sci Rep 12:22368, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HJ, Fay MP, Feuer EJ, et al. : Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 19:335-351, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Al Omari O, Jani C, Ahmed A, et al. : Lung cancer mortality in the United States between 1999 and 2019: An observational analysis of disparities by sex and race. Ann Am Thorac Soc 20:612-616, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel MI, McKinley M, Cheng I, et al. : Lung cancer incidence trends in California by race/ethnicity, histology, sex, and neighborhood socioeconomic status: An analysis spanning 28 years. Lung Cancer 108:140-149, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Jemal A, Miller KD, Ma J, et al. : Higher lung cancer incidence in young women than young men in the United States. N Engl J Med 378:1999-2009, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2022. CA Cancer J Clin 72:7-33, 2022 [DOI] [PubMed] [Google Scholar]
- 18.Zang EA, Wynder EL: Differences in lung cancer risk between men and women: Examination of the evidence. J Natl Cancer Inst 88:183-192, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Barrera-Rodriguez R, Morales-Fuentes J: Lung cancer in women. Lung Cancer (Auckl) 3:79-89, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mollerup S, Berge G, Baera R, et al. : Sex differences in risk of lung cancer: Expression of genes in the PAH bioactivation pathway in relation to smoking and bulky DNA adducts. Int J Cancer 119:741-744, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Henschke CI, Yip R, Miettinen OS: Women's susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA 296:180-184, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Warner ET, Lathan CS: Race and sex differences in patient provider communication and awareness of lung cancer screening in the health information National Trends Survey, 2013-2017. Prev Med 124:84-90, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Pinsky PF, Church TR, Izmirlian G, et al. : The National Lung Screening Trial: Results stratified by demographics, smoking history, and lung cancer histology. Cancer 119:3976-3983, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Koning HJ, van der Aalst CM, de Jong PA, et al. : Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 382:503-513, 2020 [DOI] [PubMed] [Google Scholar]
- 25.Becker N, Motsch E, Trotter A, et al. : Lung cancer mortality reduction by LDCT screening—Results from the randomized German LUSI trial. Int J Cancer 146:1503-1513, 2020 [DOI] [PubMed] [Google Scholar]
- 26.Leiter A, Veluswamy RR, Wisnivesky JP: The global burden of lung cancer: Current status and future trends. Nat Rev Clin Oncol 20:624-639, 2023 [DOI] [PubMed] [Google Scholar]
- 27.Kafle RC, Kim DY, Holt MM: Gender-specific trends in cigarette smoking and lung cancer incidence: A two-stage age-stratified Bayesian joinpoint model. Cancer Epidemiol 84:102364, 2023 [DOI] [PubMed] [Google Scholar]
- 28.Thun M, Peto R, Boreham J, et al. : Stages of the cigarette epidemic on entering its second century. Tob Control 21:96-101, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Pelosof L, Ahn C, Gao A, et al. : Proportion of never-smoker non-small cell lung cancer patients at three diverse institutions. J Natl Cancer Inst 109(7), 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boffetta P, Jarvholm B, Brennan P, et al. : Incidence of lung cancer in a large cohort of non-smoking men from Sweden. Int J Cancer 94:591-593, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Meza R, Meernik C, Jeon J, et al. : Lung cancer incidence trends by gender, race and histology in the United States, 1973-2010. PLoS One 10:e0121323, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis DR, Check DP, Caporaso NE, et al. : US lung cancer trends by histologic type. Cancer 120:2883-2892, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seijo LM, Peled N, Ajona D, et al. : Biomarkers in lung cancer screening: Achievements, promises, and challenges. J Thorac Oncol 14:343-357, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sears CR, Mazzone PJ: Biomarkers in lung cancer. Clin Chest Med 41:115-127, 2020 [DOI] [PubMed] [Google Scholar]
- 35.Howlader N, Forjaz G, Mooradian MJ, et al. : The effect of advances in lung-cancer treatment on population mortality. N Engl J Med 383:640-649, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vokes EE, Ready N, Felip E, et al. : Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 29:959-965, 2018 [DOI] [PubMed] [Google Scholar]
- 37.Kenfield SA, Wei EK, Stampfer MJ, et al. : Comparison of aspects of smoking among the four histological types of lung cancer. Tob Control 17:198-204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cornelius ME, Loretan CG, Wang TW, et al. : Tobacco product use among adults—United States, 2020. MMWR Morb Mortal Wkly Rep 71:397-405, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention : Map of Cigarette Use Among Adults. CDC, 2019. https://www.cdc.gov/statesystem/cigaretteuseadult.html [Google Scholar]
- 40.Trad C, Bayly J, Saint-Fort L, et al. : Adoption of tobacco- and smoke-free policies in a US national sample of postsecondary educational institutions. Am J Public Health 108:1366-1369, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burger EH, van der Merwe L, Volmink J: Errors in the completion of the death notification form. S Afr Med J 97:1077-1081, 2007 [PubMed] [Google Scholar]
- 42.Katsakiori PF, Panagiotopoulou EC, Sakellaropoulos GC, et al. : Errors in death certificates in a rural area of Greece. Rural Remote Health 7:822, 2007 [PubMed] [Google Scholar]
- 43.Lu TH, Shau WY, Shih TP, et al. : Factors associated with errors in death certificate completion: A national study in Taiwan. J Clin Epidemiol 54:232-238, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Mikkelsen L, Phillips DE, AbouZahr C, et al. : A global assessment of civil registration and vital statistics systems: Monitoring data quality and progress. Lancet 386:1395-1406, 2015 [DOI] [PubMed] [Google Scholar]
- 45.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators : Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1789-1858, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.GBD 2017 Causes of Death Collaborators : Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1736-1788, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]