Abstract
Pseudomonas aeruginosa drives heterogeneity of cyclic di-GMP signalling in biofilms as a division-of-labour strategy to maximize colonization and dispersal using the protein HecE.
Communities of genetically related organisms often tackle complex challenges using a division-of-labour strategy. Consider honeybees, which have defined social castes of workers, breeders and a queen to promote fitness of the colony1. Homo sapiens also engage in division-of-labour strategies with early humans specializing in distinct tasks to improve the overall strength and resilience of the community2. Bacteria, which were once thought to be simple automatons whose sole purpose was to replicate as fast as possible, also undertake division-of-labour strategies. Myxococcus xanthus, for example, creates macroscopic fruiting bodies by forming three distinct subpopulations of cells: stalk formers, resistant myxospores and a subset of cells that lyse, providing nutrients and enabling spore differentiation3. Division of labour also occurs during biofilm formation in Bacillus subtilis4 and during chain formation of cyanobacteria5. Now writing in Nature Microbiology, Manner et al.6 provide us with another example, this time for the Gram-negative pathogenic bacteria Pseudomonas aeruginosa. The authors discover a stochastic genetic switch that generates functionally distinct subpopulations of bacteria, which balances biofilm development and dispersal on surfaces through modulation of the second messenger, cyclic dimeric guanosine monophosphate (c-di-GMP).
P. aeruginosa grows in diverse environments ranging from the soil, to the lungs of human patients chronically infected with cystic fibrosis, to burn wounds and on catheters. To survive stresses in these environments, such as the host immune system, P. aeruginosa forms multicellular communities of bacteria known as biofilms. Biofilm formation is a costly process, and commitment to surface attachment can limit both colonization and spread to new areas. Thus, balancing a sessile biofilm-forming lifestyle with a motile, colonizing phenotype is key for P. aeruginosa. To maintain this balance, P. aeruginosa uses the signal c-di-GMP7. This signal is common to many bacterial species and, in most bacteria that have been studied, high intracellular concentrations of c-di-GMP promote bacterial biofilm formation while inhibiting cell motility. Although the general response of bacteria to changes in intracellular c-di-GMP are relatively well understood, how individual cells alter this signal in response to different environments or developmental processes, and whether this influences heterogeneity within bacterial populations, is just beginning to be studied.
Previous work by this same research team showed that c-di-GMP increases within seconds of P. aeruginosa attaching to a surface8. This activates surface adherence and virulence, specifically by inducing pili assembly8. Surface-attached bacteria will then divide and split between a sessile lifestyle or a dispersing lifestyle. The high c-di-GMP ‘striker’ cells remain attached to the surface, while low c-di-GMP ‘spreader’ cells, generated by asymmetric cell division, are motile and can move away to colonize new areas (Fig. 1). How cell heterogeneity influences further development of the biofilm was unclear, however, and given that biofilms promote P. aeruginosa resistance to the immune system and antibiotic therapy, understanding such processes is key for successful treatment of infections.
Fig. 1 |. The HecRE molecular switch regulates P. aeruginosa biofilm development and heterogeneity through c-di-GMP.
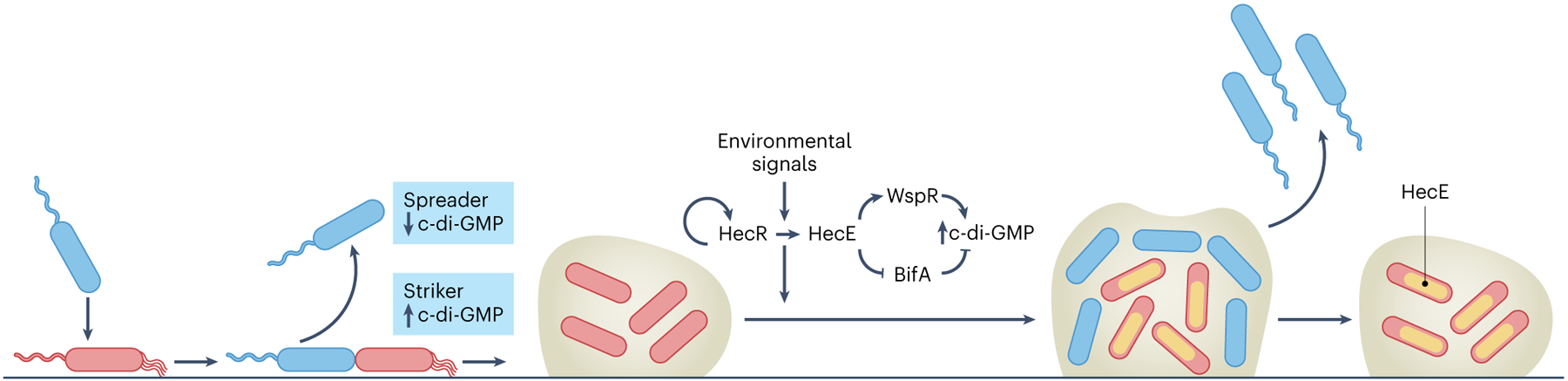
On surface encounter, P. aeruginosa attaches and undergoes asymmetric cell division. A motile, spreader cell with low c-di-GMP that colonizes new environments is generated, as well as a second, attached, striker cell with increased intracellular c-di-GMP, which initiates biofilm formation8. As the biofilm develops, environmental signals and autoregulation upregulate HecR, which induces high HecE expression in a subpopulation of cells. HecE activates the c-di-GMP synthase, WspR, and inhibits the phosphodiesterase, BifA, increasing intracellular c-di-GMP and biofilm development. Some biofilm bacteria do not induce HecE and are low in c-di-GMP. As the biofilm matures, this bacterial subpopulation disperses to colonize new areas.
In this paper, Manner et al. extended their single-cell analyses to investigate whether c-di-GMP is involved in the later stages of P. aeruginosa biofilm development. P. aeruginosa forms small colony variants (SCVs) when intracellular levels of c-di-GMP are high9, providing a simple phenotype that enabled the authors to screen transposon mutant strains of P. aeruginosa for high c-di-GMP. They discovered two genes, named hecR and hecE (for heterogeneous, environment-responsive, c-di-GMP-controlling regulator and effector, respectively), that were involved with the SCV morphotype. The authors showed that constitutive expression of hecE induced SCV formation while simultaneously increasing surface attachment, suggesting it increases intracellular c-di-GMP levels. HecE accomplishes this by activating WspR, a diguanylate cyclase that synthesizes c-di-GMP, via an unknown mechanism. It also inhibits the c-di-GMP-degrading BifA phosphodiesterase, through a direct protein–protein interaction. Overall, this increases signal levels. Both WspR and BifA were previously implicated as important modulators of c-di-GMP and biofilm formation in P. aeruginosa. In the absence of bifA and wspR, hecE no longer impacted biofilm formation, suggesting that HecE exclusively mediates changes in c-di-GMP through these factors (Fig. 1).
Intriguingly, using fluorescent reporters of HecE and c-di-GMP, the authors found that HecE expression is not uniform throughout the biofilm. Rather, stochastic expression of hecE is seen that controls P. aeruginosa biofilm formation and dispersal (Fig. 1). In early stages of the biofilm, cells expressing hecE, and those that do not, coexisted in the biofilm. However, during mid-stage biofilm formation, cells with low hecE expression dispersed because they had low c-di-GMP and higher motility. But how is this ratio of biofilm-forming and biofilm-dispersing cells determined? The proportion of cells highly expressing HecE is controlled by the transcriptional regulator HecR, which is encoded in an operon with hecE. In response to specific environmental cues, HecR activates the hecRE operon in a positive feedback loop, leading to increased HecR and HecE expression (Fig. 1). Thus, in some environments, the P. aeruginosa biofilm is geared towards enhanced biofilm development, while in other conditions more members of the community are primed for dispersal.
As the HecRE circuit is a key component of biofilm formation and dispersal, and biofilm formation is problematic during P. aeruginosa infections, understanding this pathway provides opportunities for treatment of these infections. For example, this work demonstrates that HecE inhibits biofilm dispersal by inhibiting BifA. Inhibitors of HecE would be predicted to promote dispersal of P. aeruginosa biofilms, thereby potentially clearing these infections and rendering them sensitive to antibiotic treatment. Alternatively, in some cases promotion of biofilm formation is beneficial. For example, Manner et al. found that some phages, which are viruses of bacteria, can target the P. aeruginosa biofilm extracellular matrix. As HecE, through c-di-GMP, induces extracellular matrix production, phages can specifically infect and kill HecE-expressing cells. As phages are being used to treat infections, in an approach called phage therapy, activation of the HecRE pathway could alternatively be beneficial in the presence of specific phages.
The study by Manner et al. expands our understanding of how P. aeruginosa uses c-di-GMP to optimize adherence, dispersal and virulence by generating a heterogeneous population of cells with some that stay put in a biofilm and some cells that disperse driven by the proteins HecR and HecE (Fig. 1). Although much research has yielded an understanding of the overarching principles of c-di-GMP function and signalling networks, studies such as the one by Manner et al. highlight how single-cell analyses are essential. Delving into the mechanisms behind population complexity is key to a deeper understanding of this central signalling pathway and how it regulates heterogeneity. The impact of biofilms is far reaching, from industrial applications to biofouling and infections. Understanding the genetic switches, such as hecRE, that control biofilm formation has the potential to provide powerful approaches to manipulate biofilm formation, both positively and negatively, as needed for the given application.
Acknowledgements
This work was supported by National Institutes of Health (NIH) grants GM139537 and AI58433 to C.M.W.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Pernal SF Vet. Clin. North Am. Food Anim. Pract 37, 387–400 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Nakahashi W & Feldman MW J. Theor. Biol 348, 65–79 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Muñoz-Dorado J, Marcos-Torres FJ, García-Bravo E, Moraleda-Muñoz A & Pérez J Front. Microbiol 7, 781 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Gestel J, Vlamakis H & Kolter R PLoS Biol. 13, e1002141 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossetti V & Bagheri HC Proc. R. Soc. B Biol. Sci 279, 3457–3466 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manner C et al. Nat. Microbiol 10.1038/s41564-023-01403-0 (2023). [DOI] [Google Scholar]
- 7.Jenal U, Reinders A & Lori C Nat. Rev. Microbiol 15, 271–284 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Laventie B-J et al. Cell Host Microbe 25, 140–152.e6 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Malone JG et al. PLoS Pathog. 6, e1000804 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]


