Abstract
Background and Objectives
There is an urgent need to identify novel noninvasive biomarkers for Alzheimer disease (AD) diagnosis. Recent advances in blood-based measurements of phosphorylated tau (pTau) species are promising but still insufficient to address clinical needs. Epigenetics has been shown to be helpful to better understand AD pathogenesis. Epigenetic biomarkers have been successfully implemented in other medical disciplines, such as oncology. The objective of this study was to explore the diagnostic accuracy of a blood-based DNA methylation marker panel as a noninvasive tool to identify patients with late-onset Alzheimer compared with age-matched controls.
Methods
A case-control study was performed. Blood DNA methylation levels at 46 cytosine-guanine sites (21 genes selected after a comprehensive literature search) were measured by bisulfite pyrosequencing in patients with “probable AD dementia” following National Institute on Aging and the Alzheimer's Association guidelines (2011) and age-matched and sex-matched controls recruited at Neurology Department-University Hospital of Navarre, Spain, selected by convenience sampling. Plasma pTau181 levels were determined by Simoa technology. Multivariable logistic regression analysis was performed to explore the optimal model to discriminate patients with AD from controls. Furthermore, we performed a stratified analysis by sex.
Results
The final study cohort consisted of 80 patients with AD (age: median [interquartile range] 79 [11] years; 58.8% female) and 100 cognitively healthy controls (age 77 [10] years; 58% female). A panel including DNA methylation levels at NXN, ABCA7, and HOXA3 genes and plasma pTau181 significantly improved (area under the receiver operating characteristic curve 0.93, 95% CI 0.89–0.97) the diagnostic performance of a single pTau181-based model, adjusted for age, sex, and APOE ɛ4 genotype. The sensitivity and specificity of this panel were 83.30% and 90.00%, respectively. After sex-stratified analysis, HOXA3 DNA methylation levels showed consistent association with AD.
Discussion
These results highlight the potential translational value of blood-based DNA methylation biomarkers for noninvasive diagnosis of AD.
Registration Information
Research Ethics Committee of the University Hospital of Navarre (PI17/02218).
Introduction
In a world with an increasingly aging population, early and accurate diagnosis and successful treatment of diseases related to age, such as Alzheimer disease (AD), is a top research priority.1 AD is considered a disease continuum encompassing different stages: preclinical AD (asymptomatic but with evidence of AD pathology), prodromal AD (symptomatic of brain dysfunction due to AD pathology), and eventually AD dementia.1,2 Current AD biomarkers, including core biomarkers in CSF and PET imaging tests, change across the AD continuum and mirror the characteristic neuropathologic changes described in AD, that is, extracellular β-amyloid (Aβ) plaques and neurofibrillary tangles made up of hyperphosphorylated tau protein (pTau).3 However, current AD biomarkers have a number of limitations, among others, because of limited accessibility and high costs. Thus, accurate diagnosis of patients with AD remains a challenge and is one of the reasons why clinical trials have failed in recent years.4
Most recently, the focus has shifted to the search for blood biomarkers as a source of easily accessible, noninvasive, and cost-effective alternative diagnostic methods. For instance, measurement of plasma pTau181 and other phosphoforms of Tau has revealed as a promising diagnostic tool since it can discriminate between amyloid PET–positive and amyloid PET–negative individuals along the AD continuum with an area under the receiver operating characteristic (ROC) curve (AUC) of up to 90%, being able to predict progression to AD dementia.5-9 Indeed, blood-based biomarkers could be very practical in primary care outpatient centers because screening tools in the population with complaints of memory loss in which any of the other possible causes, other than AD, have been excluded. These potential screening tools would serve as a criterion for referral to specialized centers for further specific tests (such as CSF biomarkers or PET) in case of positive suspicion. However, these blood-based biomarkers are not yet applicable in clinical practice.10,11
AD is a multifactorial disease dependent on numerous risk factors such as age, sex, and APOE ɛ4 allele and has also been associated with other genetic and environmental factors,12 which interact through epigenetic mechanisms. DNA methylation is an epigenetic modification in which a methyl group is attached at carbon 5 of a cytosine residue (5 mC) occurring in cytosine-guanine (CpG) dinucleotides. These CpG dinucleotides are frequently located in gene promoters and other regulatory regions clustered in the genome constituting “CpG islands.”13 Methylation of CpG islands is thought to be involved in the regulation of gene expression, whereas changes in methylation levels of isolated CpG dinucleotides are usually not sufficient to affect nearby gene expression. However, identifying differential methylation at CpG sites could be useful as potential epigenetic biomarkers. Different brain regions, such as entorhinal cortex, prefrontal cortex, hippocampus, or superior temporal gyrus, have been studied across the AD continuum by us and other authors14-22 in recent years. A number of AD-related DNA methylation marks have been identified located in a variety of genes including ankyrin 1 (ANK1), bridging integrator 1 (BIN1), CREB-regulated transcription coactivator 1 (CRTC1), brain-derived neurotrophic factor (BDNF), homeobox A3 (HOXA3), insulin receptor substrate 2 (IRS2), nucleoredoxin (NXN), phospholipase D family member 3 (PLD3), and triggering receptor expressed on myeloid cells 2 (TREM2), among others. These results suggest that DNA methylation is somehow involved in AD development, but these potential epigenetic marks remain inaccessible while patients are alive.
In this context, blood-based DNA methylation markers associated with AD and other neurodegenerative diseases, such as sporadic Creutzfeldt-Jakob disease,23 have begun to be studied, mainly through epigenome-wide association studies (EWASs). Whole-blood DNA methylation was explored, and a number of differentially methylated positions (DMPs) were determined in the homeobox B6 (HOXB6) gene in AD.24 DNA methylation at the oxytocin (OXT) gene promoter was proposed as a promising early biomarker of AD.25 In addition, a differentially methylated region (DMR) located in adenosine deaminase RNA–specific B2 (ADARB2) gene was detected in blood of twin pairs discordant for AD.26 Moreover, a candidate gene approach was followed to identify DNA methylation changes at BIN127 and BDNF genes in the blood samples of patients with AD.28
Despite this research, no epigenetic biomarker consistently associated with AD has been identified to date nor has a useful biomarker panel been proposed. Hence, the aim of this study was to identify and assess the performance of a blood-based panel of DNA methylation markers that could be potentially helpful in the diagnosis of AD and to explore whether this panel would improve the diagnostic value of plasma pTau181 levels.
Methods
Study Design and Participants
In this case-control study, 180 participants from the iBEAS cohort were recruited from the Dementia Clinics (Neurology Department-University Hospital of Navarre, Spain) from March 13, 2019, to June 30, 2021. iBEAS cohort was established to characterize blood-based biomarkers of epigenetic origin in patients with late-onset dementia of Alzheimer type. Diagnosis of late-onset AD was performed by neurologists as detailed in the eMethods (links.lww.com/WNL/D203). The sample size was calculated using the epiR package for providing 80% power to predict a minimum 5% significant difference in DNA methylation levels between AD cases and controls, assuming a 2-sided significance level of α = 0.05 by the independent samples t test. The sample size calculation resulted in 70 patients with AD vs 70 controls. Available imaging data of controls were also used to exclude any brain pathology that might confound our results.
Blood Samples Collection and DNA Isolation
EDTA plasma samples were obtained through venipuncture and centrifuged at 2,000g for 10 minutes, at 4°C, within 2 hours. Plasma was aliquoted into 1.5-mL tubes and stored at −80°C until testing. From the buffy coat, peripheral blood leukocyte (PBL) DNA was isolated by using the FlexiGene DNA kit (Qiagen, Redwood City, CA). A Nanodrop spectrophotometer (Thermo Fisher Scientific, Yokohama, Japan) was used to measure DNA concentration and purity.
Candidate Genes Selection
A comprehensive literature review using PubMed was conducted to select candidate epigenetic marks described before June 2021 in the brain or blood of patients with AD to be tested in the iBEAS cohort. For the search, the MeSH terms used were “Alzheimer's disease,” “DNA methylation,” “brain,” “peripheral blood” and “epigenetic.” Finally, 21 CpG sites (CpGs) with highest scores were selected to study their methylation levels in PBL DNA samples derived from the iBEAS cohort (Table 1). To facilitate reproducibility of the results, the identification alias for those CpGs reported from Illumina methylation arrays was retained. This selection compiles CpGs found (1) in hippocampal tissue by our group,14 (2) in brain tissue through EWAS or candidate gene study,16,25,27,29,30 and (3) also in PBLs through EWAS or candidate gene study.25,26,28,31-33 A score was developed based on the number and type of studies in which each of the CpGs had been found and then top-21 CpGs were prioritized (eTable 1, links.lww.com/WNL/D206). A higher weight was assigned when the CpGs overlapped both brain tissue and PBLs. For the purpose of this study, CpG designates the candidate positions based on literature search and DMPs refers to CpGs that have been found differentially methylated in the iBEAS cohort.
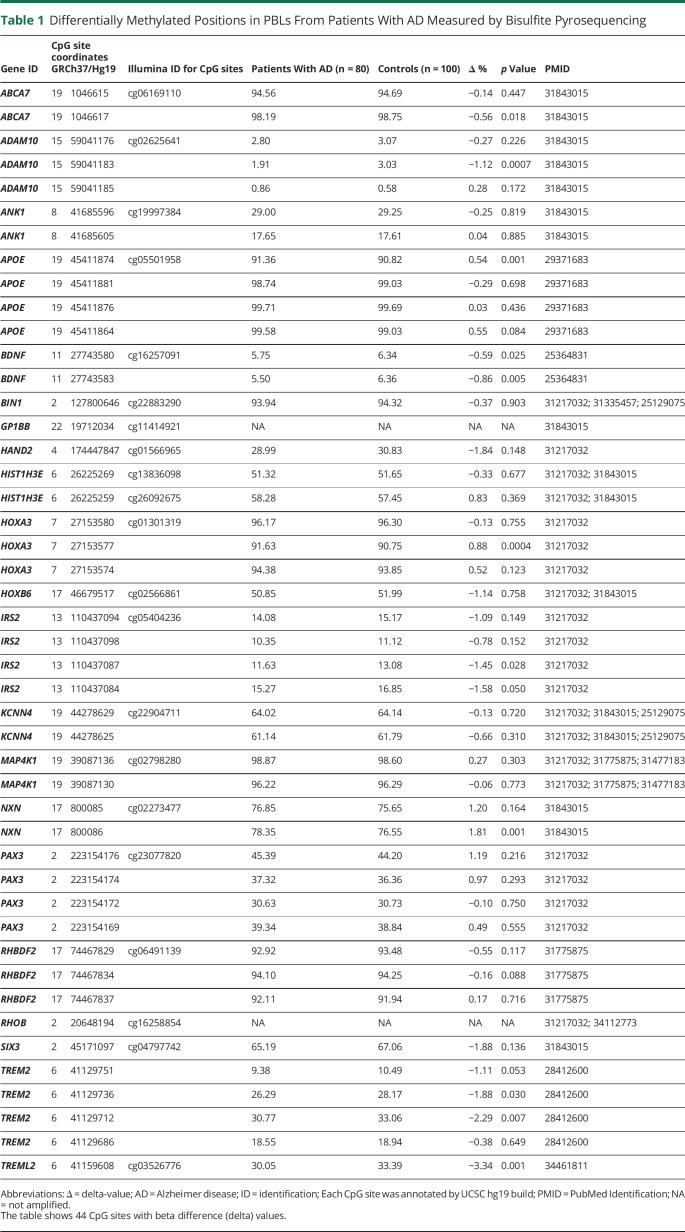
Table 1.
Differentially Methylated Positions in PBLs From Patients With AD Measured by Bisulfite Pyrosequencing
| Gene ID | CpG site coordinates GRCh37/Hg19 | Illumina ID for CpG sites | Patients With AD (n = 80) | Controls (n = 100) | Δ % | p Value | PMID | |
| ABCA7 | 19 | 1046615 | cg06169110 | 94.56 | 94.69 | −0.14 | 0.447 | 31843015 |
| ABCA7 | 19 | 1046617 | 98.19 | 98.75 | −0.56 | 0.018 | 31843015 | |
| ADAM10 | 15 | 59041176 | cg02625641 | 2.80 | 3.07 | −0.27 | 0.226 | 31843015 |
| ADAM10 | 15 | 59041183 | 1.91 | 3.03 | −1.12 | 0.0007 | 31843015 | |
| ADAM10 | 15 | 59041185 | 0.86 | 0.58 | 0.28 | 0.172 | 31843015 | |
| ANK1 | 8 | 41685596 | cg19997384 | 29.00 | 29.25 | −0.25 | 0.819 | 31843015 |
| ANK1 | 8 | 41685605 | 17.65 | 17.61 | 0.04 | 0.885 | 31843015 | |
| APOE | 19 | 45411874 | cg05501958 | 91.36 | 90.82 | 0.54 | 0.001 | 29371683 |
| APOE | 19 | 45411881 | 98.74 | 99.03 | −0.29 | 0.698 | 29371683 | |
| APOE | 19 | 45411876 | 99.71 | 99.69 | 0.03 | 0.436 | 29371683 | |
| APOE | 19 | 45411864 | 99.58 | 99.03 | 0.55 | 0.084 | 29371683 | |
| BDNF | 11 | 27743580 | cg16257091 | 5.75 | 6.34 | −0.59 | 0.025 | 25364831 |
| BDNF | 11 | 27743583 | 5.50 | 6.36 | −0.86 | 0.005 | 25364831 | |
| BIN1 | 2 | 127800646 | cg22883290 | 93.94 | 94.32 | −0.37 | 0.903 | 31217032; 31335457; 25129075 |
| GP1BB | 22 | 19712034 | cg11414921 | NA | NA | NA | NA | 31843015 |
| HAND2 | 4 | 174447847 | cg01566965 | 28.99 | 30.83 | −1.84 | 0.148 | 31217032 |
| HIST1H3E | 6 | 26225269 | cg13836098 | 51.32 | 51.65 | −0.33 | 0.677 | 31217032; 31843015 |
| HIST1H3E | 6 | 26225259 | cg26092675 | 58.28 | 57.45 | 0.83 | 0.369 | 31217032; 31843015 |
| HOXA3 | 7 | 27153580 | cg01301319 | 96.17 | 96.30 | −0.13 | 0.755 | 31217032 |
| HOXA3 | 7 | 27153577 | 91.63 | 90.75 | 0.88 | 0.0004 | 31217032 | |
| HOXA3 | 7 | 27153574 | 94.38 | 93.85 | 0.52 | 0.123 | 31217032 | |
| HOXB6 | 17 | 46679517 | cg02566861 | 50.85 | 51.99 | −1.14 | 0.758 | 31217032; 31843015 |
| IRS2 | 13 | 110437094 | cg05404236 | 14.08 | 15.17 | −1.09 | 0.149 | 31217032 |
| IRS2 | 13 | 110437098 | 10.35 | 11.12 | −0.78 | 0.152 | 31217032 | |
| IRS2 | 13 | 110437087 | 11.63 | 13.08 | −1.45 | 0.028 | 31217032 | |
| IRS2 | 13 | 110437084 | 15.27 | 16.85 | −1.58 | 0.050 | 31217032 | |
| KCNN4 | 19 | 44278629 | cg22904711 | 64.02 | 64.14 | −0.13 | 0.720 | 31217032; 31843015; 25129075 |
| KCNN4 | 19 | 44278625 | 61.14 | 61.79 | −0.66 | 0.310 | 31217032; 31843015; 25129075 | |
| MAP4K1 | 19 | 39087136 | cg02798280 | 98.87 | 98.60 | 0.27 | 0.303 | 31217032; 31775875; 31477183 |
| MAP4K1 | 19 | 39087130 | 96.22 | 96.29 | −0.06 | 0.773 | 31217032; 31775875; 31477183 | |
| NXN | 17 | 800085 | cg02273477 | 76.85 | 75.65 | 1.20 | 0.164 | 31843015 |
| NXN | 17 | 800086 | 78.35 | 76.55 | 1.81 | 0.001 | 31843015 | |
| PAX3 | 2 | 223154176 | cg23077820 | 45.39 | 44.20 | 1.19 | 0.216 | 31217032 |
| PAX3 | 2 | 223154174 | 37.32 | 36.36 | 0.97 | 0.293 | 31217032 | |
| PAX3 | 2 | 223154172 | 30.63 | 30.73 | −0.10 | 0.750 | 31217032 | |
| PAX3 | 2 | 223154169 | 39.34 | 38.84 | 0.49 | 0.555 | 31217032 | |
| RHBDF2 | 17 | 74467829 | cg06491139 | 92.92 | 93.48 | −0.55 | 0.117 | 31775875 |
| RHBDF2 | 17 | 74467834 | 94.10 | 94.25 | −0.16 | 0.088 | 31775875 | |
| RHBDF2 | 17 | 74467837 | 92.11 | 91.94 | 0.17 | 0.716 | 31775875 | |
| RHOB | 2 | 20648194 | cg16258854 | NA | NA | NA | NA | 31217032; 34112773 |
| SIX3 | 2 | 45171097 | cg04797742 | 65.19 | 67.06 | −1.88 | 0.136 | 31843015 |
| TREM2 | 6 | 41129751 | 9.38 | 10.49 | −1.11 | 0.053 | 28412600 | |
| TREM2 | 6 | 41129736 | 26.29 | 28.17 | −1.88 | 0.030 | 28412600 | |
| TREM2 | 6 | 41129712 | 30.77 | 33.06 | −2.29 | 0.007 | 28412600 | |
| TREM2 | 6 | 41129686 | 18.55 | 18.94 | −0.38 | 0.649 | 28412600 | |
| TREML2 | 6 | 41159608 | cg03526776 | 30.05 | 33.39 | −3.34 | 0.001 | 34461811 |
Abbreviations: Δ = delta-value; AD = Alzheimer disease; ID = identification; Each CpG site was annotated by UCSC hg19 build; PMID = PubMed Identification; NA = not amplified.
The table shows 44 CpG sites with beta difference (delta) values.
DNA Methylation Assays by Bisulfite Pyrosequencing
Genomic DNA isolated from PBLs (500 ng per sample) was bisulfite converted using the EpiTect Bisulfite Kit (Qiagen). Primers to amplify and sequence the target region were designed to amplify the selected CpG, in specific cases, nearby CpGs, and were designed with PyroMark Assay Design version 2.0.1.15 (Qiagen) using converted DNA as a template (eTable 2, links.lww.com/WNL/D206), and bisulfite PCR reactions were performed on a Veriti Thermal Cycler (Applied Biosystems, Foster City, CA). Next, 20 μL of biotinylated PCR product was immobilized using streptavidin-coated sepaharose beads (GE Healthcare Life Sciences, Piscataway, NJ), and 0.4 µM sequencing primer was annealed to purified DNA strands. Pyrosequencing was performed using the PyroMark Gold Q96 reagents (Qiagen) on a PyroMark Q96 ID System (Qiagen), as explained in the work of Blanco-luquin et al. 2020.34 For each particular CpG, methylation levels were calculated with PyroMark Q96 software and expressed as the percentage of methylated cytosines over the sum of total cytosines. Unmethylated and methylated DNA samples (EpiTect PCR Control DNA Set; Qiagen) were used as controls for the pyrosequencing reaction. DNA methylation levels range from 0 to 1, and delta value (Δ) measures the absolute difference in DNA methylation levels between the mean value in cases and controls.
Plasma pTau181 Measurement
Plasma pTau181 was measured in samples from a subgroup of 70 patients with AD and 70 controls using the commercially available pTau-181 V2 Advantage kit (Quanterix Corp., Billerica, MA),35 with single-molecule array (Simoa) technology at the Sant Pau Memory Unit's laboratory (Barcelona, Spain).
Statistical Analysis
Comparisons between patients with AD and controls were performed using the χ2 test for categorical variables, such as sex or APOE ɛ4 genotype, and using the Student t test or the Mann-Whitney U test for continuous variables, depending on their distribution. After excluding outliers (any value outside of first and third quartile ±1.5 × interquartile range [IQR]), the AUC together with their 95% CI provided the diagnostic accuracy of each DMP and plasma pTau181 levels. AUC values were calculated for those 15 DMPs with a p value <0.10 (Table 1) and for plasma pTau181 levels (Table 2, eFigure 1, eFigure 2A, links.lww.com/WNL/D205). To evaluate the independent relationship between each DMP and disease status, multivariable logistic regression models (LRMs) adjusted for age, sex, and APOE ɛ4 genotype were performed including continues variables. Finally, we evaluated 3 different LRMs adjusted for age, sex, and APOE ɛ4 genotype by logistic regression analysis where selected variables according to statistical criteria (univariate analysis) were included simultaneously in the model. Then, those that did not remain statistically significant were sequentially removed using the backward elimination method. Odd ratios (ORs) for each model were estimated together with their 95% CIs. The diagnostic accuracy of each model was described using the AUC together with their 95% CIs. Using a bootstrapping approach, we performed internal validation of models to evaluate their performance. Multiple imputations were performed to compare model performances before using the DeLong test. Missing values were replaced by plausible values (“imputed values”) from the median of 30 data sets by multiple imputations. Finally, stratified analysis by sex was also performed. p value was stablished at 0.05 as the cutoff point for statistical significance, except for intergroup differences of DNA methylation level which were corrected by Bonferroni. Statistical analyses were conducted with SPSS 25.0 (IBM Corp., Armonk, NY) and R (version 3.6.2, packages plugins, pROC), and figures were drawn with GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, CA).
Table 2.
DMPs in AD PBLs Measured by Bisulfite Pyrosequencing and pTau181 Levels
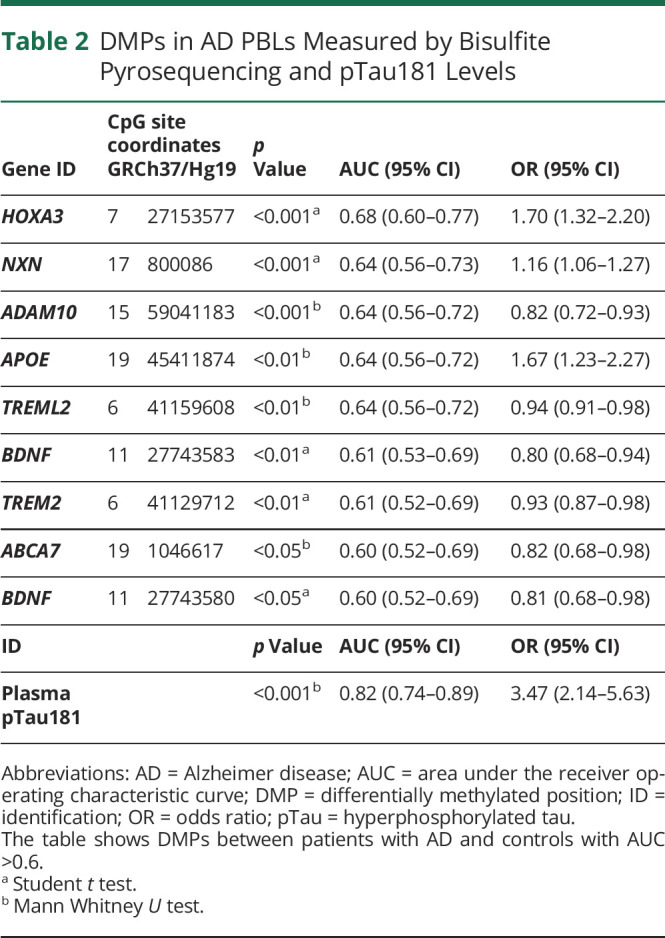
| Gene ID | CpG site coordinates GRCh37/Hg19 | p Value | AUC (95% CI) | OR (95% CI) | |
| HOXA3 | 7 | 27153577 | <0.001a | 0.68 (0.60–0.77) | 1.70 (1.32–2.20) |
| NXN | 17 | 800086 | <0.001a | 0.64 (0.56–0.73) | 1.16 (1.06–1.27) |
| ADAM10 | 15 | 59041183 | <0.001b | 0.64 (0.56–0.72) | 0.82 (0.72–0.93) |
| APOE | 19 | 45411874 | <0.01b | 0.64 (0.56–0.72) | 1.67 (1.23–2.27) |
| TREML2 | 6 | 41159608 | <0.01b | 0.64 (0.56–0.72) | 0.94 (0.91–0.98) |
| BDNF | 11 | 27743583 | <0.01a | 0.61 (0.53–0.69) | 0.80 (0.68–0.94) |
| TREM2 | 6 | 41129712 | <0.01a | 0.61 (0.52–0.69) | 0.93 (0.87–0.98) |
| ABCA7 | 19 | 1046617 | <0.05b | 0.60 (0.52–0.69) | 0.82 (0.68–0.98) |
| BDNF | 11 | 27743580 | <0.05a | 0.60 (0.52–0.69) | 0.81 (0.68–0.98) |
| ID | p Value | AUC (95% CI) | OR (95% CI) | ||
| Plasma pTau181 | <0.001b | 0.82 (0.74–0.89) | 3.47 (2.14–5.63) | ||
Abbreviations: AD = Alzheimer disease; AUC = area under the receiver operating characteristic curve; DMP = differentially methylated position; ID = identification; OR = odds ratio; pTau = hyperphosphorylated tau.
The table shows DMPs between patients with AD and controls with AUC >0.6.
Student t test.
Mann Whitney U test.
Standard Protocol Approvals, Registrations, and Patient Consents
The Research Ethics Committee of the University Hospital of Navarre approved this study (PI17/02218), and, before enrollment, written informed consent was obtained from all participants or their legal guardians.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Characteristics of the iBEAS Cohort Participants
Demographic and clinical characteristics of the iBEAS cohort participants (n = 180) are listed in eTable 3 (links.lww.com/WNL/D206). The final study cohort consisted of 80 patients (older than 65 years), who were diagnosed as dementia according to DSM-III-R and as Alzheimer's etiology following the National Institute on Aging and Alzheimer's Association criteria revised in 2011.3 Age-matched individuals (older than 65 years) with normal cognitive function evaluated by clinical interview and Mini-Mental State Examination (MMSE) (score >27) were selected as cognitively healthy controls (eTable 3). No significant differences regarding age and sex were found between groups. As expected, APOE ɛ4 carriers were overrepresented in patients with AD compared with controls, and a lower score in MMSE test and Global Deterioration Scale was observed for patients with AD. Further description of participants' characterization is described in eMethods (links.lww.com/WNL/D203).
DNA Methylation Levels of Candidate Genes in Blood
To identify blood-based DNA methylation markers to differentiate between patients with AD and controls, 46 CpGs related to 21 differentially methylated genes selected after an extensive literature search were assayed by bisulfite pyrosequencing in PBL samples from the iBEAS cohort (Table 1). Primers sets for GP1BB and RHOB genes failed to correctly amplify the corresponding amplicon and thus the study of these genes was halted. Thus, 44 CpG sites related to 19 differentially methylated genes passed to the analysis stage.
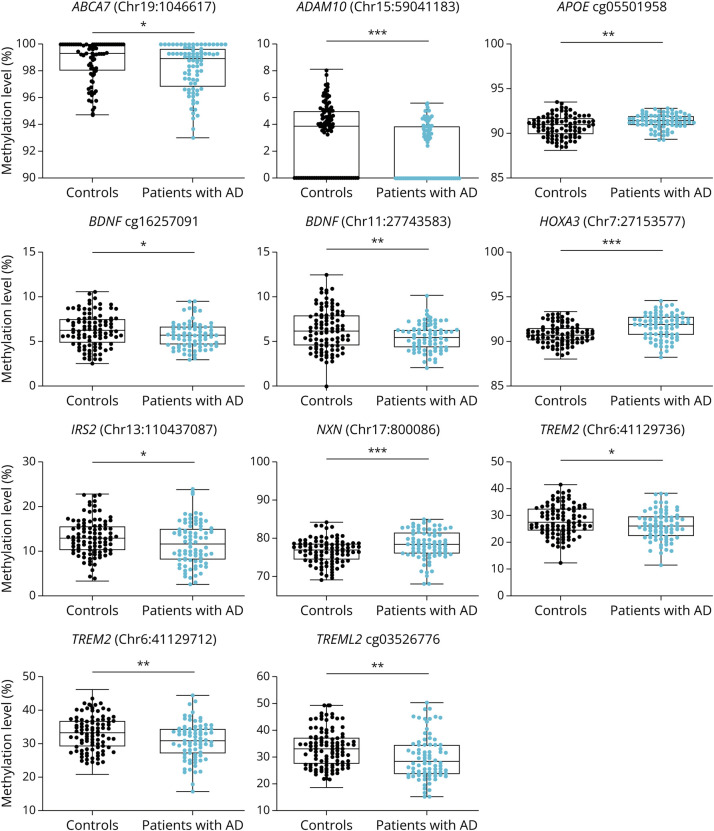
Bivariate analysis revealed statistically significant differences between groups for 11 of the 44 CpGs assessed, corresponding to 9 genes (Figure 1, Table 1). Most of the DMPs, 29 (61%), were hypomethylated in AD cases compared with controls. After correcting for multiple comparisons by the Bonferroni test (adjusted p value: 0.05/44 = 0.001), only DNA methylation levels of DMPs located in ADAM10 (chr15:59041183), HOXA3 (chr7:27153577), and NXN (chr17:800086) genes remained significantly different between groups. DNA methylation levels of DMPs located in ADAM10 gene were lower in patients with AD compared with controls (Δ = 0.011, p < 0.001) and DNA methylation levels of DMPs located in HOXA3 and NXN genes were higher in patients with AD when compared with controls (Δ = 0.009, p < 0.001; Δ = 0.018, p < 0.001, respectively).
Figure 1. DNA Methylation Levels in PBLs From AD vs Controls.
The panel shows boxplots which represent the percentage of DNA methylation for ABCA7, ADAM10, APOE, BDNF, HOXA3, IRS2, NXN, TREM2, and TREML2 genes in PBLs measured by pyrosequencing. *p < 0.05; **p < 0.01; ***p < 0.001. AD = Alzheimer disease; PBL = peripheral blood leukocyte.
Diagnostic Performance of Blood-Based DNA Methylation Markers
We next determined the performance of each of the blood-based DNA methylation markers (referred to as DMPs). Nine DMPs corresponding to 8 genes showed AUC >0.6 (Table 2, eFigure 1, links.lww.com/WNL/D205). HOXA3 (Chr7:27153577) had the highest AUC with a value of 0.683, followed by NXN (Chr17:800086) and ADAM10 (Chr15:59041183) with AUCs of 0.643 and 0.641, respectively.
Plasma Levels of pTau181
As expected,36 Simoa assay revealed higher plasma pTau181 levels in patients with AD (2.69 pg/mL, IQR = 1.56) compared with controls (1.52 pg/mL, IQR = 0.91) (p < 0.001) (eFigure 2B, links.lww.com/WNL/D205, eTable 3, links.lww.com/WNL/D206). In addition, the diagnostic accuracy of plasma pTau181 was shown to be high, with an AUC of 0.815 (Table 2, eFigure 2A).
Predictive Models of AD Status
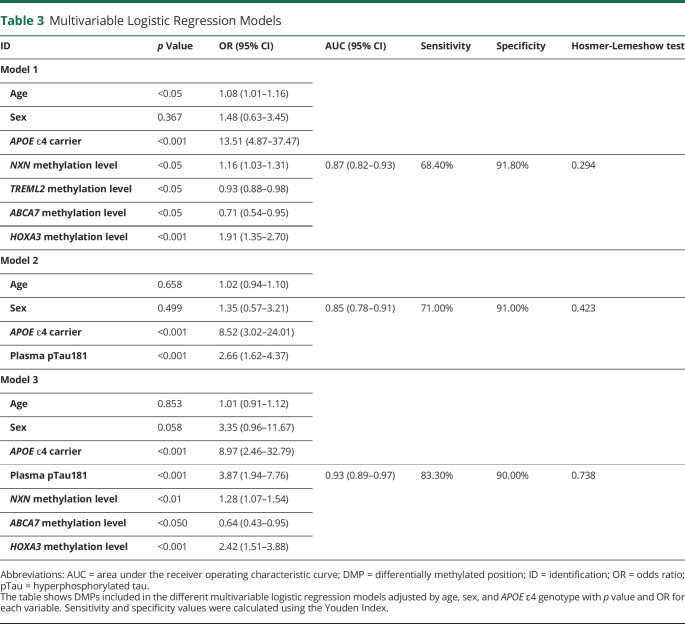
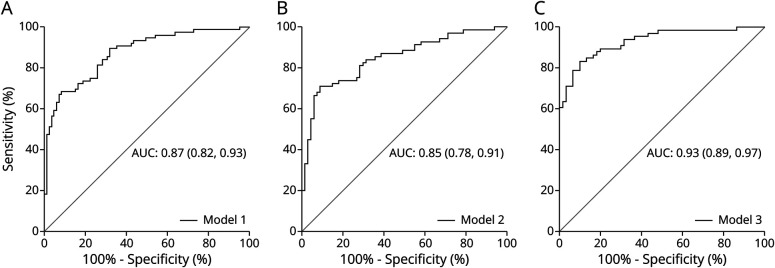
Finally, LRM analysis was used to identify variables that independently predict AD status. Evaluation of DNA methylation markers was assessed and adjusted for age, sex, and APOE ɛ4 genotype. All those 9 DMPs with AUC >0.6 (Table 2) were initially tested. The final adjusted LRM (model 1) included 4 DMPs located in NXN (Chr17:800086), triggering receptor expressed on myeloid cells like 2 (TREML2) (Chr6:41159608), ATP-binding cassette subfamily A member 7 (ABCA7) (Chr19:1046617), and HOXA3 (Chr7:27153577) genes as independent predictors of AD status (Table 3). To evaluate the accuracy of this LRM to identify patients with AD in our study, the ROC curve analysis yielded an AUC of 0.87 (Figure 2A, Table 3). Next, a LRM adjusted for age, sex, and APOE ɛ4 genotype was constructed to determine the classification accuracy of plasma pTau181 levels alone (model 2) (Table 3), showing an AUC of 0.85 (Figure 2B, Table 3). Finally, combining DNA methylation markers and plasma pTau181 levels in the same LRM resulted in an AUC of 0.93 (model 3) (Figure 2C, Table 3).
Table 3.
Multivariable Logistic Regression Models
| ID | p Value | OR (95% CI) | AUC (95% CI) | Sensitivity | Specificity | Hosmer-Lemeshow test |
| Model 1 | ||||||
| Age | <0.05 | 1.08 (1.01–1.16) | ||||
| Sex | 0.367 | 1.48 (0.63–3.45) | ||||
| APOE ɛ4 carrier | <0.001 | 13.51 (4.87–37.47) | ||||
| NXN methylation level | <0.05 | 1.16 (1.03–1.31) | 0.87 (0.82–0.93) | 68.40% | 91.80% | 0.294 |
| TREML2 methylation level | <0.05 | 0.93 (0.88–0.98) | ||||
| ABCA7 methylation level | <0.05 | 0.71 (0.54–0.95) | ||||
| HOXA3 methylation level | <0.001 | 1.91 (1.35–2.70) | ||||
| Model 2 | ||||||
| Age | 0.658 | 1.02 (0.94–1.10) | ||||
| Sex | 0.499 | 1.35 (0.57–3.21) | 0.85 (0.78–0.91) | 71.00% | 91.00% | 0.423 |
| APOE ɛ4 carrier | <0.001 | 8.52 (3.02–24.01) | ||||
| Plasma pTau181 | <0.001 | 2.66 (1.62–4.37) | ||||
| Model 3 | ||||||
| Age | 0.853 | 1.01 (0.91–1.12) | ||||
| Sex | 0.058 | 3.35 (0.96–11.67) | ||||
| APOE ɛ4 carrier | <0.001 | 8.97 (2.46–32.79) | ||||
| Plasma pTau181 | <0.001 | 3.87 (1.94–7.76) | 0.93 (0.89–0.97) | 83.30% | 90.00% | 0.738 |
| NXN methylation level | <0.01 | 1.28 (1.07–1.54) | ||||
| ABCA7 methylation level | <0.050 | 0.64 (0.43–0.95) | ||||
| HOXA3 methylation level | <0.001 | 2.42 (1.51–3.88) |
Abbreviations: AUC = area under the receiver operating characteristic curve; DMP = differentially methylated position; ID = identification; OR = odds ratio; pTau = hyperphosphorylated tau.
The table shows DMPs included in the different multivariable logistic regression models adjusted by age, sex, and APOE ɛ4 genotype with p value and OR for each variable. Sensitivity and specificity values were calculated using the Youden Index.
Figure 2. AUC Graph for Each Multivariable Logistic Regression Model.
The graphs represent the AUC (95% CI) showing the performance of diagnostic prediction for model 1 (A), model 2 (B), and model 3 (C) for distinguishing AD and controls in the iBEAS cohort. AD = Alzheimer disease; AUC = area under the receiver operating characteristic curve.
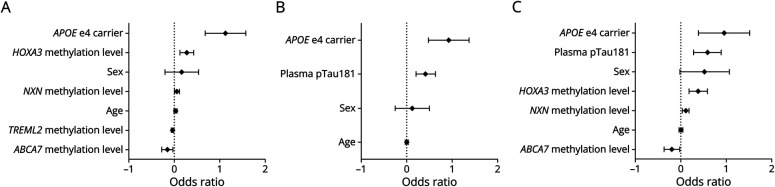
The OR and p value were provided for each variable included in the different models (Figure 3, Table 3). The variable with the strongest effect on AD status was HOXA3 methylation level. All models showed acceptable agreement between observed and predicted probability overall, as indicated by sensitivity, specificity, Hosmer-Lemeshow test, and calibration plots (Table 3 and eFigure 3, links.lww.com/WNL/D205).
Figure 3. Forest Plot Showing Odds Ratios and CIs of Each Variable Predicting Alzheimer Disease.
The panel shows odds ratio in logarithmic scale of each variable from each logistic regression for model 1 (A), model 2 (B), and model 3 (C).
An internal validation of the developed models to evaluate their performance by a bootstrapping approach corroborated our results, ruling out overfitting of models (eTable 4, links.lww.com/WNL/D206).
We determined performance differences between models by using the DeLong test. Previously, we performed multiple imputation analysis to assure comparability between models because different sample sizes had been used. The AUC of model 1 was similar to that of model 2 (p = 0.852). On the contrary, combining DNA methylation markers and plasma pTau181 in logistic regression analysis (model 3) improved the classification accuracy, as assessed by AUC, respective to model 2 (p < 0.05) and model 1 (p < 0.05) (Figure 4).
Figure 4. Comparisons of Performance Between Multivariable Logistic Regression Model.
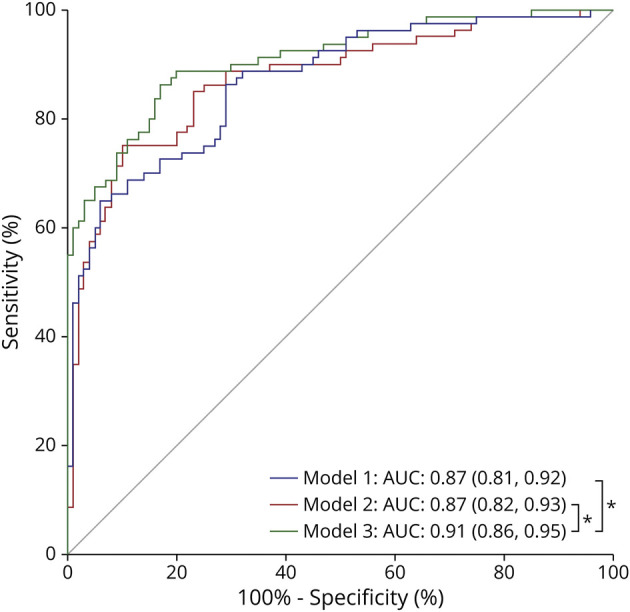
The graphs represent the AUC (95% CI) showing the performance of diagnostic prediction for model 1, model 2, and model 3 and statistical comparisons of AUCs between the models by the DeLong test after multiple imputation, for distinguishing AD and controls in the iBEAS cohort. *p < 0.05. AD = Alzheimer disease; AUC = area under the receiver operating characteristic curve.
Stratified Analysis by Sex
We also tested if results found in the whole cohort were maintained when a sex-stratified analysis was performed. After advanced age, female sex is the major risk factor for AD.37,38 In addition, sex may be a potential modifier of AD-related biomarkers and may have an effect in disease classification. Therefore, and although our models were adjusted for sex, we wanted to explore gender differences in biomarkers performance.
In a bivariate analysis, we observed that 3 DMPs, corresponding to ADAM10 (chr15:59041183), HOXA3 (chr7:27153577), and RHBDF2 (chr17:74467837) genes showed significant differences between patients with AD and controls in both female and male groups (eFigure 4, links.lww.com/WNL/D205, eTable 5, links.lww.com/WNL/D206), as also did plasma pTau181 levels (eFigure 2C, eTable 6).
Of the 3 differential epigenetic markers shared by men and women, HOXA3 (chr7:27153577) is the only one included in the predictive model proposed in this study, in addition to plasma levels of pTau181. Next, in multivariable logistic regression analysis adjusted for age and APOE ɛ4 genotype, HOXA3 (chr7:27153577) was revealed as an independent variable associated with AD status in both female and male groups with an AUC of 0.785 and 0.832, respectively. For pTau181, an independent association was only showed in the female group (AUC = 0.908). The combination of DNA methylation levels in HOXA3 (chr7:27153577) with plasma pTau181 levels only increased the diagnostic accuracy of the models for female subset (AUC = 0.932) but not for male subset, leading to a significant improvement over plasma pTau181 levels, according to the DeLong test (p < 0.05) (eFigure 5, links.lww.com/WNL/D205, eTable 6, links.lww.com/WNL/D206).
Discussion
This study provides a panel of blood-based epigenetic biomarkers to differentiate patients with AD from controls. The panel includes DNA methylation markers located at NXN, TREML2, ABCA7, and HOXA3, all genes previously associated with AD. Moreover, the addition of these epigenetic biomarkers significantly improved the diagnostic performance of a plasma pTau181-based model, adjusted for age, sex, and APOE ɛ4 genotype. The variable with the strongest effect was HOXA3 methylation levels, being the epigenetic biomarker that remained statistically significant across all models after sex-stratified analysis.
First, these results are mostly in line with those reported by other groups. NXN gene has been previously identified as differentially methylated between patients with AD and controls. Our results showed that NXN (ch17:800086) was hypermethylated in patients with AD, consistent with those revealing a hypermethylated DMR (chr17:798254-802254) in AD brain that includes our found DMP.39 NXN (cg02273477), our selected marker, has also observed to be hypermethylated in patients with Down syndrome (DS),31 which is considered a genetic form of AD. This gene encodes nucleoredoxin, which belongs to the thioredoxin family proteins that regulate the response to oxidative stress. NXN is involved in the regulation of several essential cellular processes such as proliferation, cell cycle progression, innate immunity and inflammation, and neuronal plasticity, among others.40 In a previous study by our group using an in vitro model of neurogenesis in AD, NXN was found to be hypermethylated.41 Interestingly, NXNL2, an NXN-related gene, encodes for a protein that regulates tau protein phosphorylation.42 However, the role of NXN in AD has not yet been explored.
TREML2 is located in a gene cluster in chromosome 6 along with TREM2 and TREM1 genes, both previously related to AD.43,44 TREML2, as other family members, is expressed by microglia in the CNS. Aβ protein deposition stimulates TREML2 expression during AD progression, modulating microglia activation. This receptor promotes phagocytosis of apoptotic neurons, cellular debris, and damaged proteins.44 TREML2 has been closely associated with susceptibility to AD and with pTau181 levels in CSF, as well as with the altered volume of AD brain structure.45,46 The selected CpG from TREML2 was found to be hypomethylated in AD in contrast to findings observed in twins discordant of AD.26
A risk factor for AD has been identified in the ABCA7 gene, located at chromosome 19 (rs3764650).47 Studies in mouse and in vitro models with the deletion of this gene have shown increased Aβ deposition with decreased clearance.48,49 ABCA7 encodes a family of transporters of phospholipids and cholesterol and their phagocytosis by macrophages, and it is expressed in cells of the CNS, such as microglia and neurons in the human brain.47 De Roeck et al.50 and Yu et al.22 found an association between DNA methylation levels in ABCA7 and AD. Furthermore, Haertle et al.31 also identified our selected CpG, cg06169110, hypomethylated in blood cells in DS. However, further studies are still needed to understand the role of this variant in AD.
HOXA gene cluster is located in chromosome 7. These genes encode essential transcription factors for neural development and are involved in the ankyrin-dependent axonal microtubule organization and synaptic stability, playing a crucial role in neuroprotection.e1 Several studies have previously reported some DMPs in AD brain.16,17 HOXA gene cluster also revealed differential DNA methylation in blood cells in DS.e2 Here, we found HOXA3 hypermethylation in patients with AD compared with controls; a distance of 3 bp from the CpG site (cg01301319) was previously identified as hypermethylated in the AD hippocampus.14 Furthermore, HOXA3 hypermethylation was associated with AD neuropathology.20
Blood-based biomarkers of easy application in diagnosis would be of great benefit, given the urgent need to develop new noninvasive diagnostic tools for AD. Therefore, PBLs were chosen in this study as a source of noninvasive epigenetic biomarkers to aid in AD diagnosis. To our knowledge, our study is the first to explore the diagnostic accuracy of a panel of blood-based epigenetic biomarkers based on DNA methylation in patients with AD. Our findings show that blood DNA methylation marks may improve diagnostic accuracy in identifying patients with AD with high sensitivity and specificity. Most interestingly, our proposed predictive model significantly improves the performance of another recent and well-studied tool to discriminate patients with AD from controls, namely plasma pTau181 levels detected by ultrasensitive digital immunoassay. In our study, plasma pTau181 levels showed high diagnostic accuracy (AUC = 0.85) and replicated the results of previous research.5,6,10 Remarkably, the addition of epigenetic biomarkers to the pTau181 model, resulted in a statistically significant improvement of the panel performance (AUC = 0.93), as assessed by the DeLong test (p < 0.01). Therefore, these results are worth to be tested in larger multicentric cohorts to be externally validated.
After performing sex-stratified analysis, only HOXA3 methylation and plasma pTau181 levels were shown to be different between AD cases and controls. This result strengthens HOXA3 as a unique and consistent epigenetic biomarker of this disease. On the other hand, the differences found here related to DNA methylation marks between female and male support the idea that there are sex-specific AD-related differences in DNA methylation, which requires a gender perspective in the investigation of sex-disbalanced diseases such as AD, considering that stratification reduces sample size. However, we would like to emphasize that this is a subanalysis of exploratory nature only and that it would be necessary to corroborate these results in larger samples because of reduced statistical power after stratifying the original sample.
This study has several limitations. The modest sample size of this study requires validation in larger cohorts. In addition, characterizing the cohort through existing biomarkers of amyloid and tau pathology is not available for all patients and controls. Therefore, the utility of testing DNA methylation in peripheral blood cells in neurodegeneration is unclear, making necessary their determination in other neurodegenerative diseases to be able to test their diagnostic specificity. In addition, the study of these methylation patterns in SCD and MCI will be useful to confirm their sensitivity. However, our results suggest that the origin of these DNA methylation marks could serve as future biomarkers. Finally, our analysis is limited to CpG methylation. However, methylation of non-CpG dinucleotides is gaining considerable attention in the field, particularly in neurodegenerative diseases. In non-CpG dinucleotides, cytosine is followed by a nucleotide other than guanine, such as adenine or thymine. This epigenetic modification can change the expression of nearby genes, similar to the way CpG methylation does.e3 This type of methylation can be found, although less frequently, in brain tissue and may be altered in neurodegenerative diseases, such as changes described in PSEN1, SNCA, or GSK3β.e4-e6 Thus, defining methylation levels of non-CpG dinucleotides would be another interesting strategy in the search for new biomarkers in AD.
In addition, we are aware that our study does not include all genes that have been described in the literature as differentially methylated in AD. Other genes such as PSEN1,e4 PICALMe7, and IL-1be8 have also shown methylation differences in peripheral blood and/or brain tissue of patients with AD. However, they did not score high enough in the selection of our candidate genes (eTable 1, links.lww.com/WNL/D206), which was limited for technical reasons to 21 genes (46 CpGs). To sum up, this observational case-control study endorses the idea that a panel of epigenetic biomarkers based on DNA methylation is a promising diagnostic tool to aid in AD diagnosis, especially in combination with plasma pTau181 levels. This diagnostic tool would give patients a better chance to benefit from clinical trials and other future therapeutic interventions.
Acknowledgment
The authors want to kindly thank all the collaborators of the iBEAS study group, as well as Soraya Torres (Department of Neurology, Institut d'Investigacions Biomèdiques Sant Pau (IIB Sant Pau), Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Catalunya, Spain.) and Mónica Enguita (Methodology Unit, Navarrabiomed, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Universidad Pública de Navarra (UPNA), Pamplona, 31008 Navarra, Spain), for their help. Specially, we would like to express our most sincere gratitude to the participants in the iBEAS study and to the patients and relatives who generously made this research possible. We also want to thank the nursing team of the Internal Medicine Department-Hospital García Orcoyen (Estella, Spain) for their help to setting up the study.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- ABCA7
ATP-binding cassette subfamily A member 7 gene
- ADAM10
ADAM metallopeptidase domain 10
- ADARB2
adenosine deaminase RNA specific B2
- ANK1
Ankyrin-1
- AUC
area under the ROC curve
- BDNF
brain-derived neurotrophic factor
- BIN1
amphiphysin II
- CpG
cytosine-guanine
- CRTC1
CREB-regulated transcription coactivator 1
- DMP
differentially methylated position
- DMR
differentially methylated region
- DSM-III-R
Diagnostic and Statistical Manual of Mental Disorders, Third Edition
- EWAS
epigenome-wide association study
- GP1BB
glycoprotein Ib platelet subunit beta
- HOXA
homeobox A
- HOXA3
homeobox A3
- HOXB6
homeobox B6
- IQR
interquartile range
- IRS2
insulin receptor substrate 2
- LRM
logistic regression model
- MMSE
Mini-Mental State Examination
- NXN
nucleoredoxin
- NXNL2
nucleoredoxin like 2
- OR
odd ratio
- OXT
oxytocin
- PBL
peripheral blood leukocyte
- PLD3
phospholipase D family member 3
- pTau
hyperphosphorylated tau
- RHBDF2
rhomboid 5 homolog 2
- RHOB
Ras homolog family member B
- ROC
receiver operating characteristic
- TREM1
triggering receptor expressed on myeloid cells 1
- TREM2
triggering receptor expressed on myeloid cells 2
- TREML2
triggering receptor expressed on myeloid cells like 2
Appendix 1. Authors
| Name | Location | Contribution |
| Blanca Acha, MSc | Navarrabiomed, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Universidad Pública de Navarra (UPNA), Pamplona, Spain | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Jon Corroza, MD | Department of Neurology, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Major role in the acquisition of data |
| Javier Sánchez-Ruiz de Gordoa, MD, PhD | Navarrabiomed, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Universidad Pública de Navarra (UPNA); Department of Neurology, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Carolina Cabello | Navarrabiomed, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Universidad Pública de Navarra (UPNA); Department of Neurology, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Major role in the acquisition of data; analysis or interpretation of data |
| Maitane Robles | Navarrabiomed, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Universidad Pública de Navarra (UPNA), Pamplona, Spain | Major role in the acquisition of data |
| Iván Méndez-López, MD, PhD | Navarrabiomed, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Universidad Pública de Navarra (UPNA); Department of Internal Medicine, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Major role in the acquisition of data; study concept or design |
| Mónica Macías, PhD | Navarrabiomed, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Universidad Pública de Navarra (UPNA), Pamplona, Spain | Major role in the acquisition of data; analysis or interpretation of data |
| Sara Zueco | Navarrabiomed, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Universidad Pública de Navarra (UPNA), Pamplona, Spain | Major role in the acquisition of data |
| Miren Roldan | Navarrabiomed, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Universidad Pública de Navarra (UPNA), Pamplona, Spain | Major role in the acquisition of data; analysis or interpretation of data |
| Amaya Urdánoz-Casado, PhD | Navarrabiomed, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Universidad Pública de Navarra (UPNA), Pamplona, Spain | Major role in the acquisition of data; analysis or interpretation of data |
| Ivonne Jericó, MD, PhD | Navarrabiomed, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Universidad Pública de Navarra (UPNA); Department of Neurology, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Maria Elena Erro, MD, PhD | Navarrabiomed, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Universidad Pública de Navarra (UPNA); Department of Neurology, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Daniel Alcolea, MD, PhD | Department of Neurology, Institut d'Investigacions Biomèdiques Sant Pau (IIB Sant Pau), Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Catalunya; Centro de Investigación Biomédica en Red en Enfermedades Neurodegenerativas, CIBERNED, Madrid, Spain | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Alberto Lleo, MD, PhD | Department of Neurology, Institut d'Investigacions Biomèdiques Sant Pau (IIB Sant Pau), Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Catalunya; Centro de Investigación Biomédica en Red en Enfermedades Neurodegenerativas, CIBERNED, Madrid, Spain | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Idoia Blanco-Luquin, PhD | Navarrabiomed, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Universidad Pública de Navarra (UPNA), Pamplona, Spain | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Maite Mendioroz, MD, PhD | Navarrabiomed, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Universidad Pública de Navarra (UPNA); Department of Neurology, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Appendix 2. Coinvestigators
| Name | Location | Role | Contribution |
| Mikel San Miguel, MD | Department of Neurology, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Neurologist | Recruitment of participants and sample collection |
| Pedro Clavero, MD, PhD | Department of Neurology, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Neurologist | Recruitment of participants |
| María Martín-Bujanda, MD | Department of Neurology, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Neurologist | Recruitment of participants |
| Rosa Larumbe, MD | Department of Neurology, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Neurologist | Recruitment of participants |
| Paula Tellechea, MD | Department of Neurology, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Neurologist | Recruitment of participants and sample collection |
| Itsaso Elizalde | Primary Care Department, Osasunbidea, Universidad Pública de Navarra, IdiSNA (Navarra Institute for Health Research), Pamplona, Navarra, Spain | Nurse | Sample collection |
| Marian Garde | Department of Neurology, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Neurologist | Sample collection |
| Idoia Marañón | Department of Neurology, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Neurologist | Sample collection |
| María Ferrer | Department of Microbiology, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Nurse, Lab Technician | Sample collection |
| Beatriz Nuin | Department of Neurology, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Neurologist | Sample collection |
| María Carmen Navarro, MD | Department of Neurology, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Neurologist | Recruitment of participants and sample collection |
| Aiora Ostolaza, MD | Department of Neurology, Hospital Universitario de Navarra-IdiSNA (Navarra Institute for Health Research), Pamplona, Spain | Neurologist | Recruitment of participants and sample collection |
Study Funding
The authors sincerely appreciate funding support by the Spanish Government through grants from the Institute of Health Carlos III (FIS PI17/02218), jointly funded by the European Regional Development Fund (ERDF), European Union, A way of shaping Europe; the Department of Industry of Government of Navarra (PI058 iBEAS-Plus and PI055 iBEAS-Plus); Institute of Health Carlos III (FIS PI20/01330 and AC19/00103 to AL) and CIBERNED (Program 1, Alzheimer Disease). The project leading to these results has received funding from la Caixa Foundation (ID 100010434) co-funded by Fundación Luzón (HR20-01109_BIOP-AD) under the project code LCF/PR/PR15/51100006.
Disclosure
B. Acha is supported by a PFIS fellowship from the Spanish Government (FI18/00150). M. Macías is beneficiary of a grant Río Hortega from the Spanish Government (CM20/00240). A. Urdánoz-Casado received a grant Doctorandos industriales 20182020 and a Predoctoral grant (2019) founded by the Department of Industry and Health of the Government of Navarra. D. Alcolea participated in advisory boards from Fujirebio-Europe and Roche Diagnostics and received speaker honoraria from Fujirebio-Europe, Roche Diagnostics, Nutricia, Krka Farmacéutica S.L., Zambon S.A.U. and Esteve Pharmaceuticals S.A. D. Alcolea declares a filed patent application (WO2019175379 A1 Markers of synaptopathy in neurodegenerative disease). A. Lleo has served at scientific advisory boards from Fujirebio-Europe, Nutricia, Biogen, Roche, and Grifols, and has filed a patent application of synaptic markers in neurodegenerative diseases. M. Mendioroz received a grant (LCF/PR/PR15/51100006) founded by Fundación Bancaria la Caixa and Fundación Caja-Navarra, and Contrato de intensificación from the Institute of Health Carlos III (INT19/00029). All other authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Porsteinsson AP, Isaacson RS, Knox S, Sabbagh MN, Rubino I. Diagnosis of early Alzheimer's disease: clinical practice in 2021. J Prev Alzheimers Dis. 2021;8(3):371-386. doi: 10.14283/jpad.2021.23 [DOI] [PubMed] [Google Scholar]
- 2.Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263-269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings JL, Morstorf T, Zhong K. Alzheimer's disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6(4):37. doi: 10.1186/alzrt269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brickman AM, Manly JJ, Honig LS, et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer's disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 2021;17(8):1353-1364. doi: 10.1002/alz.12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19(5):422-433. doi: 10.1016/s1474-4422(20)30071-5 [DOI] [PubMed] [Google Scholar]
- 7.Lleó A, Zetterberg H, Pegueroles J, et al. Phosphorylated tau181 in plasma as a potential biomarker for Alzheimer's disease in adults with Down syndrome. Nat Commun. 2021;12(1):4304. doi: 10.1038/s41467-021-24319-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmqvist S, Tideman P, Cullen N, et al. Prediction of future Alzheimer's disease dementia using plasma phospho-tau combined with other accessible measures. Nat Med. 2021;27(6):1034-1042. doi: 10.1038/s41591-021-01348-z [DOI] [PubMed] [Google Scholar]
- 9.Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi: 10.1038/s41591-020-0762-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P-tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med. 2020;26(3):379-386. doi: 10.1038/s41591-020-0755-1 [DOI] [PubMed] [Google Scholar]
- 11.Karikari TK, Benedet AL, Ashton NJ, et al. Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer's Disease Neuroimaging Initiative. Mol Psychiatry. 2021;26(2):429-442. doi: 10.1038/s41380-020-00923-z [DOI] [PubMed] [Google Scholar]
- 12.Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137-152. doi: 10.1038/nrneurol.2011.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9(6):465-476. doi: 10.1038/nrg2341 [DOI] [PubMed] [Google Scholar]
- 14.Altuna M, Urdánoz-Casado A, Sánchez-Ruiz de Gordoa J, et al. DNA methylation signature of human hippocampus in Alzheimer's disease is linked to neurogenesis. Clin Epigenetics. 2019;11(1):91. doi: 10.1186/s13148-019-0672-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakulski KM, Dolinoy DC, Sartor MA, et al. Genome-wide DNA methylation differences between late-onset Alzheimer's disease and cognitively normal controls in human frontal cortex. J Alzheimers Dis. 2012;29(3):571-588. doi: 10.3233/jad-2012-111223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Jager PL, Srivastava G, Lunnon K, et al. Alzheimer's disease: early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat Neurosci. 2014;17(9):1156-1163. doi: 10.1038/nn.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lunnon K, Smith R, Hannon E, et al. Methylomic profiling implicates cortical deregulation of ANK1 in Alzheimer's disease. Nat Neurosci. 2014;17(9):1164-1170. doi: 10.1038/nn.3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao JS, Keleshian VL, Klein S, Rapoport SI. Epigenetic modifications in frontal cortex from Alzheimer's disease and bipolar disorder patients. Transl Psychiatry. 2012;2:e132. doi: 10.1038/tp.2012.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez-Mut JV, Heyn H, Vidal E, et al. Whole genome grey and white matter DNA methylation profiles in dorsolateral prefrontal cortex. Synapse. 2017;71(6):e21959. doi: 10.1002/syn.21959 [DOI] [PubMed] [Google Scholar]
- 20.Smith RG, Hannon E, De Jager PL, et al. Elevated DNA methylation across a 48-kb region spanning the HOXA gene cluster is associated with Alzheimer's disease neuropathology. Alzheimers Dement. 2018;14(12):1580-1588. doi: 10.1016/j.jalz.2018.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson CT, Roussos P, Garg P, et al. Genome-wide12 DNA methylation profiling in the superior temporal gyrus reveals epigenetic signatures associated with Alzheimer's disease. Genome Med. 2016;8:5. doi: 10.1186/s13073-015-0258-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu L, Chibnik LB, Srivastava GP, et al. Association of Brain DNA methylation in SORL1, ABCA7, HLA-DRB5, SLC24A4, and BIN1 with pathological diagnosis of Alzheimer disease. JAMA Neurol. 2015;72(1):15-24. doi: 10.1001/jamaneurol.2014.3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dabin LC, Guntoro F, Campbell T, et al. Altered DNA methylation profiles in blood from patients with sporadic Creutzfeldt-Jakob disease. Acta Neuropathol. 2020;140(6):863-879. doi: 10.1007/s00401-020-02224-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roubroeks JAY, Smith AR, Smith RG, et al. An epigenome-wide association study of Alzheimer's disease blood highlights robust DNA hypermethylation in the HOXB6 gene. Neurobiol Aging. 2020;95:26-45. doi: 10.1016/j.neurobiolaging.2020.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lardenoije R, Roubroeks JAY, Pishva E, et al. Alzheimer's disease-associated (hydroxy)methylomic changes in the brain and blood. Clin Epigenetics. 2019;11(1):164. doi: 10.1186/s13148-019-0755-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konki M, Malonzo M, Karlsson IK, et al. Peripheral blood DNA methylation differences in twin pairs discordant for Alzheimer's disease. Clin Epigenetics. 2019;11(1):130. doi: 10.1186/s13148-019-0729-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salcedo-Tacuma D, Melgarejo JD, Mahecha MF, et al. Differential methylation levels in CpGs of the BIN1 gene in individuals with Alzheimer disease. Alzheimer Dis Assoc Disord. 2019;33(4):321-326. doi: 10.1097/WAD.0000000000000329 [DOI] [PubMed] [Google Scholar]
- 28.Chang L, Wang Y, Ji H, et al. Elevation of peripheral BDNF promoter methylation links to the risk of Alzheimer's disease. PLoS One. 2014;9(11):e110773. doi: 10.1371/journal.pone.0110773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu D, Wang Y, Jing H, Meng Q, Yang J. Novel DNA methylation loci and genes showing pleiotropic association with Alzheimer's dementia: a network Mendelian randomization analysis. Epigenetics. 2022;17(7):746-758. doi: 10.1080/15592294.2021.1959735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith RG, Pishva E, Shireby G, et al. A meta-analysis of epigenome-wide association studies in Alzheimer's disease highlights novel differentially methylated loci across cortex. Nat Commun. 2021;12(1):3517. doi: 10.1038/s41467-021-23243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haertle L, Müller T, Lardenoije R, et al. Methylomic profiling in trisomy 21 identifies cognition- and Alzheimer's disease-related dysregulation. Clin Epigenetics. 2019;11(1):195. doi: 10.1186/s13148-019-0787-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozaki Y, Yoshino Y, Yamazaki K, et al. DNA methylation changes at TREM2 intron 1 and TREM2 mRNA expression in patients with Alzheimer's disease. J Psychiatr Res. 2017;92:74-80. doi: 10.1016/j.jpsychires.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 33.Shao Y, Shaw M, Todd K, et al. DNA methylation of TOMM40-APOE-APOC2 in Alzheimer's disease. J Hum Genet. 2018;63(4):459-471. doi: 10.1038/s10038-017-0393-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanco-Luquin I, Acha B, Urdánoz-Casado A, et al. Early epigenetic changes of Alzheimer's disease in the human hippocampus. Epigenetics. 2020;15(10):1083-1092. doi: 10.1080/15592294.2020.1748917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thijssen EH, Verberk IMW, Kindermans J, et al. Differential diagnostic performance of a panel of plasma biomarkers for different types of dementia. Alzheimers Dement (Amst). 2022;14(1):e12285. doi: 10.1002/dad2.12285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tissot C, L Benedet A, Therriault J, et al. Plasma pTau181 predicts cortical brain atrophy in aging and Alzheimer's disease. Alzheimers Res Ther. 2021;13(1):69. doi: 10.1186/s13195-021-00802-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349-1356. [PubMed] [Google Scholar]
- 38.Rahman A, Jackson H, Hristov H, et al. Sex and gender driven modifiers of Alzheimer's: the role for estrogenic control across age, race, medical, and lifestyle risks. Front Aging Neurosci. 2019;11:315. doi: 10.3389/fnagi.2019.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez-Mut JV, Heyn H, Vidal E, et al. Human DNA methylomes of neurodegenerative diseases show common epigenomic patterns. Translational Psychiatry. 2016;6:e718. doi: 10.1038/tp.2015.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Idelfonso-García OG, Alarcón-Sánchez BR, Vásquez-Garzón VR, et al. Is nucleoredoxin a master regulator of cellular redox homeostasis? Its implication in different pathologies. Antioxidants (Basel). 2022;11(4):670. doi: 10.3390/antiox11040670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanco-Luquin I, Acha B, Urdánoz-Casado A, et al. NXN gene epigenetic changes in an adult neurogenesis model of Alzheimer's disease. Cells. 2022;11(7):1069. doi: 10.3390/cells11071069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaillard C, Ouechtati F, Clérin E, et al. The metabolic signaling of the nucleoredoxin-like 2 gene supports brain function. Redox Biol. 2021;48:102198. doi: 10.1016/j.redox.2021.102198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Celarain N, de Gordoa JSR, Zelaya MV, et al. TREM2 upregulation correlates with 5-hydroxymethycytosine enrichment in Alzheimer's disease hippocampus. Clin Epigenetics. 2016;8:37. doi: 10.1186/s13148-016-0202-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang SY, Gong PY, E Y, Zhang YD, Jiang T. The Role of TREML2 in Alzheimer's disease. J Alzheimers Dis. 2020;76(3):799-806. doi: 10.3233/JAD-200406 [DOI] [PubMed] [Google Scholar]
- 45.Cruchaga C, Kauwe JS, Harari O, et al. GWAS of cerebrospinal fluid tau levels identifies risk variants for Alzheimer's disease. Neuron. 2013;78(2):256-268. doi: 10.1016/j.neuron.2013.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang SY, Xue X, Duan R, et al. A TREML2 missense variant influences specific hippocampal subfield volumes in cognitively normal elderly subjects. Brain Behav. 2020;10(4):e01573. doi: 10.1002/brb3.1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamazaki K, Yoshino Y, Mori T, et al. Gene expression and methylation analysis of ABCA7 in patients with Alzheimer's disease. J Alzheimers Dis. 2017;57(1):171-181. doi: 10.3233/JAD-161195 [DOI] [PubMed] [Google Scholar]
- 48.Fu Y, Hsiao JH, Paxinos G, Halliday GM, Kim WS. ABCA7 mediates phagocytic clearance of amyloid-β in the brain. J Alzheimers Dis. 2016;54(2):569-584. doi: 10.3233/JAD-160456 [DOI] [PubMed] [Google Scholar]
- 49.Kim WS, Li H, Ruberu K, et al. Deletion of Abca7 increases cerebral amyloid-β accumulation in the J20 mouse model of Alzheimer's disease. J Neurosci. 2013;33(10):4387-4394. doi: 10.1523/JNEUROSCI.4165-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Roeck A, Van Broeckhoven C, Sleegers K. The role of ABCA7 in Alzheimer's disease: evidence from genomics, transcriptomics and methylomics. Acta Neuropathol. 2019;138(2):201-220. doi: 10.1007/s00401-019-01994-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- eReferences are listed at; links.lww.com/WNL/D204.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.