Abstract
Background and Objectives
Deep brain stimulation (DBS) of the ventral tegmental area (VTA) is a surgical treatment option for selected patients with refractory chronic cluster headache (CCH). We aimed to identify clinical and structural neuroimaging factors associated with response to VTA DBS in CCH.
Methods
This prospective observational cohort study examines consecutive patients with refractory CCH treated with VTA DBS by a multidisciplinary team in a single tertiary neuroscience center as part of usual care. Headache diaries and validated questionnaires were completed at baseline and regular follow-up intervals. All patients underwent T1-weighted structural MRI before surgery. We compared clinical features using multivariable logistic regression and neuroanatomic differences using voxel-based morphometry (VBM) between responders and nonresponders.
Results
Over a 10-year period, 43 patients (mean age 53 years, SD 11.9), including 29 male patients, with a mean duration of CCH 12 years (SD 7.4), were treated and followed up for at least 1 year (mean follow-up duration 5.6 years). Overall, there was a statistically significant improvement in median attack frequency from 140 to 56 per month (Z = −4.95, p < 0.001), attack severity from 10/10 to 8/10 (Z = −4.83, p < 0.001), and duration from 110 to 60 minutes (Z = −3.48, p < 0.001). Twenty-nine (67.4%) patients experienced ≥50% improvement in attack frequency and were therefore classed as responders. There were no serious adverse events. The most common side effects were discomfort or pain around the battery site (7 patients) and transient diplopia and/or oscillopsia (6 patients). There were no differences in demographics, headache characteristics, or comorbidities between responders and nonresponders. VBM identified increased neural density in nonresponders in several brain regions, including the orbitofrontal cortex, anterior cingulate cortex, anterior insula, and amygdala, which were statistically significant (p < 0.001).
Discussion
VTA DBS showed no serious adverse events, and, although there was no placebo control, was effective in approximately two-thirds of patients at long-term follow-up. This study did not reveal any reliable clinical predictors of response. However, nonresponders had increased neural density in brain regions linked to processing of pain and autonomic function, both of which are prominent in the pathophysiology of CCH.
Introduction
Cluster headache is a primary headache disorder characterized by attacks of severe unilateral headache associated with cranial autonomic symptoms.1 Cluster headache is most commonly an episodic disorder, with attacks occurring in bouts separated by remissions of longer than 3 months; however, approximately 15% have chronic cluster headache (CCH) with no remissions.2 A proportion of those with CCH are refractory to the available preventive treatments, in which case it represents a major medical problem with a high degree of disability.
Ventral tegmental area deep brain stimulation (VTA DBS) has been reported in case series to be effective in 60%–80% of patients with treatment-refractory CCH.3-5 Currently, it is unknown why some patients respond to VTA DBS while others do not. Potential reasons include clinical characteristics, comorbidities, differences in headache pathophysiology, neuroanatomic differences, or in lead location of the DBS implant.
Currently, patient selection is a risk-benefit equation which takes into account severity of symptoms, failure of response to noninvasive treatments, comorbidities, psychological state, risks of surgery, and patient wishes.6 The identification of biomarkers for treatment response to VTA DBS would be useful to better predict the individual risk-benefit ratio. If the 20%–40% of nonresponders could be identified before surgery, the rare but potentially serious risks of invasive DBS surgery in those who are unlikely to respond could be avoided. Moreover, a better understanding of the pathophysiology of CCH and the mechanism of action of VTA DBS is likely to contribute to better future diagnostics and therapeutics.
Objectives
To identify clinical and structural neuroimaging factors which are associated with response to VTA DBS in CCH.
Methods
Population
This prospective observational cohort study investigates consecutive patients who were treated with VTA DBS between 2009 and 2019 at a single tertiary neuroscience center, the National Hospital for Neurology and Neurosurgery, Queen Square, London, UK. No sample size calculation was performed, and we included all patients treated during the study period. All patients included in the study met International Classification of Headache Disorders, 3rd edition, criteria for CCH.1 Patients were aged 18 and older, with no upper age limit. DBS was considered in all patients who had suffered from treatment-refractory CCH for 2 or more years. Owing to the invasive nature of the procedure, the criteria used for refractory CCH were stricter than the European Headache Federation consensus criteria.7 CCH was defined as treatment-refractory if there had been treatment failure to adequate trial of verapamil and at least 4 of lithium, topiramate, melatonin, gabapentin, pregabalin, and sodium valproate. Many patients had also trialled one or more of methysergide, baclofen, and/or levetiracetam, and 18 patients had already failed to respond to occipital nerve stimulation. Patients who had attacks lasting less than 30 minutes or more than 5 attacks per day had also undergone a trial of indomethacin to exclude paroxysmal hemicrania. No headache treatments were changed for at least 3 months before DBS implantation.
All patients underwent MRI before DBS implant for surgical planning. Patients who had contraindications to MRI or refractory medical conditions that would increase the risk from surgery such as uncontrollable hypertension or anticoagulation (that could not be temporarily stopped) were excluded from surgery.
Surgical Procedure
DBS was performed unilaterally in those with strictly unilateral attacks and bilaterally in those with a history of side-variable attacks. Surgery for electrode implantation was performed under general anesthesia in the vast majority of patients. After attachment of the stereotactic frame (Leksell Coordinate Frame G), T1-weighted and T2-weighted stereotactic MRI was obtained (Magnetom Espree, 1.5 T).
Targeting the VTA was performed using commercially available surgical planning software (Framelink, Medtronic). The VTA was defined at a level immediately above the mammillary bodies, anteromedial to the red nucleus and posterolateral to the mammillothalamic tract.8 Medtronic 3,389 electrodes were implanted in the initial participants, and Boston Scientific Vercise Cartesia directional leads in a smaller subset of the most recent 7 patients. Electrode location was verified intraoperatively with stereotactic MRI scan in patients without occipital nerve stimulation and with a stereotactic CT scan in patients with implanted occipital nerve stimulation hardware.
DBS programming commenced within a few weeks of the surgical procedure. Settings were adjusted based on patient clinical response and lack of adverse effects. Start of stimulation was postponed in those patients experiencing a stun effect (i.e., a temporary resolution of symptoms for days or weeks after implantation without stimulation), until symptoms returned to baseline.4
Assessment of Treatment Response
Patients were instructed to complete a headache diary for a baseline period of 1 month before surgery, for a 1-month period every 3 months for the first year after surgery, then annually thereafter. Clinical outcomes were collected and entered prospectively onto a database. Data were collected on attack frequency (number of attacks per month); typical headache severity (on 0–10 verbal rating scale [VRS]); typical headache duration (in minutes); headache load (a composite measure calculated as the monthly sum of headache VRS multiplied by headache hours for each day); the Headache Impact Test-6 (HIT-6) measure of headache-related disability9; and the Hospital Anxiety and Depression Scale (HADS) questionnaire.10
Statistical analysis of the clinical data was performed using IBM SPSS version 28. Normality assumptions were based on visual inspection of histograms and the Kolmogorov-Smirnov test. Missing data were not imputed. Descriptive data were summarized as means with SD or medians with ranges depending on the distribution of data.
Data were compared from baseline to the point of last follow-up and unless otherwise specified are stated as percentage improvement. The last available observation was used for participants who were lost to follow-up or died during the study period. Whole group comparison of follow-up to baseline data was performed using the Wilcoxon signed-rank test. p values shown are uncorrected for multiple comparisons.
For analysis of responders vs nonresponders, response was defined as a ≥50% improvement in attack frequency. Comparison of clinical characteristics of responders with nonresponders was performed using multivariable logistic regression, with the variables included defined a priori.
DBS Target
Volumes of tissue activation were estimated using field simulation software (GuideXT TM, Brainlab). Virtual electrodes were modeled and adjusted into the lead artifact using postoperative T1-weighted MR images. Parameters of stimulation from the most recent clinical visit were applied. All devices were programmed with a frequency of 185 Hz and a pulse width of 60 ms. Amplitudes were variable among participants and determined the volume of activated tissue. Ventral contacts were most often stimulated. When monopolar configuration was used, the implantable pulse generator acted as anode and the single contact as cathode. All volumes of tissue activation were exported into a NIfTI format and coregistered to the symmetrical MNI ICBM152 1-mm nonlinear template as previously described.8 Right-sided volumes of tissue activation were flipped to the left using the fslswapdim tool—an advanced tool that reorders the data storage to permit changes between axial, sagittal, and coronal slicing; when used in this mode, the same left-right convention will be maintained. Group averages were created using the fslmaths tool for the responders and nonresponders.
Voxel-Based Morphometry
Preimplantation 3D T1-weighted MRI images (MPRAGE) were used for voxel-based morphometry (VBM) analysis. All scans were acquired on a single 1.5T Siemens Espree MRI scanner with a spatial resolution of 1 mm3. Structural data were analyzed with FSL-VBM,11 an optimized VBM protocol12 using FSL tools.13 First, brain-extraction and gray matter segmentation was performed on structural images, before nonlinear registration to the MNI 152 standard space.14 The resulting images were averaged and flipped along the x-axis to create a left-right symmetric, study-specific gray matter template. Second, native gray matter images were nonlinearly registered to this study-specific template and “modulated” to correct for local expansion or contraction because of the nonlinear component of the spatial transformation. Then, the modulated gray matter images were smoothed with an isotropic Gaussian kernel with a sigma of 2 mm. Finally, voxelwise general linear model was applied using permutation-based nonparametric testing and threshold-free cluster enhancement (TFCE), correcting for multiple comparisons across space. Two-group difference (2-sample unpaired t-test) was performed with the groups being “responders” and “nonresponders” with contrasts showing increased voxels in each group when tested against the other.
Standard Protocol Approvals, Registrations, and Patient Consents
Clinical data collection and MRI scans were performed as part of clinical practice under supervision of our organization's Clinical Effectiveness Supervisory Committee on the basis of a humanitarian intervention, with arrangements for clinical governance, consent, and audit or research as advised by National Institute for Health and Care Excellence guidelines.15 Ethical approval for the radiologic analysis was granted by West London REC 3 (REC reference number: 10/H0706/68). Written informed consent for the procedure and collection of anonymized clinical and radiologic data were obtained from all participants in the study.
Data Availability
Deidentified data are available on reasonable request from the corresponding author.
Results
Population
Forty-three patients with treatment-refractory CCH were treated with VTA DBS during the study period. Four additional patients met criteria and were offered the procedure but declined. Fifteen patients received right, 16 left, and 12 bilateral DBS implantation. The mean age at the time of implant was 53.4 years (SD 11.9, range 25–77), and 30 (70%) were men. The mean duration of chronic headache at the time of implant was 12.0 years (SD 7.4). Patients had previously failed a mean of 8.4 preventive medications (SD 1.3, range 6–11), including verapamil (43/43 of the patients), lithium (41/43), topiramate (42/43), gabapentin (40/43), pregabalin (38/42), melatonin (36/43), sodium valproate (27/43), methysergide (29/43), baclofen (15/43), and levetiracetam (19/43). Seven patients had failed to respond to noninvasive vagus nerve stimulation. Eighteen patients (42%) had previously been treated with invasive occipital nerve stimulation, from which 10 had no response and 8 had a partial response. Almost all patients (41/43, 95%) were within the highly disabled range on the HIT-6 questionnaire.
Clinical Outcomes
Twenty-nine patients (67%) experienced a stun effect after the procedure, of whom 15 were temporarily rendered pain-free.
The mean duration of follow-up was 6.1 years (SD 3.1, range 1.0–11.7). Overall, at time of last follow-up, there was a statistically significant improvement in attack severity, frequency, duration, and headache load, but no statistically significant improvement in HIT-6 score or anxiety or depression measured by the HADS score (Table 1). Twenty-nine patients (67.4%) were classed as responders (50% or greater improvement in attack frequency), and 4 (9.3%) were pain-free.
Table 1.
Improvement in Headache Metrics After VTA DBS
| Baseline (median) | Follow-up (median) | Za | p Valuea | |
| Attack frequency (per mo) | 140 | 56 | −4.95 | <0.001* |
| Attack severity (0–10 VRS scale) | 10 | 8 | −4.83 | <0.001* |
| Attack duration (min) | 110 | 60 | −3.48 | <0.001* |
| Headache load | 700 | 229 | −3.98 | <0.001* |
| HIT-6 | 70 | 66 | −2.16 | 0.03 |
| HADS-A | 11 | 10.5 | −0.89 | 0.37 |
| HADS-D | 13 | 10.5 | −0.96 | 0.34 |
Abbreviations: HADS-A = Hospital Anxiety and Depression Scale–anxiety subscale; HADS-D = Hospital Anxiety and Depression Scale–depression subscale; HIT-6 = headache impact test-6; VRS = verbal rating scale; VTA DBS = ventral tegmental area deep brain stimulation.
Wilcoxon signed-rank test.
*Statistically significant results if Bonferroni correction used.
Demographics and baseline clinical characteristics were similar between responders and nonresponders, with the possible exception of right-side attacks being more likely to respond than either left-sided or side-variable attacks (Table 2).
Table 2.
Baseline Patient Characteristics in Responders and Nonresponders
| Responders n = 29 |
Nonresponders n = 14 |
OR (95% CI) | p Value | |
| Age (y), mean (SD) | 54.1 (12.3) | 51.9 (11.3) | — | — |
| Sex (male patients) | 21 (72%) | 8 (57%) | 0.54 (0.11–2.66) | 0.451 |
| Duration of CCH (y), mean (SD) | 11.3 (7.7) | 13.6 (6.5) | — | — |
| No. of oral preventive treatments failed, mean (SD) | 8.3 (1.5) | 8.6 (1.0) | — | — |
| Laterality of attacks | ||||
| Right | 13 (45%) | 2 (14%) | 9.78 (1.04–91.84) | 0.046 |
| Left | 9 (31%) | 7 (50%) | 1.28 (0.24–6.85) | 0.776 |
| Side variable | 7 (24%) | 5 (36%) | Reference group | — |
| Baseline attack frequency (attacks per mo), median, IQR | 140 (91) | 154 (91) | — | — |
| Baseline attack severity (0–10 on VRS scale), median, IQR | 10 (1) | 9.5 (1.5) | — | — |
| Baseline attack duration (min), median, IQR | 120 (147.5) | 105 (90) | — | — |
| Baseline headache load, median, IQR | 700 (651.5) | 685.5 (738.75) | 1.00 (1.00–1.00) | 0.238 |
| Baseline HIT-6 score, median, IQR | 71 (9.5) | 68 (9.25) | — | — |
| Baseline HADS-A score, median, IQR | 12 (8) | 10.5 (7.75) | — | — |
| Baseline HADS-D score, median, IQR | 13 (9) | 12 (7) | — | — |
| Diagnosis of affective disorder (depression or anxiety) | 14 (48%) | 9 (64%) | 0.56 (0.08–2.61) | 0.378 |
| Other pain disorder (including migraine and nonheadache pain) | 13 (45%) | 7 (50%) | 1.42 (0.31–6.48) | 0.648 |
| Smoking history | 16 (55%) | 6 (42%) | — | — |
| Triptan responsea | 23/28c (82%) | 10/14 (71%) | — | — |
| Oxygen responsea | 18/24c (75%) | 9/13c (69%) | — | — |
| GON block responseb | 16/27c (59%) | 6/14 (43%) | — | — |
Abbreviations: CCH = chronic cluster headache; GON = greater occipital nerve; HADS = Hospital Anxiety and Depression Scale; HIT-6 = headache impact test-6; IQR = interquartile range; OR = odds ratio; V1/V2/V3 = first, second, and third divisions of the trigeminal nerve, respectively; VRS = verbal rating scale.
Response defined as 50% improvement in attack frequency at the time of last follow-up.
Defined as greater than 50% improvement in pain severity within 15 minutes.
Defined as greater than 50% improvement in pain severity within 2 weeks.
Not all patients had tried subcutaneous sumatriptan and/or oxygen because of contraindications, and 2 patients had not previously had a greater occipital nerve block.
Adverse Events
There were no serious adverse events. The most common side effects were discomfort or pain around the battery site (7 patients), diplopia and/or oscillopsia (6 patients), and neck stiffness (4 patients) (Table 3). Diplopia or oscillopsia could be resolved by altering stimulation amplitude in all patients other than one who had a preexisting trochlear nerve palsy after a previous head injury. Four nonresponders had the DBS system explanted, 2 of them secondary to infection of the peripheral hardware, and one because of postsurgical neuropathic pain around the head wound site. Two patients were lost to follow-up, and 4 patients died from unrelated conditions during the study period, all at least 1 year after the DBS implant.
Table 3.
Adverse Events

| Adverse event | No. of patients | Proportion |
| Battery site discomfort or pain | 9 | 21% |
| Transient diplopia or oscillopsia | 6 | 14% |
| Neck stiffness | 4 | 9% |
| Lead site pain | 3 | 7% |
| Lead migration | 1 | 2% |
| Swelling over battery site | 1 | 2% |
Voxel-Based Morphometry Results
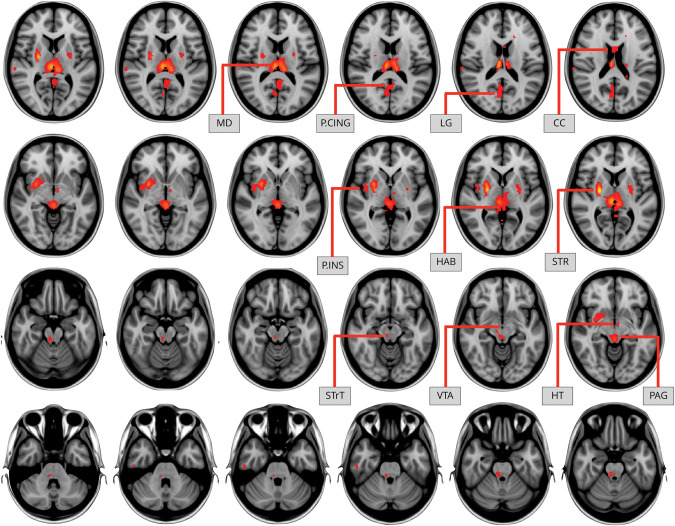
VBM showed differences in several brain regions between responders and nonresponders. Before correction for multiple comparisons, responders showed increased neural density in the posterior cingulate cortex, lingual gyrus, corpus callosum, posterior insula, habenula, striatum, VTA, hypothalamus and periaqueductal gray; however, the TFCE clusters did not survive multiple comparison correction. Nonresponders had increased neural density in the dorsal anterior cingulate cortex, anterior insula, amygdala, visual cortex, and the orbitofrontal cortex which was statistically significant after multiple comparison correction (1-p corrected = 0.999) (Figures 1 and 2).
Figure 1. Increased Neural Density in Responders.
Differences shown did not survive multiple comparison correction at 1-p corrected = 0.999. CC = corpus callosum; HAB = habenula; HT = hypothalamus; LG = lingual gyrus; MD = mediodorsal thalamus; P.CING = posterior cingulate cortex; P.INS = posterior insula; PAG = periaqueductal gray; SRT = striatum; STrT = spinal trigeminal tract; VTA = ventral tegmental area.
Figure 2. Increased Neural Density in Nonresponders.
Differences shown are statistically significant after multiple comparison correction (1-p corrected = 0.999). A.CING = anterior cingulate cortex; A.INS = anterior insula; AMY = amygdala; DA.CING = dorsal anterior cingulate cortex; OCC = occipital love; OFC = orbitofrontal cortex; OFC, orbitofrontal cortex; VS = ventral striatum.
DBS Target
All active electrodes were placed in the target in the VTA with a mean targeting error of 0.9 mm (SD = 0.6 mm). Group average volumes of tissue activation for the entire group, the responders, and nonresponders are presented in Figure 3. Table 4 shows the coordinates for the maximum intensity voxels and center of gravity for the group average volumes. There was no difference between the responder and the nonresponder groups, although the nonresponder group had a tendency of being more lateral, posterior, and inferior.
Figure 3. Volumes of Tissue Activation.
Group average volumes of tissue activation for the entire group (green), responders (red), and nonresponders (blue).
Table 4.
DBS Electrode Position
| Maximum intensity voxel (MNI ICBM) mm | Centre of gravity voxel (MNI ICBM) mm | |||||
| X | Y | Z | X | Y | Z | |
| Group mean | −4 | −13 | −9 | −4 | −13 | −8 |
| Responders | −4 | −13 | −9 | −4 | −13 | −8 |
| Nonresponders | −5 | −14 | −10 | −5 | −13 | −8 |
Abbreviations: DBS = deep brain stimulation; MNI ICBM = Montreal Neurological Institute International Consortium of Brain Mapping standardized stereotactic space.
Discussion
In the population studied in this prospective observational cohort study, VTA DBS showed no serious adverse events, was effective in approximately two-thirds of patients with treatment-refractory CCH, and remained effective at long-term follow-up. VBM analysis has been used to show that neural density differs between responders and nonresponders in several brain regions which are commonly linked to pain processing and central processing of autonomic function, both of which are relevant to the pathophysiology CCH.
The pathophysiology of cluster headache is imperfectly understood but believed to involve interactions between the hypothalamus and the trigeminovascular system. The circadian and circannual periodicity of cluster headache attacks and bouts suggest hypothalamic involvement. Positron emission tomography and functional MRI studies have demonstrated activation in the region of the posterior hypothalamus during attacks of cluster headache.16-18 Based on this finding, DBS was first used in a patient with treatment refractory CCH in 2001.19 Although initially described as the posterior hypothalamic region, the DBS target has been more precisely located at the VTA.20 Published case series suggest it is effective in 60%–80% of patients and remains effective with long-term follow-up.3-5 A single randomized sham-controlled trial including 11 patients with CCH was negative. However, the study was limited by the short blinded phase of only 1 month that overlaps with the possible stun period, and at 1 year follow-up, 6 (55%) were responders with no safety concerns.21 A randomized sham-controlled trial with a longer blinded phase is required to exclude a placebo effect, although this seems unlikely given the patients' lack of response to multiple other treatments including occipital nerve stimulation surgery and recognition that attack recurrence is often seen in patients who are unaware that the battery has run flat or stimulation has been turned off.3,22
Given the good response to treatment in approximately two-thirds of this highly disabled population, we therefore recommend considering DBS in similarly treatment-refractory patients with CCH, providing it is performed in centers which are experienced in its use and where there are arrangement for clinical governance, consent, and audit, as per National Institute for Health and Care Excellence guidelines.15 In the future, there may be newer less-invasive treatments which should be trialed before considering DBS. Noninvasive vagus nerve stimulation and monoclonal antibodies to calcitonin gene-related peptide are 2 newer treatments for headache disorders, but both seem to only be effective in episodic and not CCH.23,24
Previously, no clinical characteristics have been identified that reliably predict VTA DBS response in CCH, although the presence of bilateral (side-variable) attacks has been suggested to be a possible predictor of poor response.5 The increased likelihood of right-sided attacks responding to VTA DBS in this study is not easily explained. There is a slight preponderance for right-sided attacks in cluster headache.25 Taken together with better responses in those with right-sided attacks, it suggests that the pain neuromatrix may have some form of lateralization which both makes patients more prone to right-sided attacks and more likely to respond to treatment if attacks are right-sided. We hypothesized that patients with comorbid affective disorders or other chronic pain conditions may be less likely to respond because these has been shown to be negative predictive factors of response to occipital nerve stimulation for chronic headache disorders, but this was not borne out by the results.26
VBM has been used to show that gray matter concentration in an area in lobule VI of the cerebellum is associated with treatment responsiveness to verapamil.27 Brain morphometry studies have revealed neuroimaging predictors of response to DBS in Parkinson disease28,29 and obsessive compulsive disorder.30 Therefore, we posited that differences in brain structure may be associated with treatment response to VTA DBS in CCH. Indeed, several brain regions were identified which differ between responders and nonresponders. The regions with increased neural density in responders (thalamus, periaqueductal gray, hypothalamus) are known to be involved in modulation of peripheral pain signals, whereas the regions with increased neural density in nonresponders (anterior cingulate cortex, anterior insula, frontal cortex) are involved in the perception and expectation of pain and potentially the placebo effect, as well as the cortical representation of the autonomic nervous system.31,32
A variety of brain areas have been found to differ in patients with cluster headache compared with healthy controls in previous neuroimaging studies. The first published study in cluster headache using VBM found increased bilateral posterior hypothalamic gray matter volume, but this has not been confirmed by other studies.33,34 In our study, there was a small area of increased neural density in the left hypothalamic region in responders to DBS; however, the difference to nonresponders was not statistically significant. Another study using VBM found that patients with cluster headache had decreased gray matter volume in several brain regions involved in pain processing, possibly suggesting deficient top-down modulation of antinociceptive circuits in patients with cluster headache.35 However, other than the left insula, these areas do not overlap with the regions in which we found to correlate with response to VTA DBS.
Our finding of increased neural density in the amygdala and frontal cortex of nonresponders may suggest dysfunction in the corticolimbic system. Increased volumes of these brain regions and associated connectivity abnormalities have been shown in a previous study of CCH.36 Interestingly, the opposite finding of smaller amygdala volume has been shown to be a predictor of persistence in other chronic pain syndromes.37 This could be further investigated in future studies using connectivity measures, quantitative analysis of amygdala volume, and/or cortical thickness measures of the relevant frontal brain regions.
Our finding of morphometric brain differences between responders and nonresponders to VTA DBS suggests that with larger data sets in the future, structural neuroimaging may be able to improve prediction of likelihood of response for an individual patient. This may allow the identification of likely nonresponders before surgery and thereby avoid an invasive and costly operation and conversely to consider offering this treatment at an earlier stage in those who are highly likely to respond.
Identifying predictors of response may shed light on the mechanism of action of DBS, which is currently uncertain. A probabilistic tractography study in 7 patients who underwent DBS for CCH showed that the largest treatment response was associated with activation in an area which lay on a tract which connects the hypothalamus, prefrontal, and mesial temporal regions in the forebrain, with brainstem regions including the nucleus of the solitary tract, periaqueductal gray, trigeminal nucleus, and tract.8 This tract may correspond with the trigeminohypothalamic tract that has been demonstrated in rats.38 The often-delayed response to DBS, and previous findings that acute stimulation is unable to abort acute attacks, suggest that DBS may act by modulating the process of central sensitization, rather than the process of attack generation.5,39 More than half of the patients in our study experienced between 50% and 99% improvement. The fact that most patients did not have complete symptom resolution may support this mechanism of action. A previous PET study has shown stimulation-induced changes in a variety of cortical regions involved in pain processing, including deactivation in the middle temporal gyrus, posterior cingulate cortex, and contralateral anterior insula.40 These brain regions overlap with the regions we found to have increased neural density in nonresponders to VTA DBS, and the increased neural density in these regions may reflect a greater degree of central sensitization in the nonresponders which is more difficult to reverse by DBS.
Interestingly, despite the improvement in headache frequency, severity, and duration, we did not find a statistically significant improvement in disability or affective scores after VTA DBS (Table 1). The lack of a statistically significant (once multiple comparisons are taken into account) improvement in HIT-6 may be because it is not validated for use in cluster headache. The lack of improvement in HADS scores suggests than the mechanism of anxiety and depression in these patients may be different to that of cluster headache, although this is surprising given the association between central sensitization disorders and depression and anxiety.41
A challenge to neuroimaging studies in CCH is accounting for the attack lateralization. Cluster headache is a strictly unilateral disorder (although a minority of patients may have side-alternating attacks). Many previous neuroimaging studies in CCH have flipped the images along the x-axis so that brain regions are analyzed as ipsilateral or contralateral to the side of pain.16,34,42,43 Other studies have used the true left and right hemispheres regardless of the side of the headache, to take into account the lateralization of brain functions.44 Structural asymmetry has been shown in previous VBM MRI studies in healthy controls, the most right-handed persons having a larger left hemisphere.12,45 Most of our patients were right-handed, but 5 were left-handed (who may either have right hemisphere dominance and therefore larger right hemisphere or bilateral representation). A subset of our patients had bilateral side-variable attacks, with varying proportions of attacks on each side; therefore, we elected not to flip scans so that the true left and right were preserved and so that all treated patients could be included.
In conclusion, although it remains to be proven in clinical trials, VTA DBS seems to be an effective long-term treatment which may be considered in patients with CCH who are refractory to medical treatment. Neuroanatomic differences may explain why some patients do not respond. A randomized sham-controlled trial with a longer blinded phase is required to confirm the efficacy of VTA DBS for CCH.
Acknowledgment
We would like to thank our headache specialist nurses for their help with completion of the clinical database and management of the patients. We also thank the patients and their families for their help with this project. This research study was supported by researchers at the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre. No funding was received specifically for this study.
Glossary
- CCH
chronic cluster headache
- HADS
Hospital Anxiety and Depression Scale
- HIT-6
Headache Impact Test-6
- TFCE
threshold-free cluster enhancement
- VBM
voxel-based morphometry
- VRS
verbal rating scale
- VTA DBS
ventral tegmental area deep brain stimulation
Appendix. Authors

| Name | Location | Contribution |
| Sanjay Cheema, MBBS, BSc | Headache and Facial Pain Group, UCL Queen Square Institute of Neurology, London, UK; The National Hospital for Neurology and Neurosurgery, London, UK | Drafting/revision of the manuscript for content, including medical writing for content; drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Francisca Ferreira, MD | The National Hospital for Neurology and Neurosurgery, London, UK; Functional Neurosurgery Unit, UCL Queen Square Institute of Neurology, London, UK; Wellcome Centre for Human Neuroimaging, 12 Queen Square, London, UK; UCL EPSRC Centre for Doctoral Training in Intelligent Integrated Imaging in Healthcare (i4health), London, UK | Drafting/revision of the manuscript for content, including medical writing for content; drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Olga Parras, MD | The National Hospital for Neurology and Neurosurgery, London, UK; Functional Neurosurgery Unit, UCL Queen Square Institute of Neurology, London, UK | Drafting/revision of the manuscript for content, including medical writing for content; drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Susie Lagrata, MSc | The National Hospital for Neurology and Neurosurgery, London, UK | Drafting/revision of the manuscript for content, including medical writing for content; drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Salwa Kamourieh, MBBS, BSc, PhD | Headache and Facial Pain Group, UCL Queen Square Institute of Neurology, London, UK; The National Hospital for Neurology and Neurosurgery, London, UK | Drafting/revision of the manuscript for content, including medical writing for content; drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Ashkan Pakzad, AFHEA | Centre for Medical Image Computing, University College London, London, UK; Department of Medical Physics and Biomedical Engineering, University College London, London, UK | Drafting/revision of the manuscript for content, including medical writing for content; drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Ludvic Zrinzo, MD, PhD, FRCSEd (Neuro.Surg) | The National Hospital for Neurology and Neurosurgery, London, UK; Functional Neurosurgery Unit, UCL Queen Square Institute of Neurology, London, UK; | Drafting/revision of the manuscript for content, including medical writing for content; drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Manjit Matharu, BSc, MBChB, PhD, FRCP | Headache and Facial Pain Group, UCL Queen Square Institute of Neurology, London, UK; The National Hospital for Neurology and Neurosurgery, London, UK | Drafting/revision of the manuscript for content, including medical writing for content; drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Harith Akram, MBChB, FRCS (Neuro.Surg) | The National Hospital for Neurology and Neurosurgery, London, UK; Functional Neurosurgery Unit, UCL Queen Square Institute of Neurology, London, UK | Drafting/revision of the manuscript for content, including medical writing for content; drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
The authors report no targeted funding.
Disclosure
S. Cheema is supported by a research fellowship sponsored by Abbott; F. Ferreira reports no disclosures relevant to the manuscript; O. Parras reports no disclosures relevant to the manuscript; S. Lagrata has received payment for educational presentations and attending advisory board meetings from Allergan, Eli Lilly, Novartis, TEVA, and Lundbeck Ltd and has received payment for consultancy work from Salvia; S. Kamourieh reports no disclosures relevant to the manuscript; A. Pakzad reports no disclosures relevant to the manuscript; L. Zrinzo acts as a consultant for and has received payment for educational presentations from Medtronic & Boston Scientific; M. Matharu is chair of the medical advisory board of the CSF Leak Association; has served on advisory boards for Allergan, Autonomic Technologies Inc, Eli Lilly, Novartis, Pfizer, Salvia, and TEVA; has received payment for educational presentations from Allergan, electroCore, Eli Lilly, Novartis, and TEVA; has received grants from Abbott, Medtronic, and electroCore; and has a patent on system and method for diagnosing and treating headaches (WO2018051103A1, issued); H. Akram reports no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Headache Classification Committee of the International Headache Society. The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211. [DOI] [PubMed] [Google Scholar]
- 2.Fischera M, Marziniak M, Gralow I, Evers S. The incidence and prevalence of cluster headache: a meta-analysis of population-based studies. Cephalalgia. 2008;28(6):614-618. doi: 10.1111/j.1468-2982.2008.01592.x [DOI] [PubMed] [Google Scholar]
- 3.Leone M, Franzini A, Broggi G, Bussone G. Hypothalamic stimulation for intractable cluster headache: long-term experience. Neurology. 2006;67(1):150-152. doi: 10.1212/01.wnl.0000223319.56699.8a [DOI] [PubMed] [Google Scholar]
- 4.Akram H, Miller S, Lagrata S, et al. Ventral tegmental area deep brain stimulation for refractory chronic cluster headache. Neurology. 2016;86(18):1676-1682. doi: 10.1212/WNL.0000000000002632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leone M, Franzini A, Cecchini AP, Bussone G. Success, failure, and putative mechanisms in hypothalamic stimulation for drug-resistant chronic cluster headache. Pain. 2013;154(1):89-94. doi: 10.1016/j.pain.2012.09.011 [DOI] [PubMed] [Google Scholar]
- 6.Leone M, May A, Franzini A, et al. Deep brain stimulation for intractable chronic cluster headache: proposals for patient selection. Cephalalgia. 2004;24(11):934-937. doi: 10.1111/j.1468-2982.2004.00742.x [DOI] [PubMed] [Google Scholar]
- 7.Mitsikostas DD, Edvinsson L, Jensen RH, et al. Refractory chronic cluster headache: a consensus statement on clinical definition from the European Headache Federation. J Headache Pain. 2014;15(1):79. doi: 10.1186/1129-2377-15-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akram H, Miller S, Lagrata S, et al. Optimal deep brain stimulation site and target connectivity for chronic cluster headache. Neurology. 2017;89(20):2083-2091. doi: 10.1212/WNL.0000000000004646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosinski M, Bayliss MS, Bjorner JB, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 2003;12(8):963-974. doi: 10.1023/a:1026119331193 [DOI] [PubMed] [Google Scholar]
- 10.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 11.Douaud G, Smith S, Jenkinson M, et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130(pt 9):2375-2386. doi: 10.1093/brain/awm184 [DOI] [PubMed] [Google Scholar]
- 12.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 pt 1):21-36. doi: 10.1006/nimg.2001.0786 [DOI] [PubMed] [Google Scholar]
- 13.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208-S219. doi: 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- 14.Andersson J, Jenkinson M, Smith S. Non-linear Registration Aka Spatial Normalisation. FMRIB Technical Report TR07JA2. FMRIB Centre; 2007. [Google Scholar]
- 15.National Institute for Health and Care Excellence. Deep Brain Stimulation for Intractable Trigeminal Autonomic Cephalalgias; 2011. Accessed April 3, 2023. nice.org.uk/guidance/ipg381/chapter/1-Guidance. [Google Scholar]
- 16.May A, Bahra A, Buchel C, Frackowiak RS, Goadsby PJ. Hypothalamic activation in cluster headache attacks. Lancet. 1998;352(9124):275-278. doi: 10.1016/S0140-6736(98)02470-2 [DOI] [PubMed] [Google Scholar]
- 17.Sprenger T, Boecker H, Tolle TR, Bussone G, May A, Leone M. Specific hypothalamic activation during a spontaneous cluster headache attack. Neurology. 2004;62(3):516-517. doi: 10.1212/wnl.62.3.516 [DOI] [PubMed] [Google Scholar]
- 18.Morelli N, Pesaresi I, Cafforio G, et al. Functional magnetic resonance imaging in episodic cluster headache. J Headache Pain. 2009;10(1):11-14. doi: 10.1007/s10194-008-0085-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leone M, Franzini A, Bussone G. Stereotactic stimulation of posterior hypothalamic gray matter in a patient with intractable cluster headache. N Engl J Med. 2001;345(19):1428-1429. doi: 10.1056/NEJM200111083451915 [DOI] [PubMed] [Google Scholar]
- 20.Matharu MS, Zrinzo L. Deep brain stimulation in cluster headache: hypothalamus or midbrain tegmentum? Curr Pain Headache Rep. 2010;14(2):151-159. doi: 10.1007/s11916-010-0099-5 [DOI] [PubMed] [Google Scholar]
- 21.Fontaine D, Lazorthes Y, Mertens P, et al. Safety and efficacy of deep brain stimulation in refractory cluster headache: a randomized placebo-controlled double-blind trial followed by a 1-year open extension. J Headache Pain. 2010;11(1):23-31. doi: 10.1007/s10194-009-0169-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoenen J, Di Clemente L, Vandenheede M, et al. Hypothalamic stimulation in chronic cluster headache: a pilot study of efficacy and mode of action. Brain. 2005;128(pt 4):940-947. doi: 10.1093/brain/awh411 [DOI] [PubMed] [Google Scholar]
- 23.de Coo IF, Marin JC, Silberstein SD, et al. Differential efficacy of non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: a meta-analysis. Cephalalgia. 2019;39(8):967-977. doi: 10.1177/0333102419856607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dodick DW, Goadsby PJ, Lucas C, et al. Phase 3 randomized, placebo-controlled study of galcanezumab in patients with chronic cluster headache: results from 3-month double-blind treatment. Cephalalgia. 2020;40(9):935-948. doi: 10.1177/0333102420905321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gobel CH, Karstedt S, Heinze A, Koch B, Gobel H. Phenotype of cluster headache: clinical variability, persisting pain between attacks, and comorbidities-an observational cohort study in 825 patients. Pain Ther. 2021;10(2):1121-1137. doi: 10.1007/s40122-021-00267-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller S, Watkins L, Matharu M. Predictors of response to occipital nerve stimulation in refractory chronic headache. Cephalalgia. 2018;38(7):1267-1275. doi: 10.1177/0333102417728747 [DOI] [PubMed] [Google Scholar]
- 27.Tso AR, Brudfors M, Danno D, et al. Machine phenotyping of cluster headache and its response to verapamil. Brain. 2021;144(2):655-664. doi: 10.1093/brain/awaa388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Younce JR, Campbell MC, Perlmutter JS, Norris SA. Thalamic and ventricular volumes predict motor response to deep brain stimulation for Parkinson's disease. Parkinsonism Relat Disord. 2019;61:64-69. doi: 10.1016/j.parkreldis.2018.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yim Y, Kim SJ, Jung SC, et al. Pretreatment brain volumes can affect the effectiveness of deep brain stimulation in Parkinson's disease patients. Sci Rep. 2020;10(1):22065. doi: 10.1038/s41598-020-79138-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liebrand LC, Zhutovsky P, Tolmeijer EK, et al. Deep brain stimulation response in obsessive-compulsive disorder is associated with preoperative nucleus accumbens volume. Neuroimage Clin. 2021;30:102640. doi: 10.1016/j.nicl.2021.102640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci U S A. 2005;102(36):12950-12955. doi: 10.1073/pnas.0408576102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sklerov M, Dayan E, Browner N. Functional neuroimaging of the central autonomic network: recent developments and clinical implications. Clin Auton Res. 2019;29(6):555-566. doi: 10.1007/s10286-018-0577-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.May A, Ashburner J, Buchel C, et al. Correlation between structural and functional changes in brain in an idiopathic headache syndrome. Nat Med. 1999;5(7):836-838. doi: 10.1038/10561 [DOI] [PubMed] [Google Scholar]
- 34.Naegel S, Holle D, Desmarattes N, et al. Cortical plasticity in episodic and chronic cluster headache. Neuroimage Clin. 2014;6:415-423. doi: 10.1016/j.nicl.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Absinta M, Rocca MA, Colombo B, Falini A, Comi G, Filippi M. Selective decreased grey matter volume of the pain-matrix network in cluster headache. Cephalalgia. 2012;32(2):109-115. doi: 10.1177/0333102411431334 [DOI] [PubMed] [Google Scholar]
- 36.Ferraro S, Medina JP, Nigri A, et al. Mesocorticolimbic system abnormalities in chronic cluster headache patients: a neural signature? Cephalalgia. 2022;42(10):1039-1049. doi: 10.1177/03331024221092416 [DOI] [PubMed] [Google Scholar]
- 37.Vachon-Presseau E, Tetreault P, Petre B, et al. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain. 2016;139(pt 7):1958-1970. doi: 10.1093/brain/aww100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malick A, Strassman RM, Burstein R. Trigeminohypothalamic and reticulohypothalamic tract neurons in the upper cervical spinal cord and caudal medulla of the rat. J Neurophysiol. 2000;84(4):2078-2112. doi: 10.1152/jn.2000.84.4.2078 [DOI] [PubMed] [Google Scholar]
- 39.Leone M, Franzini A, Broggi G, Mea E, Cecchini AP, Bussone G. Acute hypothalamic stimulation and ongoing cluster headache attacks. Neurology. 2006;67(10):1844-1845. doi: 10.1212/01.wnl.0000247273.93084.49 [DOI] [PubMed] [Google Scholar]
- 40.May A, Leone M, Boecker H, et al. Hypothalamic deep brain stimulation in positron emission tomography. J Neurosci. 2006;26(13):3589-3593. doi: 10.1523/JNEUROSCI.4609-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams LM, Turk DC. Psychosocial factors and central sensitivity syndromes. Curr Rheumatol Rev. 2015;11(2):96-108. doi: 10.2174/1573397111666150619095330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teepker M, Menzler K, Belke M, et al. Diffusion tensor imaging in episodic cluster headache. Headache. 2012;52(2):274-282. doi: 10.1111/j.1526-4610.2011.02000.x [DOI] [PubMed] [Google Scholar]
- 43.Yang FC, Chou KH, Fuh JL, et al. Altered hypothalamic functional connectivity in cluster headache: a longitudinal resting-state functional MRI study. J Neurol Neurosurg Psychiatry. 2015;86(4):437-445. doi: 10.1136/jnnp-2014-308122 [DOI] [PubMed] [Google Scholar]
- 44.Kiraly A, Szabo N, Pardutz A, et al. Macro- and microstructural alterations of the subcortical structures in episodic cluster headache. Cephalalgia. 2018;38(4):662-673. doi: 10.1177/0333102417703762 [DOI] [PubMed] [Google Scholar]
- 45.Watkins KE, Paus T, Lerch JP, et al. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cereb Cortex. 2001;11(9):868-877. doi: 10.1093/cercor/11.9.868 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data are available on reasonable request from the corresponding author.