Abstract
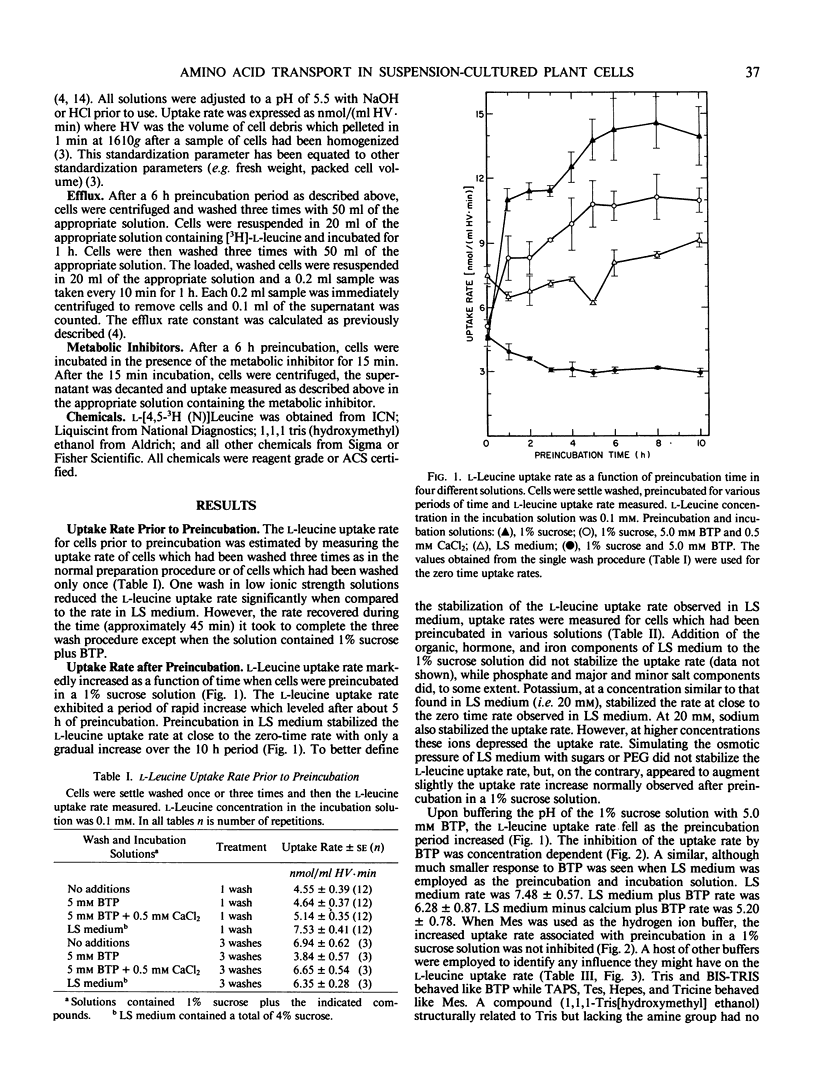
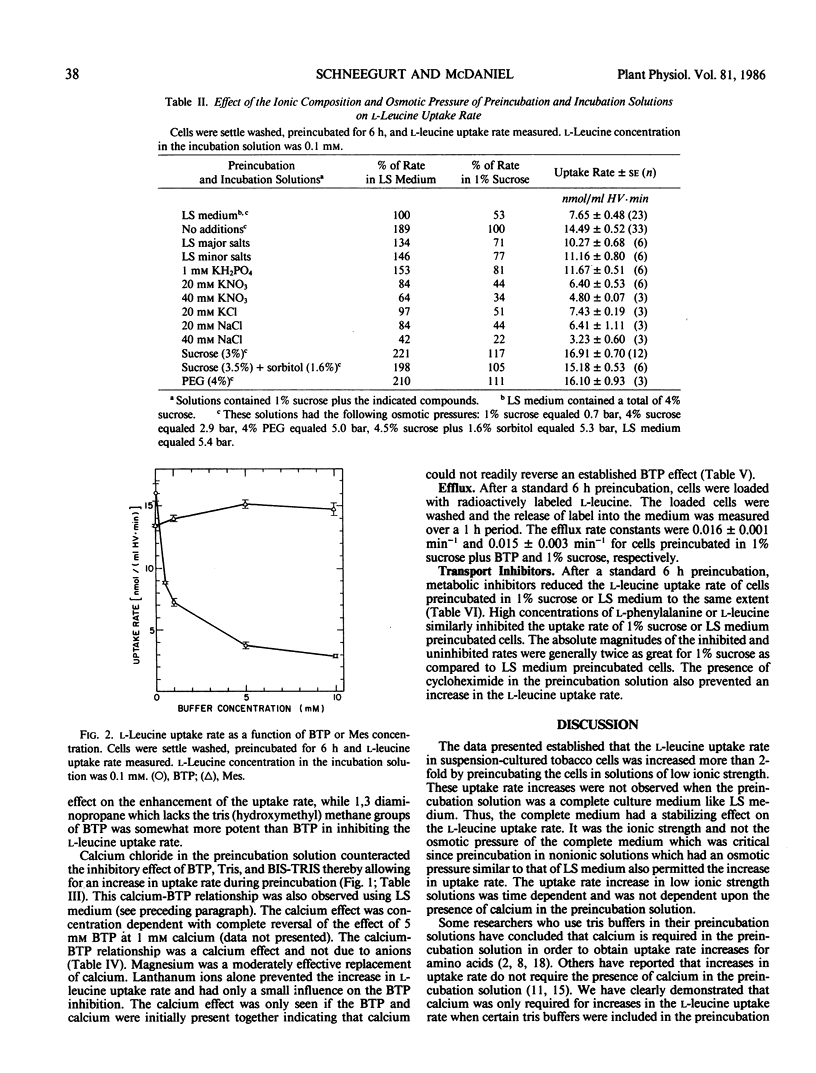
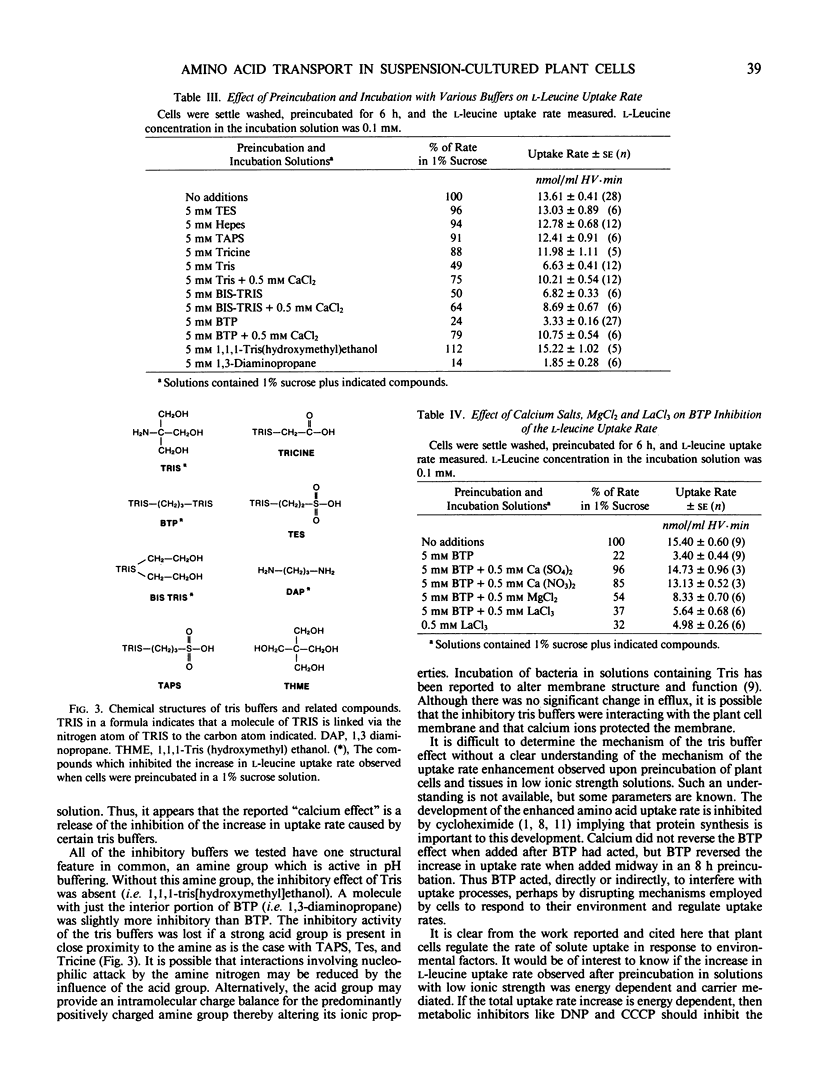
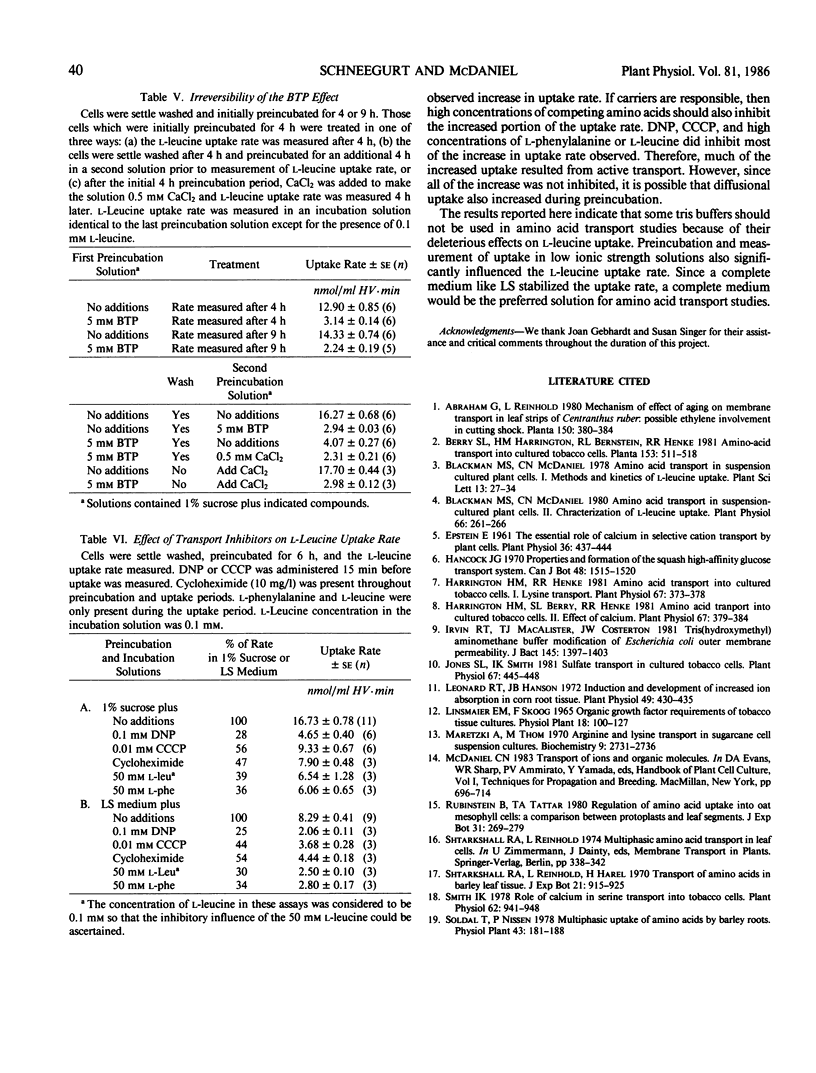
The rate at which l-leucine was transported into suspension-cultured Nicotiana tabacum cv Wisconsin 38 cells increased more than 2-fold over a period of hours when the cells were preincubated in a 1% sucrose solution. This increase in uptake rate was eliminated if certain tris buffers were included in the preincubation solution while other buffers had little effect. Calcium could reverse the effect of the inhibitory buffers only if the buffer and calcium were present together from the beginning of the preincubation period. It was the amine group of the inhibitory buffers which was responsible for the inhibition. Preincubation in a complete culture medium (EM Linsmaier, F Skoog 1965 Physiol Plant 18: 100-127) led to minimal changes in l-leucine uptake rate over a 10 hour preincubation period indicating that the uptake rate was stabilized by this medium. The complete medium stabilized the l-leucine uptake rate as a result of its ionic composition and not because of its osmolarity. Most of the increased uptake rate observed after preincubation in a 1% sucrose solution could be inhibited by 2,4-dinitrophenol or carbonyl cyanide m-chlorophenyl hydrazone, or high concentrations of l-phenyl-alanine or l-leucine. Therefore much of the increase could be accounted for by an increase in active transport of l-leucine.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackman M. S., McDaniel C. N. Amino Acid Transport in Suspension-cultured Plant Cells: II. CHARACTERIZATION OF l-LEUCINE UPTAKE. Plant Physiol. 1980 Aug;66(2):261–266. doi: 10.1104/pp.66.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. The essential role of calcium in selective cation transport by plant cells. Plant Physiol. 1961 Jul;36(4):437–444. doi: 10.1104/pp.36.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington H. M., Berry S. L., Henke R. R. Amino Acid Transport into Cultured Tobacco Cells: II. EFFECT OF CALCIUM. Plant Physiol. 1981 Feb;67(2):379–384. doi: 10.1104/pp.67.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington H. M., Henke R. R. Amino Acid Transport into Cultured Tobacco Cells: I. LYSINE TRANSPORT. Plant Physiol. 1981 Feb;67(2):373–378. doi: 10.1104/pp.67.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin R. T., MacAlister T. J., Costerton J. W. Tris(hydroxymethyl)aminomethane buffer modification of Escherichia coli outer membrane permeability. J Bacteriol. 1981 Mar;145(3):1397–1403. doi: 10.1128/jb.145.3.1397-1403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. L., Smith I. K. Sulfate Transport in Cultured Tobacco Cells : EFFECTS OF CALCIUM AND SULFATE CONCENTRATION. Plant Physiol. 1981 Mar;67(3):445–448. doi: 10.1104/pp.67.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hanson J. B. Induction and development of increased ion absorption in corn root tissue. Plant Physiol. 1972 Mar;49(3):430–435. doi: 10.1104/pp.49.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretzki A., Thom M. Arginine and lysine transport in sugarcane cell suspension cultures. Biochemistry. 1970 Jun 23;9(13):2731–2736. doi: 10.1021/bi00815a022. [DOI] [PubMed] [Google Scholar]
- Smith I. K. Role of calcium in serine transport into tobacco cells. Plant Physiol. 1978 Dec;62(6):941–948. doi: 10.1104/pp.62.6.941. [DOI] [PMC free article] [PubMed] [Google Scholar]


