Abstract
目的
探究尿酸水平对慢性肾脏病(CKD)3-4期免疫球蛋白A肾病(IgAN)患者的临床病理及预后的影响。
方法
回顾性收集2008年12月–2020年1月在西京医院经肾活检确诊的263例CKD 3-4期IgAN患者的临床和病理资料,根据空腹血尿酸水平是否>420 μmol/L将其分为高尿酸组(n=102)及尿酸正常组(n=161),比较两组的临床病理特征。以进展至终末期肾病或死亡为终点事件,通过Kaplan-Meier法比较两组患者的肾脏存活率,采用Cox和LASSO回归分析尿酸与预后的关系。
结果
与尿酸正常组相比,高尿酸组男性及高血压病史的患者占比更多,血尿素氮(BUN)更高,估计肾小球滤过率(eGFR)、高密度脂蛋白(HDL)更低;在病理方面,高尿酸组患者肾小球硬化比例、系膜细胞增生、肾小管萎缩或肾间质纤维化较高,差异均有统计学意义(P<0.05)。Kaplan-Meier曲线显示两组肾脏生存率差异有统计学意义(P<0.0001); LASSO回归显示高尿酸是影响CKD 3-4期IgAN患者预后情况的危险因素,进一步的多因素Cox分析显示,与尿酸正常组相比,高尿酸组发生复合结局风险更高〔风险比(HR)=1.61,95%可信区间(CI):1.10~2.34〕。当尿酸作为连续性变量时,尿酸质量浓度每增加1 mg/dL,发生复合结局的HR增加1.18 (95%CI:1.08~1.29)。
结论
高尿酸是CKD 3-4期IgAN患者肾脏预后不良的危险因素,降低尿酸水平可能有效改善高风险IgAN患者预后。
Keywords: IgA肾病, 慢性肾脏病, 高尿酸血症, 预后, 回顾性
Abstract
Objective
To investigate the effect of uric acid on the clinicopathological characteristics and prognosis of immunoglobulin A nephropathy (IgAN) in patients with stage 3-4 chronic kidney disease (CKD).
Methods
The clinical and pathological data of 263 IgAN patients who had stage 3-4 CKD and who had confrimed diagosis through renal biopsy at the First Affiliated Hospital of Air Force Medical University between December 2008 and January 2020 were retrospectively collected. According to the levels of uric acid, the patients were divided into a hyperuricemia group (n=102) and a normal uric acid group (n=161), and the clinicopathological characteristics of the two groups were compared accordingly. With progression to end-stage renal disease or death as the endpoint event, the renal survival rate of the two groups was compared by the Kaplan-Meier method and the relationship between uric acid and the prognosis was analyzed by Cox regression and LASSO regression.
Results
Compared with the normal uric acid group, the hyperuricemia group had a significantly higher proportion of male patients and patients with a history of hypertension, a significantly higher level of blood urea nitrogen, and lower levels of estimated glomerular filtration rate and high-density lipoprotein. In terms of pathology, patients in the hyperuricemia group had significantly higher proportion of glomerulosclerosis, higher mesangial hypercellularity, and higher tubular atrophy/interstitial fibrosis (P<0.05). Kaplan-Meier curve showed that there was a significant difference in renal survival rate between the two groups (P<0.0001). LASSO regression showed that high uric acid was a risk factor for the prognosis of IgAN patients with stage 3-4 CKD. Further multivariate Cox analysis showed that, compared with the normal uric acid group, the hyperuricemia group had a higher risk of incurring composite outcomes (hazard ratio [HR]=1.61, 95% confidence interval [CI]: 1.10-2.34). When uric acid was used as a continuous variable, the increase of 1 mg/dL in uric acid concentration was associated with an increased HR of 1.18 (95% CI: 1.08-1.29) for the composite outcome.
Conclusion
High uric acid is a risk factor for poor renal prognosis in IgAN patients with stage 3-4 CKD and reducing uric acid levels may effectively improve the prognosis of high-risk IgAN patients.
Keywords: IgA nephropathy, Chronic kidney disease, Hyperuricemia, Prognosis, Retrospective
免疫球蛋白A肾病(immunoglobulin A nephropathy, IgAN)是全球最常见的原发性肾小球肾炎,其中15%~50%的病例有从无症状到终末期肾病(end-stage kidney disease, ESKD)的临床表现[1]。估计肾小球滤过率(estimated glomerular filtration rate, eGFR)降低和尿蛋白定量>1 g/d被认为是IgAN进展的危险因素[2],这类肾功能不全合并大量蛋白尿的高风险IgAN患者发生心血管事件、ESKD和死亡风险显著增加[3]。慢性肾脏病(chronic kidney disease, CKD)3-4 期的IgAN患者往往有广泛且不可逆的肾功能损伤,目前也尚无最佳的治疗方案[4-5]。因此,早期识别危险因素和个体化治疗对于延缓高风险IgAN患者的疾病进展和改善预后至关重要。基线肾功能、高血压和病理变化等已被证实与IgAN的进展有关[6]。高尿酸血症是嘌呤代谢障碍导致的一种常见的代谢性疾病,发病率逐年增高[7]。研究发现,尿酸(uric acid, UA)可通过诱导炎症、内皮功能障碍和激活肾素-血管紧张素系统参与CKD的发生发展[8-9]。多项研究证实UA是IgAN进展至ESKD的危险因素[10],然而UA水平与高风险IgAN患者预后的关系尚未可知[11-12]。有证据显示别嘌呤醇对所有类型CKD 3-4期患者的肾脏进展无减缓作用[13],与安慰剂相比,非布索坦并未缓解3期CKD合并无症状高尿酸血症患者的肾功能下降[14],降尿酸治疗能否延缓eGFR较低的CKD患者的肾功能仍存在争议[10]。因此,本研究旨在探究高尿酸血症对CKD 3-4期IgAN患者的预后影响,为临床预后观察和治疗提供证据。
1. 资料和方法
1.1. 研究对象
回顾性收集我院2008年12月–2020年1月经肾活检诊断为CKD 3-4期IgAN的患者。纳入标准:①年龄≥18岁且有完整随访记录;②eGFR 15~60 mL/(min·1.73 m2);③24 h尿蛋白定量≥1000 mg/d。排除标准:①继发性IgAN患者:紫癜性肾炎、狼疮肾炎、乙型肝炎相关性肾炎、干燥综合征等;②合并症:糖尿病、严重心血管疾病等;③随访时间<6个月(达到终点除外);④肾活检标本中光镜下肾小球数目<8个或肾活检报告缺失;⑤资料不全。随访时间截至2023年5月。本研究经西京医院伦理委员会批准(伦理号:KY20213027-1)并遵循《世界医学会赫尔辛基宣言》。
1.2. 观察指标
通过电子病历系统收集患者活检时的临床特征和生存情况。收集患者一般情况包括性别、年龄、体质量指数(body mass index, BMI)、高血压病史、治疗方案等;实验室指标包括平均动脉压、血常规、血尿素氮(blood urea nitrogen, BUN)、UA、eGFR、24 h尿蛋白定量等。
1.3. 病理资料
记录病理报告中的光镜、电镜、荧光、肾小球硬化比例(肾小球硬化数目/肾小球总数)结果。根据2017年牛津病理分型[15]进行分型:①系膜细胞增生(M):M0:系膜增殖积分≤0.5,M1:系膜增殖积分>0.5;②内皮细胞增生(E): E0:无内皮细胞增生,E1:有内皮细胞增生;③节段性硬化或粘连(S):S0:无节段性硬化或粘连,S1:有节段性硬化或粘连;④肾小管萎缩或肾间质纤维化(T):T0:≤25%,T1:26%~50%,T2:>50%;⑤细胞或纤维细胞新月体(C):C0:无新月体, C1:<25%, C2:≥25%。
1.4. 变量定义
高尿酸血症定义为非同日2次检测空腹血尿酸水平>420 μmol/L(成年人,不分男女性)[16] 并据此将其分为高尿酸组和尿酸正常组;ESKD定义为eGFR<15 mL/(min·1.73 m2),或开始长期肾脏替代治疗,或接受肾移植[1];eGFR采用慢性肾脏疾病流行病学合作组织(CKD-EPI)制定的公式估算[17]。CKD的分类和定义基于KDIGO于2021年提供的临床实践指南[1]。
临床结局:发生ESKD或死亡的复合事件。
1.5. 统计学方法
所有统计在SPSS 25.0和R 4.2.3中完成。符合正态分布的计量资料用 表示,采用独立样本t检验进行组间比较。非正态分布的计量资料使用中位数(四分位数间距)表示,采用非参数检验进行组间比较。计数资料采用频数(百分率)表示,组间比较采用χ2检验。用Kaplan-Meier生存曲线计算肾脏累积生存率,并采用log-rank检验进行组间比较。采用单因素Cox回归分析筛选出P<0.05的变量,在LASSO回归中进一步验证,并将单因素指标纳入多因素Cox回归模型,尿酸分别作连续变量和分类变量纳入模型。使用前向逐步选择算法来识别独立的预测因子。使用R中的“rms”包进行Cox回归,使用“glmnet”包进行LASSO回归并通过10次交叉验证,得出的最佳惩罚参数λ值对应的最优影响因素。P<0.05为差异有统计学意义。
表示,采用独立样本t检验进行组间比较。非正态分布的计量资料使用中位数(四分位数间距)表示,采用非参数检验进行组间比较。计数资料采用频数(百分率)表示,组间比较采用χ2检验。用Kaplan-Meier生存曲线计算肾脏累积生存率,并采用log-rank检验进行组间比较。采用单因素Cox回归分析筛选出P<0.05的变量,在LASSO回归中进一步验证,并将单因素指标纳入多因素Cox回归模型,尿酸分别作连续变量和分类变量纳入模型。使用前向逐步选择算法来识别独立的预测因子。使用R中的“rms”包进行Cox回归,使用“glmnet”包进行LASSO回归并通过10次交叉验证,得出的最佳惩罚参数λ值对应的最优影响因素。P<0.05为差异有统计学意义。
2. 结果
2.1. 基线资料
见表1。共收集到2831例经活检证实的IgAN患者,最终共纳入263例CKD 3-4期患者。其中男性180例(68.4%),女性83例(31.6%)。高尿酸组102例(38.8%),尿酸正常组161例(61.2%)。与尿酸正常组相比,高尿酸组男性及高血压病史的患者占比更多, BUN更高,eGFR、HDL更低,且差异有统计学意义(P<0.05)。两组之间BMI、24 h尿蛋白定量等方面差异无统计学意义(P>0.05)。
表 1. Baseline characteristics of the subjects.
研究对象的基线特征
| Characteristic | All patients (n=263) | Hyperuricemia group (n=102) |
Normal uric acid group (n=161) |
P |
| BMI: body mass index; RAAS: renin-angiotensin system; MAP: mean arterial pressure; UTP: urinary total protein; WBC: white blood cell; eGFR: estimated glomerular filtration rate; HDL: high-density lipoprotein; AST: aspartate aminotransferase. 1 All patients received supportive treatment if they can tolerate it. 2 The Oxford classification: mesangial hypercellularity (M0, mesangial score≤0.5; M1, mesangial score>0.5), endocapillary hypercellularity (E0, absent; E1, present), segmental glomerulosclerosis (S0, absent; S1, present), tubular atrophy/interstitial fibrosis (T0, ≤25%; T1, 26%-50 %; T2, >50%), and cellular or fibrocellular crescents (C0, absent; C1, <25%; and C2, ≥25% of the glomeruli). | ||||
| Age/yr., median (P25, P75) | 36.00 (29.00, 47.00) | 33.00 (28.00, 43.75) | 41.00 (31.00, 49.00) | 0.001 |
| Male/case (%) | 180 (68.4) | 86 (84) | 94 (58) | <0.001 |
| BMI/(kg/m2), median (P25, P75) | 24.02 (21.48, 26.64) | 24.36 (21.62, 27.25) | 23.88 (21.47, 26.08) | 0.257 |
| RAAS blockade before biopsy/case (%) | 91 (34.6) | 35 (34.3) | 56 (34.8) | 0.938 |
| Glucocorticoid before biopsy/case (%) | 36 (13.7) | 15 (14.7) | 21 (13.0) | 0.702 |
| Hypertension/case (%) | 183 (69.6) | 80 (78.4) | 103 (63.9) | 0.013 |
| MAP/mmHg, median (P25, P75) | 102.67 (93.00, 113.67) | 105.67 (96.33, 116.33) | 102.00 (90.67, 112.66) | 0.066 |
| UTP/(mg/d), median (P25, P75) | 1912 (1372.00, 2998.00) | 1869 (1345.00, 2894.50) | 1930 (1390.00, 3041.00) | 0.846 |
| WBC/(109 L-1), median (P25, P75) | 7.19 (6.08, 8.76) | 7.19 (5.88, 8.53) | 7.17 (6.15, 8.95) | 0.541 |
| eGFR/(mL/[min·1.73 m2]), median (P25, P75) | 41.64 (30.17, 49.98) | 35.95 (26.79, 46.49) | 44.34 (35.18, 52.59) | <0.001 |
| Blood urea nitrogen/(mmol/L), median (P25, P75) | 8.31 (6.80, 11.49) | 10.24 (7.60, 13.46) | 7.54 (6.07, 10.10) | <0.001 |
| Serum uric acid/(mg/dL), median(P25, P75) | 6.52 (5.66, 7.88) | 8.39 (7.60, 10.71) | 5.87(5.06, 6.41) | <0.001 |
| Serum glucose/(mmol/L), median (P25, P75) | 4.77 (4.38, 5.20) | 4.77 (4.37, 5.16) | 4.80 (4.39, 5.27) | 0.459 |
Serum potassium/(mmol/L), 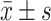
|
4.27±0.51 | 4.33±0.49 | 4.23±0.51 | 0.120 |
| Glomeruli sclerosis ratio (median [P25, P75]) | 36.36 (16.00, 55.55) | 41.67 (27.12, 57.70) | 29.00 (12.50, 50.00) | <0.001 |
| Total protein/(g/L), median (P25, P75) | 62.80 (55.70, 68.30) | 63.45 (56.20, 68.30) | 62.20 (54.50, 68.40) | 0.320 |
| HDL/(mmol/L), median (P25, P75) | 1.08 (0.88, 1.35) | 1.00 (0.87, 1.25) | 1.17 (0.90, 1.39) | 0.007 |
| Triglyceride/(mmol/L), median (P25, P75) | 2.14 (1.45, 3.09) | 2.35 (1.54, 3.29) | 2.03 (1.38, 2.95) | 0.124 |
| AST/(U/L), median (P25, P75) | 17.00 (15.00, 22.00) | 18.00 (15.00, 22.00) | 17.00 (15.00, 22.00) | 0.782 |
| Indirect bilirubin/(μmol/L), median (P25, P75) | 5.80 (4.30, 7.90) | 5.80 (4.19, 7.85) | 5.90 (4.30, 7.90) | 0.491 |
Hemoglobin/(g/L), 
|
128.06±23.11 | 127.12±24.25 | 128.65±22.42 | 0.640 |
| Treatment/case (%)1 | <0.001 | |||
| Only supportive treatment | 128 (48.6) | 54 (52.9) | 74 (45.9) | |
| Immunosuppressants | 135 (51.3) | 48 (47.1) | 87 (54.0) | |
| CKD stage/case (%) | ||||
| Stage 3 | 199 (75.6) | 64 (62.7) | 135 (83.9) | |
| Stage 4 | 64 (24.3) | 38 (37.3) | 26 (16.1) | |
| Oxford classification/case (%)2 | ||||
| M1 | 166 (63.1) | 74 (72.5) | 92 (57.1) | 0.012 |
| E1 | 55 (20.9) | 20 (19.6) | 35 (21.7) | 0.679 |
| S1 | 233 (88.6) | 92 (90.2) | 141 (87.6) | 0.515 |
| T1 | 91 (34.6) | 39 (38.2) | 52 (32.3) | 0.001 |
| T2 | 113 (42.9) | 52 (50.9) | 61 (37.9) | 0.001 |
| C1 | 95 (36.1) | 35 (34.3) | 60 (37.3) | 0.689 |
| C2 | 26 (9.8) | 12 (11.8) | 14 (8.7) | 0.692 |
| Follow-up/month, median (P25, P75) | 57.32 (32.05, 82.96) | 41.62 (15.79, 61.22) | 66.51 (45.82, 96.29) | |
| Combined event/case (%) | 124 (47.1) | 60 (58.8) | 64 (39.8) | |
| ESKD | 110 (41.8) | 53 (51.9) | 57 (35.4) | |
| Death | 14 (5.3) | 7 (6.9) | 7 (4.3) | |
2.2. 肾脏病理情况比较
与尿酸正常组相比,高尿酸组肾小球硬化比例、系膜细胞增生、肾小管萎缩或肾间质纤维化较高,差异有统计学意义(P<0.05)。两组之间在内皮细胞增生、节段性硬化或粘连和新月体形成方面差异无统计学意义(P>0.05)(表1)。
2.3. 肾脏累积生存率
本研究中位随访时间 57.32(32.05, 82.96)个月,共124例(47.1%)患者出现ESKD或死亡。高尿酸组有60例(58.8%)出现复合结局,包括53例(51.9%)ESKD和7例(6.9%)死亡。尿酸正常组有64例(39.8%)出现复合结局,其中57例(35.4%)发生ESKD,7例(4.3%)死亡。Kaplan-Meier 生存曲线分析结果显示,合并高尿酸血症的IgAN患者肾脏存活率低于非高尿酸组,log-rank检验提示差异有统计学意义(P<0.0001),见图1。
图 1.
Comparison of cumulative renal survival rates between the hyperuricemia group and the normal uric acid group
高尿酸组与尿酸正常组肾脏累积存活率比较
Red and blue shades represent the range of confidence intervals for each group.
2.4. 影响肾脏预后的危险因素
以是否发生复合事件为结局变量,进行单因素和多因素Cox回归分析探究尿酸对CKD 3-4期IgAN预后的影响。单因素Cox回归结果显示,当UA作为二分类变量时,高尿酸组发生复合结局的风险更高〔风险比(hazard ratio, HR)=2.42,95%可信区间(confidence interval, CI):1.69~3.46〕,当UA作为连续性变量时,发生复合结局的风险为HR=1.28(95%CI:1.17~1.40)(表2)。见图2,考虑混杂因素年龄、BMI、白细胞、血红蛋白、天冬氨酸氨基转移酶、间接胆红素、BUN、血钾、eGFR、肾小球硬化比例、高血压病史、治疗方案、M、S、T的影响,进一步进行LASSO回归,结果显示尿酸是影响预后的危险因素。
表 2. Univariate and multivariate Cox analyses of IgAN patients with stage 3-4 CKD.
CKD 3-4期IgAN患者的单因素和多因素Cox分析
| Characteristic | Univariate Cox | Multivariate Cox (UA as continuous variable) |
Multivariate Cox (UA as dichotomous variables) |
|||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||
| BMI: body mass index; NLR: neutrophil to lymphocyte ratio; Hb: hemoglobin; IB: indirect bilirubin; HUA: high uric acid; AST: aspartate aminotransferase. 1 The Oxford classification denotes the same as that in Table 1. | ||||||||
| Age | 0.96 (0.95-0.98) | <0.001 | 0.97 (0.95-0.99) | 0.002 | 0.97 (0.95-0.99) | <0.001 | ||
| BMI | 0.94 (0.90-0.99) | 0.026 | ||||||
| WBC | 1.07 (1.00-1.15) | 0.044 | ||||||
| Hb | 0.98 (0.98-0.99) | <0.001 | ||||||
| AST | 0.97 (0.95-1.00) | 0.048 | ||||||
| IB | 0.91 (0.85-0.97) | 0.005 | ||||||
| Blood urea nitrogen | 1.15 (1.11-1.20) | <0.001 | ||||||
| HUA (as dichotomous variables) | 2.42 (1.69-3.46) | <0.001 | 1.61 (1.10-2.34) | 0.013 | ||||
| HUA (as continuous variable) | 1.28 (1.17-1.40) | <0.001 | 1.18 (1.08-1.29) | <0.001 | ||||
| Serum potassium | 2.21 (1.59-3.07) | <0.001 | 1.46 (1.04-2.06) | 0.03 | ||||
| eGFR | 0.93 (0.91-0.95) | <0.001 | 0.93 (0.91-0.95) | <0.001 | 0.92 (0.90-0.94) | <0.001 | ||
| Glomeruli sclerosis ratio | 1.02 (1.01-1.03) | <0.001 | ||||||
| Hypertension | 1.97 (1.3-2.99) | 0.002 | ||||||
| Treatment immunosuppressants |
0.66 (0.46-0.95) | 0.024 | ||||||
| Oxford classification1 | ||||||||
| M1 | 2.17 (1.46-3.23) | <0.001 | 1.71 (1.11, 2.64) | 0.015 | 1.83 (1.19, 2.80) | 0.006 | ||
| S1 | 2.92 (1.36-6.27) | 0.006 | ||||||
| T1 | 3.28 (1.65-6.53) | 0.001 | 2.78 (1.36, 5.68) | 0.005 | 2.79 (1.37, 5.68) | 0.005 | ||
| T2 | 5.69 (2.93-11.08) | <0.001 | 1.96 (0.95, 4.02) | 0.067 | 1.88 (0.92, 3.84) | 0.083 | ||
图 2.
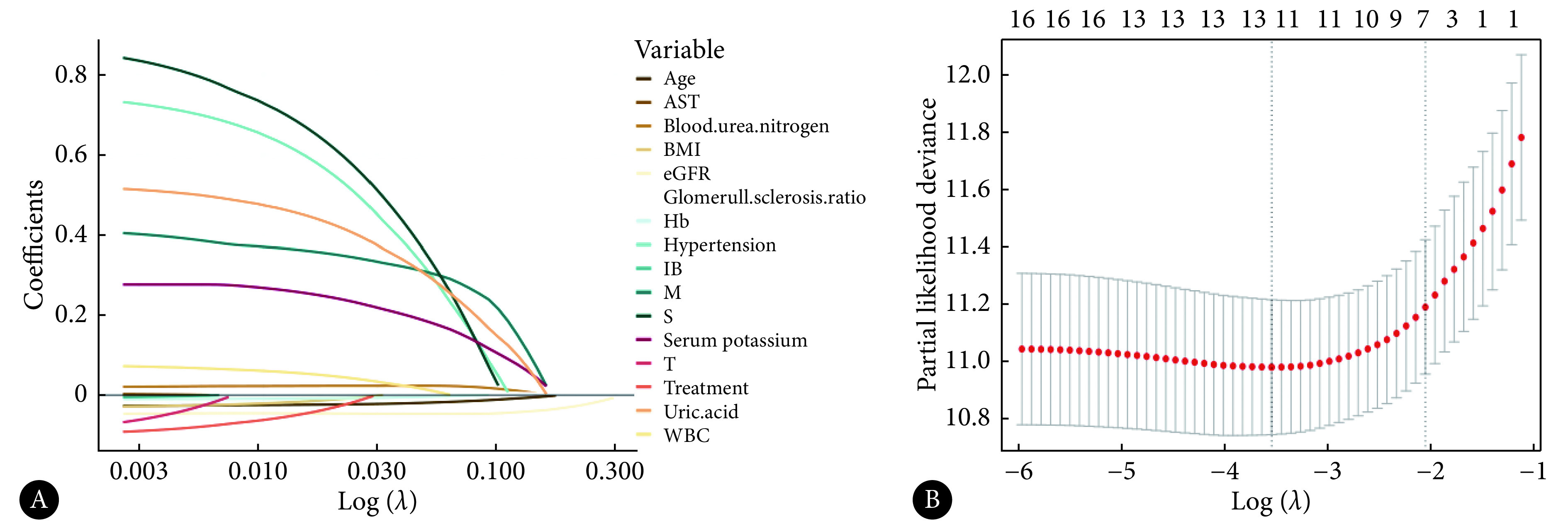
LASSO regression analysis of IgAN patients with stage 3-4 CKD
CKD 3-4期IgAN患者的LASSO 回归分析
AST, BMI, eGFR and WBC denote the same as those in Table 1; Hb, IB, M, S and T denote the same as those in Table 2. A, The selection process of variables fitted by the LASSO regression model after univariate Cox regression selection. Each colored line represents a variable, and the abscissa below is the Log value corresponding to the best penalty parameter λ. B, The process of screening the variables in the dataset with the change of the value of the penalty parameter λ. The ordinate is the minimum mean square error, the upper ordinate is the number of independent variables, the lower ordinate is the Log value corresponding to the λ value, and the first dashed line (left) represents the optimal solution of the λ value.
多因素Cox分析结果显示,当尿酸作为二分类变量时,与尿酸正常组相比,高尿酸组发生复合结局风险更高(HR=1.61,95%CI:1.10~2.34)。当尿酸作为连续性变量时,尿酸质量浓度每增加1 mg/dL,发生复合结局的HR增加1.18(95%CI:1.08~1.29)(表2)。
3. 讨论
IgAN是我国最常见的原发性肾小球疾病,约20%的IgAN患者在肾活检时表现为CKD 3-4期[18],且近80%的患者会在7~10年内进展为ESKD[19]。体内嘌呤物质代谢产生UA,约2/3的UA经肾脏排泄。研究发现,高尿酸血症与CKD的进展密切相关[10]。本研究结果显示,约38.8%的CKD 3-4期IgAN患者发生高尿酸血症,与吕天洋等[20]和袁茜宁等[21]报道的结果相近。在高尿酸组,男性患者占比更多,患高血压病史的人更多,BUN更高,eGFR、HDL更低,与既往报道结果一致[22-23]。一项Meta分析显示,UA水平和高血压发生风险呈正相关,每增加1 mg/dL的UA,高血压的总体风险就会增加13%[24]。UA可通过激活肾素-血管紧张素-醛固酮系统、胰岛素抵抗等机制引起血压的升高[8]。研究发现高脂血症与高尿酸血症在代谢机制上互为因果,嘌呤代谢为UA时,会产生大量自由基,促进体内脂质过氧化,导致HDL下降,促进肾动脉硬化、减少肾灌注及竞争性抑制,从而抑制尿酸排泄,导致高尿酸血症[25]。血UA水平与肾功能进展密切相关[26],本研究发现高尿酸组BUN增加,eGFR降低,提示IgAN合并高尿酸血症患者的肾功能受损更为严重。
在病理变化方面,本研究显示,高尿酸组患者肾小球硬化比例、M、T均显著高于尿酸正常组。研究发现,高UA水平诱导肾小球、小管间质和血管损伤,导致IgAN患者肾功能进一步恶化并进展为ESKD,特别是在IgAN合并CKD 3a期患者中,高尿酸血症患者肾小球球性硬化的比例是尿酸正常人的3倍[27]。以上结果提示降低UA水平可能有效减轻IgAN患者肾脏病理损伤。
多因素Cox和LASSO回归分析均显示尿酸是CKD 3-4期IgAN患者肾脏预后不良的危险因素。Kaplan-Meier生存曲线显示高尿酸组的肾脏生存率低于尿酸正常组。一项Meta分析结果显示尿酸水平升高可加重IgAN患者的肾功能和病理变化,进而加重患者预后,与本研究结果一致[12]。SRIVASTAVA等[28]纳入了3885例CKD 2-4期患者,发现当eGFR≥45 mL/(min·1.73 m2)时,较高的血尿酸水平与肾功能衰竭的风险呈独立相关,尿酸与全因死亡率的关系呈J型。尿酸影响IgAN合并CKD患者预后的潜在机制可能与血流动力学因素如肾小球静水压力的增加、血流量减少和非血流动力学因素如刺激炎症、激活血管收缩途径、抑制内皮细胞功能等有关[10]。
多项研究试图解答降尿酸治疗是否可延缓CKD的进展,但尚无一致结论。SIRCAR等[29]发现非布司他可改善CKD的预后,但日本一项大型队列研究认为非布司他无明显作用[14]。BADVE等[13]的研究发现别嘌呤醇对延缓CKD 3-4期的进展无作用。因此,尽管本研究认为尿酸是影响CKD 3-4期IgAN的危险因素,但早期干预高尿酸血症是否可改善高风险IgAN患者的预后仍需在多中心、大样本的RCT研究中进一步验证。
综上所述,CKD 3-4期IgAN伴高尿酸血症患者的肾脏损害和病理变化更重,尿酸是影响IgAN合并CKD 3-4期患者预后的重要危险因素。在IgAN患者进展至CKD 3-4期之前控制尿酸水平可能有助于延缓疾病进展,改善患者预后。
* * *
作者贡献声明 余紫娴负责论文构思、数据审编、正式分析、可视化和初稿写作,秦云龙负责正式分析、研究方法和验证,袁进国负责监督指导、验证和审读与编辑写作,赵晋负责经费获取和审读与编辑写作,孙世仁负责论文构思、经费获取、监督指导、初稿写作和审读与编辑写作。所有作者已经同意将文章提交给本刊,且对将要发表的版本进行最终定稿,并同意对工作的所有方面负责。
利益冲突 所有作者均声明不存在利益冲突
Funding Statement
国家自然科学基金(No. 82170722)和空军军医大学博士后蓝剑基金(No. lj20220102)资助
Contributor Information
紫娴 余 (Zixian YU), Email: 13765394869@163.com.
世仁 孙 (Shiren SUN), Email: sunshiren@medmail.com.cn.
References
- 1.Kidney Disease: Improving Global Outcomes Glomerular Diseases Work Group KDIGO 2021 Clinical Practice Guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 2.BARBOUR S J, COPPO R, ZHANG H, et al Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern Med. 2019;179(7):942–952. doi: 10.1001/jamainternmed.2019.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.RODRIGUES J C, HAAS M, REICH H N IgA nephropathy. Clin J Am Soc Nephrol. 2017;12(4):677–686. doi: 10.2215/CJN.07420716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.JIA Q, MA F, ZHAO J, et al Effect of corticosteroids combined with cyclophosphamide or mycophenolate mofetil therapy for IgA nephropathy with stage 3 or 4 chronic kidney disease: a retrospective cohort study. Front Pharmacol. 2022;13:946165. doi: 10.3389/fphar.2022.946165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ZHAO J, MA F, BAI M Low-dose corticosteroid combined with mycophenolate mofetil for IgA nephropathy with stage 3 or 4 CKD: a retrospective cohort study. Clin Ther. 2021;43(5):859–870. doi: 10.1016/j.clinthera.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 6.路延双, 刘文虎 IgA肾病预后相关因素的研究新进展. 临床和实验医学杂志. 2020;19(15):1671–1674. doi: 10.3969/j.issn.1671-4695.2020.15.030. [DOI] [Google Scholar]
- 7.黄叶飞, 杨克虎, 陈澍洪 高尿酸血症/痛风患者实践指南. 中华内科杂志. 2020;59(7):519–527. doi: 10.3760/cma.j.cn112138-20200505-00449. [DOI] [Google Scholar]
- 8.JOHNSON R J, BAKRIS G L, BORGHI C, et al Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the national kidney foundation. Am J Kidney Dis. 2018;71(6):851–865. doi: 10.1053/j.ajkd.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SHARAF EL DIN U A A, SALEM M M, ABDULAZIM D O Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: a review. J Adv Res. 2017;8(5):537–548. doi: 10.1016/j.jare.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.郝凤杰, 郭珲, 焦艳艳 高尿酸血症与IgA肾病牛津病理分型及肾存活率相关性分析. 临床军医杂志. 2022;50(2):191–193. [Google Scholar]
- 11.ZHANG K, TANG L, JIANG S S, et al Is hyperuricemia an independent prognostic factor for IgA nephropathy: a systematic review and meta-analysis of observational cohort studies. Renal Failure. 2022;44(1):70–80. doi: 10.1080/0886022X.2021.2019589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GOLDBERG A, GARCIA-ARROYO F, SASAI F, et al Mini review: reappraisal of uric acid in chronic kidney disease. Am J Nephrol. 2021;52(10−11):837–844. doi: 10.1159/000519491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.BADVE S V, PASCOE E M, TIKU A, et al Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med. 2020;382(26):2504–2513. doi: 10.1056/NEJMoa1915833. [DOI] [PubMed] [Google Scholar]
- 14.KIMURA K, HOSOYA T, UCHIDA S, et al Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis. 2018;72(6):798–810. doi: 10.1053/j.ajkd.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 15.TRIMARCHI H, BARRATT J, CATTRAN D C, et al Oxford classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91(5):1014–1021. doi: 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 16.中华医学会内分泌学分会 中国高尿酸血症与痛风诊疗指南(2019) 中华内分泌代谢杂志. 2020;36(1):1–13. doi: 10.3760/cma.j.issn.1000-6699.2020.01.001. [DOI] [Google Scholar]
- 17.INKER L A, ENEANYA N D, CORESH J, et al New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LE W, LIANG S, HU Y, et al Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant. 2012;27(4):1479–1485. doi: 10.1093/ndt/gfr527. [DOI] [PubMed] [Google Scholar]
- 19.LV J, ZHANG H, ZHOU Y, et al Natural history of immunoglobulin A nephropathy and predictive factors of prognosis: a long-term follow up of 204 cases in China. Nephrology (Carlton) 2008;13(3):242–246. doi: 10.1111/j.1440-1797.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- 20.吕天洋, 张静, 贾红彦 原发性IgA肾病并发血尿酸升高患者影响因素分析. 中国实验诊断学. 2019;23(9):1536–1537. [Google Scholar]
- 21.袁茜宁, 董怡晨, 卿玲玲 尿酸血症对不同蛋白尿水平IgA肾病患者临床及病理特征的影响. 中国现代医药杂志. 2022;24(12):16–22. [Google Scholar]
- 22.任慧敏, 杨淑芬, 陆晨 IgA肾病伴高尿酸血症的临床、病理特点及相关因素分析. 临床肾脏病杂志. 2021;21(9):715–720. doi: 10.3969/j.issn.1671-2390.2021.09.003. [DOI] [Google Scholar]
- 23.WU Y Y, QIU X H, YE Y, et al Risk factors analysis for hyperuricemic nephropathy among CKD stages 3-4 patients: an epidemiological study of hyperuricemia in CKD stages 3-4 patients in Ningbo, China. Ren Fail. 2018;40(1):666–671. doi: 10.1080/0886022X.2018.1487859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.GRAYSON P C, KIM S Y, LAVALLEY M, et al Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthrit Care Res. 2011;63(1):102–110. doi: 10.1002/acr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.SYRJANEN J, MUSTONEN J, PASTERNACK A Hypertriglyceridaemia and hyperuricaemia are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant. 2000;15(1):34–42. doi: 10.1093/ndt/15.1.34. [DOI] [PubMed] [Google Scholar]
- 26.GENG Y H, ZHANG Z, ZHANG J J, et al Hyperuricemia aggravates the progression of IgA nephropathy. Int Urol Nephrol. 2022;54(9):2227–2237. doi: 10.1007/s11255-022-03125-4. [DOI] [PubMed] [Google Scholar]
- 27.MORIYAMA T, ITABASHI M, TAKEI T, et al High uric acid level is a risk factor for progression of IgA nephropathy with chronic kidney disease stage G3a. J Nephrol. 2015;28(4):451–456. doi: 10.1007/s40620-014-0154-0. [DOI] [PubMed] [Google Scholar]
- 28.SRIVASTAVA A, KAZE A D, MCMULLAN C J, et al Uric acid and the risks of kidney failure and death in individuals with CKD. Am J Kidney Dis. 2018;71(3):362–370. doi: 10.1053/j.ajkd.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SIRCAR D, CHATTERJEE S, WAIKHOM R, et al Efficacy of febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: a 6-month, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis. 2015;66(6):945–950. doi: 10.1053/j.ajkd.2015.05.017. [DOI] [PubMed] [Google Scholar]



