Abstract
Intrahepatic cholangiocarcinoma (iCCA) can originate from the large bile duct group (segment bile ducts and area bile ducts), small bile duct group (septal bile ducts and interlobular bile ducts), and terminal bile duct group (bile ductules and canals of Hering) of the intrahepatic biliary tree, which can be histopathological corresponding to large duct type iCCA, small duct type iCCA and iCCA with ductal plate malformation pattern, and cholangiolocarcinoma, respectively. The challenge in pathological diagnosis of above subtypes of iCCA falls in the distinction of cellular morphologies, tissue structures, growth patterns, invasive behaviors, immunophenotypes, molecular mutations, and surgical prognoses. For these reasons, this expert consensus provides nine recommendations as a reference for standardizing and refining the diagnosis of pathological subtypes of iCCA, mainly based on the 5th edition of the World Health Organization Classification of Tumours of the Digestive System.
Keywords: Intrahepatic cholangiocarcinoma, Pathology, Histological type, Immunohistochemistry, Molecular variation
Graphical abstract
Introduction
Intrahepatic cholangiocarcinoma (iCCA) is a highly aggressive tumor that arises from the lining epithelium and peribiliary glands from the second order intrahepatic bile ducts to the smallest intrahepatic bile branches, and it expresses cholangiocyte-specific markers.1–5 iCCA accounts for 10–20% of primary liver cancers6 and 20–30% of cholangiocarcinomas.7,8 Based on a study in China, hepatocellular carcinoma (HCC) and iCCA account for 90.2% and 8.2% of 26,684 cases of surgically resected primary hepatic malignant tumors, respectively.9 The age-standardized incidence rates of iCCA in China did not rise significantly from 2006 to 2015 (2.0/100,000–2.2/100,000), but there was a significant increase in patients age over 65 years (3.4/100,000–4.5/100,000) during the study period.10 iCCA is a highly malignant tumor with a 5-year overall survival rate of less than 50% even after surgical resection with curative intent.5,11
The clinicopathological features of iCCA and extrahepatic cholangiocarcinoma are significantly different.12 In the past, there was no refined diagnostic mode on iCCA owing mainly to its inconsistent histological types and unclear diagnostic criteria for its subtypes. However, notable progresses have been made in the pathological research of iCCA in the past decade, some of which have been adopted by the World Health Organization (WHO) Classification of Tumours of the Digestive System (fifth edition). However, the criteria for histological classification of iCCA, the differential diagnosis of histological variation, the selection of immunohistochemical (IHC) markers, and the detection of molecular targets need to be refined in order to be better applied in practice. To our knowledge, no guidelines for contouring the iCCA pathological subtype diagnosis have been previously reported. As a consensus on the diagnosis of histological subtypes can help to standardize the routine pathological diagnosis of iCCA, thus providing an elaborate pathological basis for individualized treatment in clinical practice, the present consensus on pathological diagnosis of iCCA was jointly formulated by the expert team in China.
Methodology
Forty-seven experts who represented six professional academic groups were selected to form an expert consensus group based on their expertise and contribution to research and scholarship in the field of liver pathology, surgery, oncology, and basic scientific disciplines in China. All participants disclosed potential conflicts of interest and they did not receive any financial compensation for participation. The expert consensus group decided to engage in small-consensus draft team brainstorming discussions aligned with the expertise and interests of the participants, which was then discussed as a large group and vetted by the rest of the participants. The literature search was conducted on multiple electronic data libraries that included the PubMed, China National Knowledge Infrastructure, Web of Science, Medline, and EMBASE databases up to May 2022. No limits were incorporated into the literature search to obtain all available evidence. A review of the incorporated articles was then undertaken to identify and synthesize evidence to support the creation of recommendations based on the available evidence.
Draft versions of the present consensus were repeatedly presented, discussed, and subsequently revised at several online-to-offline expert consensus meetings to come up with a consensus on the applicability and challenges of each recommendation in clinical practice. Then, the members of expert consensus group received literature specific to the revised consensus statements and indicated their agreement opinions. Consensus was defined as universal agreement of the participants and categorized based on the Grading of Recommendations Assessment, Development, and Evaluations (GRADE) system. The quality of evidence scale ranged from high, moderate, to low and very low, and the recommendation levels for clinical practice were strong and conditional (Supplementary Table 1).13,14
Structure and heterogeneity of the intrahepatic biliary system
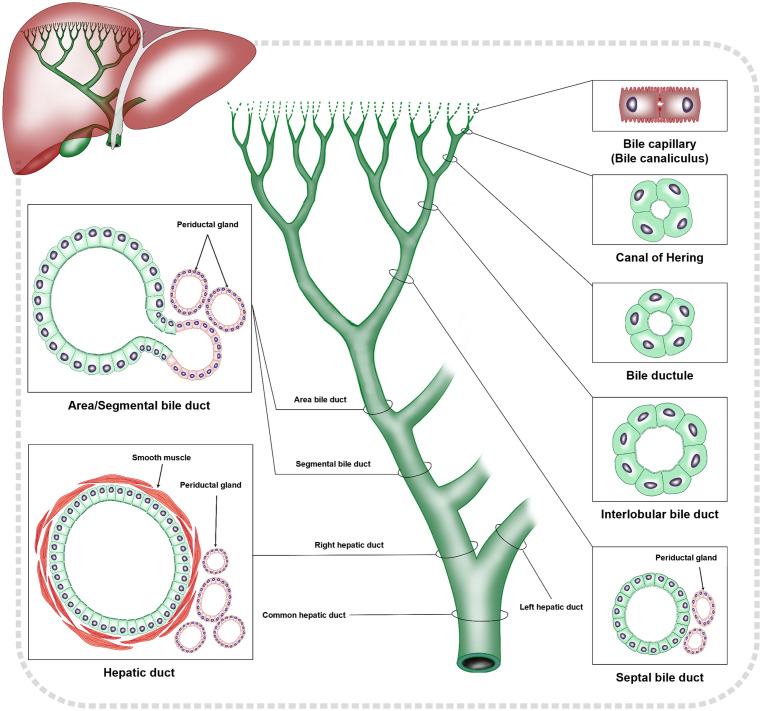
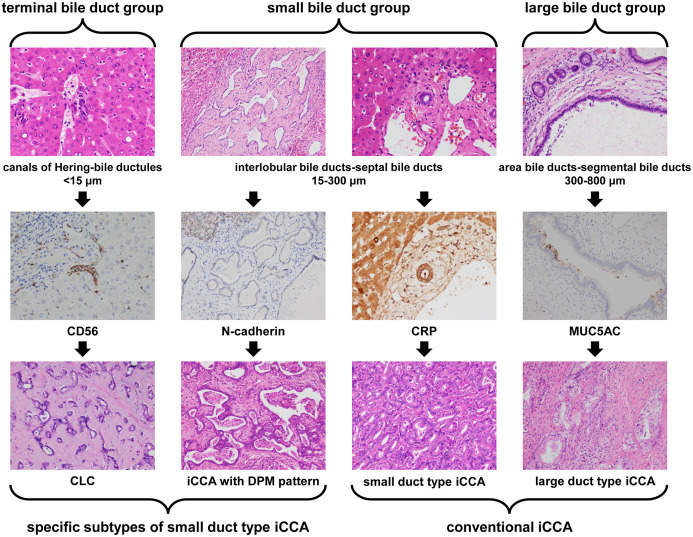
iCCA originates from the lining epithelium of the intrahepatic bile duct tree, the peribiliary glands and hepatic stem/progenitor cells.15 In addition, mature hepatocytes can also give rise to iCCA caused by reprogramming mechanisms.1,8 Based on the caliber of bile ducts, the intrahepatic biliary tree can be divided into a terminal bile duct group with canals of Hering (CoH) and bile ductules, < 15 µm, a small bile duct group with interlobular bile ducts and septal bile ducts, 15–300 µm, and a large bile duct group with areas of bile ducts and segmental bile ducts of 300–800 µm (Fig. 1). The histogenesis and the anatomic sites of each group is the basement of histopathologic classification of iCCA [corresponding to cholangiolocarcinoma (CLC), small duct type iCCA and iCCA with ductal plate malformation (DPM) pattern, as well as large duct type iCCA, respectively].16–18 iCCA with different histological subtypes is highly heterogeneous in its cell origin, tissue structure, immunophenotype, and molecular mutations. For example, iCCA can express not only biliary lineage markers such as cytokeratin 19 (CK19) which indicates a cholangiocyte origin, but also stem cell lineage markers such as cluster of differentiation 56 (CD56) which indicates a hepatic stem/progenitor cell origin.19
Fig. 1. Schematic diagram of structural characteristics of intrahepatic biliary system.
Cholangiocytes are also diverse in their morphology and immunophenotyping. For example, IHC staining is positive for epithelial membrane antigen on the cell membrane at the edge of glandular lumen of the small interlobular bile ducts similar to the staining pattern in CoH, while medium-sized interlobular bile ducts have a cytoplasmic staining pattern similar to that of septal bile ducts and large bile ducts.19–21 In addition, the septal bile ducts in the small bile duct group are invisible in the gross specimens of liver. However, from these bile ducts, columnar cells, mucus-secreting cells, and peribiliary glands which are gradually appear.2,22 Thus, the heterogeneity of cholangiocytes should be considered in the diagnosis of histological subtypes of iCCA.
Recommendation 1: The histological and anatomical characteristics of terminal bile ducts (CoH and bile ductules), small bile ducts (interlobular bile ducts and septal bile ducts) and large bile ducts (area bile ducts and segmental bile ducts) in the intrahepatic biliary system serve as the histological and anatomical foundation for classification of the iCCA histological subtypes (i.e., CLC, small duct type iCCA and iCCA with DPM pattern, and large duct type iCCA), to form the basis for better understanding and refining the diagnosis of the histological subtypes of iCCA (moderate-quality evidence and strong recommendation).
Gross Patterns of iCCA
Mass forming pattern
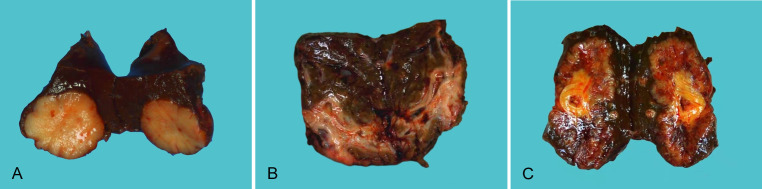
This pattern is common in the peripheral area of the liver.23,24 Grossly, the tumor is a gray-white, nodular solid mass formed in the liver parenchyma with a clear boundary (Fig. 2A). The surrounding liver tissues are normal or accompanied by chronic viral hepatitis or liver cirrhosis. Histopathologically, it is the most common pattern of small duct type iCCA.
Fig. 2. Gross pattern of iCCA.
(A) Mass forming pattern; (B) Periductal infiltrating pattern; (C) Mixed pattern. iCCA, intrahepatic cholangiocarcinoma.
Periductal infiltrating pattern
This pattern often involves large bile ducts near the hilar area.23,24 The tumor grows longitudinally along the bile duct wall in an infiltrating manner (Fig. 2B) and may be accompanied by diseases such as intrahepatic biliary ductal stones, sclerosing cholangitis, and biliary parasites. It remains as a common pattern of large duct type iCCA.
Mixed pattern (mass forming pattern + periductal infiltrating pattern)
The tumor penetrates the bile duct wall and invades into the surrounding liver parenchyma while growing along the bile duct wall, thereby forming a nodular mass which is embedded within a residual bile duct cavity (Fig. 2C).23,24 This is a common gross pattern of large duct type iCCA. Periductal infiltrating and mixed pattern iCCAs have a 45% higher risk of long-term mortality than the mass forming pattern iCCA.25 The mixed pattern iCCA is more prone to portal vein invasion, biliary invasion, or lymph node metastasis.26 Nevertheless, some scholars argue that the gross pattern of iCCA is not significantly correlated with the postoperative overall survival rate or metastasis-free survival rate.27 iCCA with the intraductal growing pattern has similar morphological characteristics to those of intraductal papillary tumor.28 Therefore, the previously reported intraductal growing pattern is no longer regarded as a growth pattern of iCCA by the Union for International Cancer Control, and the WHO categorizes it as a unique entity of intraductal papillary tumor with associated invasive carcinoma.24,29,30
Sampling of iCCA Gross Specimens
The surgeon should indicate the hepatic lobes or segments where the tumor was located on the pathological examination application form, because it is necessary for the pathological diagnosis of whether the tumor arose from intrahepatic or extrahepatic biliary ducts. The surgical margins (both liver and bile duct) can be stained with dye or marked with a surgical suture for accurate sampling. iCCA has a lymph node metastatic incidence rate of 30–60%.31,32 Metastasis to lymph nodes tend to occur in the regions of the hepatoduodenal ligament, along the common hepatic artery, and posterior to the pancreas.33 Any freely sampled lymph nodes including their locations and numbers sent for testing should be marked clearly and separately. The clinicians are recommended to perform a regional lymphadenectomy containing six or more lymph nodes for histological examination. If the regional lymph nodes are negative, but the number ordinarily examined is not met, classify as N0 staging.34
The seven-point baseline sampling method is recommended for mass forming and mixed pattern iCCA specimens.13,35 The maximum diameter and number of tumors as well as the presence of macroscopic tumor invasion of vessels and adjacent organs are crucial in gross pathological examination, as they directly affect the pathological tumor–node–metastasis stage of iCCA.34 Microvascular invasion is an important pathological predictor of postoperative prognosis of iCCA patients36,37 and it should be pathologically graded as in HCC.13,35,38 It is more encouraging to employ some IHC biomarkers (i.e. CD34 and D2-40) to distinguish the blood vessels and lymphatics as different types of microtumor thrombus may represent different recurrence patterns.39,40
The periductal infiltrating pattern iCCA specimens should basically be collected in the following manner: After the invaded bile duct wall has been dissected longitudinally, the length of the invaded bile duct, the thickness of the duct wall, and the shortest distances of the tumor from the surgical margins are measured. Samples are taken from the junction between the involved bile duct wall, the surrounding liver parenchyma and the surgical margins of the liver and bile duct to study the depth and extent of bile duct invasion.
Recommendation 2: The gross pattern of iCCA is related to the histological subtype. For mass forming and mixed pattern iCCAs, the seven-point baseline sampling method should be used. For periductal infiltrating pattern iCCA, specimens are taken from the junction between the invaded bile duct wall, the liver parenchyma, and the surgical margins to study the extent of iCCA invasion and presence of any precancerous lesions (moderate-quality evidence and strong recommendation).
Histopathological characteristics of iCCA
Pathological concepts of iCCA
Almost all iCCAs are adenocarcinomas (> 95%).24,41 Generally, the diagnosis of iCCA is pathologically represented as an adenocarcinoma which originates from lining epithelium and/or peribiliary gland cells of intrahepatic bile ducts. Other rare malignant tumors of intrahepatic large bile duct include adenosquamous carcinoma, squamous cell carcinoma, mucinous carcinoma, signet ring cell carcinoma, lymphoepithelioma-like carcinoma (with highly expressed programmed death ligand 1 and better prognosis in patients with Epstein–Barr virus infection42), and sarcomatoid carcinoma (including multiple molecular targets43), etc. All these tumors need to be separately diagnosed and classified because of specific differences in specific pathogenesis, morphology, biological behavior, and clinical prognosis.44 The WHO classification does not recommend using the term cholangiocellular carcinoma.24
Histological types of iCCA
Large duct type iCCA
Cell origin
This type of iCCA arises from the lining epithelium or peribiliary gland of intrahepatic large bile ducts. It accounts for 41–59% of all iCCA cases.18,45 In large duct type iCCA, the serum cancer antigen 19–9 and carcinoembryonic antigen levels can be increased to varying degrees.46 There may be precancerous lesions such as intraepithelial neoplasia of bile duct or intrahepatic papillary tumor adjacent to the cancer boundary.
Histological characteristics
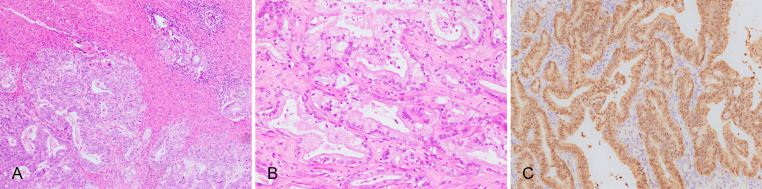
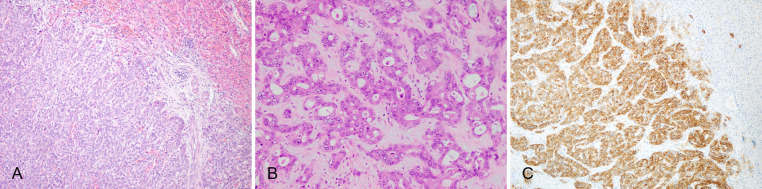
Large duct type iCCA has cuboidal or columnar cells, which are mainly moderately–poorly differentiated, arranged in irregular ductal or tubular patterns (Fig. 3A), with mucus-secreting cells or intraluminal mucus (Fig. 3B). These features are similar to those seen in hilar cholangiocarcinoma. A prominent desmoplastic response is commonly observed in large duct type iCCA, which correlates with the high invasiveness of the tumor and its resistance to chemotherapy and targeted drugs.47,48 There are often portal area, blood vessel and lymphatic and perineural invasion and lymph node metastasis.
Fig. 3. Large duct type iCCA.
(A) Columnar tumor cells arranged in irregular glandular structures, invading multiple portal areas adjacent to the carcinoma; (B) High columnar tumor cells secret mucus; (C) S100 calcium-binding protein P-positive in IHC staining. iCCA, intrahepatic cholangiocarcinoma; IHC, immunohistochemical.
IHC markers and special staining
S100 calcium-binding protein P (S100P; Fig. 3C), mucin 5AC (MUC5AC), MUC6, and trefoil factor 1 (TFF1) are usually positive, among them, S100P is a highly sensitive (95%) and moderately specific (71%) marker for large duct type iCCA.49 However, the expression results can be affected by antibodies from different clones. For instance, it has been reported that in large duct type iCCA, the positive rates of S100P varied from 29–95%49 or from 13–56%.50 Positive Alcian blue and Periodic acid Schiff (AB-PAS) staining is indicative of presence of mucus-secreting cells.
Representative molecular alterations
The rates of the KRAS mutations vary from 15% to 30%, which are frequently correlated with poor postoperative prognosis. The mutation of other target genes, such as BRAF, EGFR, and HER2 might be harbored.4,46
Surgical prognosis
Comparing the large duct type and the small duct type iCCAs, the postoperative 5-year recurrence-free survival rates were 10% and 38%, respectively, and the 5-year overall survival rates were 20% and 60%, respectively.46 Positive S100P staining indicates strong invasiveness and poor prognosis.51,52
Recommendation 3: Large duct type iCCA are mainly composed of cuboidal or columnar cells with mucinous cytoplasm, and they are arranged in irregular ductal or tubular pattern with abundant fibrous stroma. The tumor usually shows a more aggressive growth pattern which is commonly associated with portal area, blood vessel, lymphatic and perineural invasion and lymph node metastases. S100P, MUC5AC, MUC6, TFF1, and mucus staining should be positive on IHC. KRAS Mutation is frequent. Large duct type iCCA has worse long-term prognosis than the other subtypes of iCCA (moderate-quality evidence and strong recommendation).
Small duct type iCCA
Cell origin
It originates from interlobular bile ducts or septal bile ducts,8 accounting for 41–58% of iCCA cases.18,45 Biliary hamartoma and biliary adenoma are regarded as possible precancerous lesions.22,53–56
Histological characteristics
The tumor cells are cuboidal or low columnar with scant cytoplasm, mostly moderately–highly differentiated and lack of mucus-secreting. However, mucus-secreting cells may be present in small duct type iCCA arising from septal bile duct.2 Most tumor tissues are arranged into dense and regular small ductal structures (Fig. 4A, B). Obvious fibrous stroma is frequently observed in the central area of the tumor of which the constitutive cells may be highly relevant to the immunotherapeutic response.48 Usually, there is no capsule around the tumor, and there are few invasions into adjacent portal area, blood vessel, lymphatic vessel, or nerve. Small duct type iCCA can also show a lot of morphological variations, such as the branching duct shape, branching trabecular shape, microcystic shape, cribriform shape, papillary shape, and solid shape, which may increase the difficulty of subtype diagnosis.46,49
Fig. 4. Small duct type iCCA.
(A) Cuboidal tumor cells arranged in dense small glandular structures, uncovered by a capsule; (B) Tumor arranged in a solid or trabecular like structure; (C) N-cadherin-positive in IHC staining. iCCA, intrahepatic cholangiocarcinoma; IHC, immunohistochemical.
IHC markers and special staining
C-reactive protein (CRP), N-cadherin (Fig. 4C) and CD56 are usually positive. It is reported that the sensitivity and specificity are 97% and 95% in CRP, and 87% and 84% in N-cadherin, respectively.46,49 Mucus staining is usually negative. However, the positive rates of S100P, TFF1, and mucus staining in small duct type iCCA can reach up to 51.4%, 15%, and 54.1%, respectively.19,57 As above indicators are partly overlapping, the differential diagnosis between small duct type iCCA and large duct type iCCA should be carefully combined with the features of morphology and immunohistochemistry (Table 1).
Table 1. Histological subtypes and pathological characteristics of iCCA.
| Histological subtype | Large duct type iCCA | Small duct type iCCA |
|---|---|---|
| Gross pattern | Peritubular infiltrating pattern, mixed pattern | Mass forming pattern |
| Cell morphology | Columnar-cuboidal | Cuboidal-low columnar |
| Tissue structure | Irregular large ductal or tubular adenocarcinoma | Dense small ductal adenocarcinoma |
| Invasiveness | ++ – +++ | – –+ |
| Mucus staining | ++ – +++ | – –+ |
| Immunohistochemistry | S100P, MUC5AC, MUC6, TFF1 | CRP, N-cadherin, CD56 |
| Genetic variation | KRAS gene mutation | IDH1/2 gene mutation, FGFR2 gene fusion/rearrangement |
iCCA, intrahepatic cholangiocarcinoma; S100P, S100 calcium-binding protein P; MUC, mucin; TFF, trefoil factor 1; CRP, C-reactive protein; CD56, cluster of differentiation 56.
Representative molecular alterations
IDH1/2 mutation and FGFR2 fusion/rearrangement are the most representative of the druggable molecular alterations in small duct type iCCA.4,24,45,46
Surgical prognosis
Generally, the surgical prognosis of it is much better than that of the large duct type iCCA.46,58 The prognosis of small duct type iCCA with mutant IDH1/2 is better than that with wild-type IDH1/2.59
Recommendation 4: Small duct type iCCA, which is predominantly composed of cuboidal or low columnar cells, is often constituted with dense small ductal structures, and a deficiency of mucus-secreting cells, and it frequently presents with multiple histological variations. It rarely invades portal area, blood vessel, lymphatic vessel, nerve, with few lymph node metastases. IHC usually shows positive CRP, N-cadherin and CD56 staining, but mucus staining usually shows a negative result. IDH1/2 mutation and FGFR2 fusion/rearrangement are its representative targetable mutations. The surgical prognosis of small duct type iCCA is better than that of the large duct type iCCA (moderate-quality evidence and strong recommendation).
CLC
Cell origin
CLC had been considered as a specific subtype of combined hepatocellular-cholangiocarcinoma with stem cell features, while the following molecular analysis showed that CLC was an independent entity without the traits of HCC.60 The available evidence suggests that CLC originates from the cells of the terminal branches of intrahepatic bile duct tree, as well as hepatic stem cells or progenitor cells,8,19,30 making up 0.6–1% of primary liver cancers61 and 13–40% of iCCAs. The discrepancy may come partly from different diagnostic criteria,62,63 and it is often complicated with liver diseases such as chronic viral hepatitis B.64 The WHO classification does not recommend using the term cholangiolocellular carcinoma.24
Histological characteristics
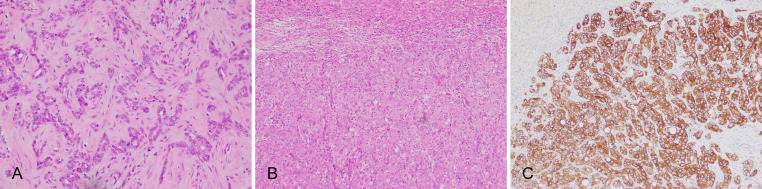
CLC is a unique morphological subtype simulating CoH, bile ductules,8 or ductular reaction, and a lumen diameter < 15 µm can be used as one of the diagnostic criteria.65 CLC cells have a small cuboidal shape, a high nuclear-to-cytoplasmic ratio, oval nuclei, pale cytoplasm, insufficient mucus, without or with minimal ductal atypia and high differentiation, characterized by a loose, angular small ductular, cord-like or branched arrangement in a hyalinized collagen fiber stroma (Fig. 5A). CLC seldom invades adjacent portal area, blood vessel, or lymphatic vessel. CLC and small duct type iCCA can be complicated with HCC-like iCCA components.2,63,64,66 These components are formed by polygonal tumor cells which are rich in eosinophilic cytoplasm and arranged in a trabecular or nest-like pattern, thus resembling HCC (Fig. 5B). However, these cells express cholangiocyte-lineage markers such as CK7/CK19 (Fig. 5C) and stem cell markers such as epithelial cell adhesion molecule, but they do not express hepatocyte-lineage markers which is essentially different from combined hepatocellular-cholangiocarcinoma that is defined by unequivocal presence of both hepatocytic and cholangiocytic differentiation within the same tumor.67 The HCC-like iCCA components are thought to be originating from hepatic precursor cells45,63,68,69 or are the manifestation of poorly differentiated CLC.63 HCC-like iCCA may be similar to HCC on imaging features or clinical features and needs to be distinguished from HCC.70
Fig. 5.
CLC and HCC-like ICC. (A) CLC, Small cuboidal tumor cells in sparse branching glandular structures, with hyaline degeneration of fibrous stroma; (B) HCC-like iCCA, with polygonal tumor cells in trabecular patterns; (C) HCC-like iCCA, cytokeratin 19-positive IHC staining. CLC, cholangiolocarcinoma; HCC, hepatocellular carcinoma; iCCA, intrahepatic cholangiocarcinoma; IHC, immunohistochemistry.
IHC markers and molecular alterations
They are similar to those of small duct type iCCA. Moreover, positive staining of CD56 is more frequent in CLC compared with classical iCCA.66,70
Surgical prognosis
It is better than that of small and large duct type iCCAs.19,24,61,64
Recommendation 5: CLC is composed of small cuboidal tumor cells with low atypia forming a sparsely branched small ductular configuration embedded in a hyalinized fibrous stroma. The IHC markers and molecular variations are similar to those of small duct type iCCA. CLC has lower invasiveness and better surgical prognosis than that of small and large duct type iCCAs (moderate-quality evidence and strong recommendation).
iCCA with DPM pattern
Cell origin
This type of tumor mimics DPM, which is found in Caroli’s disease, congenital hepatic fibrosis or von Meyenburg complexes from each parts of intrahepatic biliary system, and is considered as a rare subtype of small duct type iCCA.24,71,72 Whether its occurrence is associated with DPM remains controversial.73,74 iCCA with DPM pattern is often complicated by liver diseases such as viral hepatitis B.75
Histological characteristics
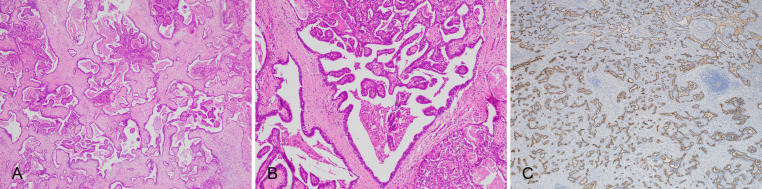
It has saccate, adenoid, or fissure-like tumor ducts lined by a single layer of flat, small cuboidal or low columnar epithelium, with clear and sparse cytoplasm, small and pale nuclei, and a few mucus-secreting cells. The lumens are dilated as irregular cystic lumens of varying sizes and the lining epithelium has polypoid protrusions into the lumen (Fig. 6A, B). The lumens in the central region of the tumor are large, with dense fibrotic stroma, while those in the peripheral region are small, without a capsule, and grows invasively with rare blood vessel and lymphatic invasion as well as lymph node metastasis.63
Fig. 6. iCCA with DPM pattern.
(A) Irregular cystic dilated tumor glandular ducts with abundant fibrous stroma; (B) Polypoid protuberance in the tumor crypt; (C) IHC staining for mucin 1 shows positive results on the marginal surface of the lumen. DPM, ductal plate malformation; iCCA, intrahepatic cholangiocarcinoma; IHC, immunohistochemistry.
IHC markers and molecular alterations
They are similar to those of small duct type iCCA (Fig. 6C). In addition, ARID1A mutation and absent expression may occur.70,76–78
Surgical prognosis: Postoperative recurrence and metastasis are rare.75
Recommendation 6: iCCA with DPM pattern is formed by small cuboidal or low columnar cells characterized by irregular cystic dilatation of lumen with polypoid protrusions in the lumen. IHC markers and molecular mutations of iCCA with DPM pattern are similar to those of small duct type iCCA. iCCA with DPM pattern is less aggressive and has a better prognosis after surgery (moderate-quality evidence and strong recommendation).
Indeterminate/Mixed Pattern iCCA
Multiple histological subtypes of iCCA sometimes co-exist, and they account for 56.5% of iCCA,19,24,49,64,71,77,79 representing the high intratumoral heterogeneity of iCCA (Fig. 7). Among them, the tumor dominated by CLC components has the better prognosis.65 To objectively assess the biological characteristics of this kind of iCCA, it is recommended to diagnose iCCA according to the dominant and other components of the tumor and to illustrate the proportions of all histological components in the pathological report. It is reported that according to conventional histologic assessment, 32.4% of iCCA initially fall into the mixed category including those with mixtures of typical large duct and small duct types and those with nontypical histology, such as columnar cell tumors without mucin, cuboidal cell tumors with abundant mucin production, or poorly differentiated tumors. However, by just choosing the appropriate IHC profiles can classify most iCCA cases (96%) into the definite histological subtypes.46 Hence, it is recommended to pair large duct type iCCA markers with small duct type iCCA markers to help with the subtype diagnosis. Combining with published experimental data, S100P, MUC5AC, CRP, and N-cadherin are recommended as the first option IHC panel for iCCA subclassification, and MUC6, TFF1, CD56, MUC1, and AB-PAS mucus staining as beneficial supplements to the diagnosis if necessary.21,24,50,57,69,78,79 This combination should continuously be optimized in pathological practice.
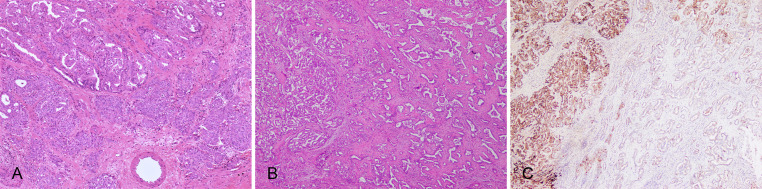
Fig. 7. iCCA with multiple histological subtypes.
(A) Large duct type iCCA (upper left) and small duct type iCCA (bottom right) components in the same tumor; (B) Small duct type iCCA (left) and DPM pattern iCCA (right) components in the same tumor; (C) IHC staining for CRP shows strong positive results on small duct type iCCA (left) components and weak positive results on DPM pattern iCCA (right) components. CRP, C-reactive protein; DPM, ductal plate malformation; iCCA, intrahepatic cholangiocarcinoma; IHC, immunohistochemistry.
Recommendation 7: iCCA is a highly heterogeneous tumor, with histological subtypes which cannot always subtly match the IHC phenotypes and molecular alterations. Great efforts should be made to establish the model of diagnosis of pathological subtypes for iCCA. The comprehensive clinical characteristics, gross pattern, cell morphology, histological structure, immunophenotype, mucus secretion, and molecular mutations should be considered in its diagnosis and differential diagnosis. A four-antibody panel of IHC markers (S100P, MUC5AC, CRP, and N-cadherin) is recommended for distinguishing the histological subtypes of iCCA (low-quality evidence and conditional recommendation).
Targeted therapy and immunotherapy biomarkers of iCCA
Targeted therapy and immunotherapy biomarkers of iCCA should be detected based on the National Comprehensive Cancer Network as well as the Chinese Society of Clinical Oncology Clinical Practice Guidelines for biliary tract cancers to provide a reference for targeted inhibitor therapy for unresectable and metastatic disease.80,81 Table 2 shows the authorized drugs and their relevance to managing patients with different subtypes of iCCA.78,79,82,83 An increasing number of studies confirm that drugs designed for several targetable genetic aberrations, such as IDH and FGFR2, show the therapeutic potential and correlation with histological subtypes,84–86 while only a small proportion of patients harboring these specific gene alterations could obtain survival benefit. On this account, agents targeting programmed death ligand 1 and programmed death receptor 1 immune checkpoint are currently under investigation and is emerged as a new paradigm of therapy.87,88 Referring multiple valuable biomarkers, such as KRAS and tumor mutational burden (TMB), to predict whether the patients with iCCA could benefit from clinical trial provides a new idea for further expansion of the appropriate population for targeted therapy and immunotherapy.89 Therefore, it is warranted to provide some associated pathologically detectable targets to meet clinical requirements.
Table 2. Targeted therapy and immunotherapy biomarkers of iCCA.
| Molecular variation | Mutation frequency* | Targeted drug | Pathological type | Test methods |
|---|---|---|---|---|
| FGFR2 fusion/rearrangement | 2–13% | Pemigatinib; Infigratinib | Common in small duct type iCCA | FISH, second generation sequencing |
| IDH1/2 mutation | 7.5–16% | Ivosidenib | Common in small duct type iCCA | First/second generation sequencing |
| NTRK fusion | 5% | Entrectinib; Larotrectinib | iCCA | FISH, RT-PCR, second generation sequencing |
| RET fusion | 1.80% | Pralsetinib | iCCA | FISH, second generation sequencing |
| BRAFV600E mutation | 4.3–11.1% | Dabrafenib + trametinib | iCCA | RT-PCR, first/second generation sequencing |
| HER2 amplification | 1.8–8% | Trastuzumab + pertuzumab | iCCA | Immunohistochemistry, FISH |
| Second generation sequencing | ||||
| MSI-H/dMMR | 2–4.8% | Pembrolizumab | iCCA | PCR, immunohistochemistry |
| TMB-H | 10.40% | Pembrolizumab | iCCA | Second generation sequencing |
Recommendation 8: Rapid progress in clinical research on targeted therapy and immunotherapy of iCCA has led to higher requirements for screening and detection of biomarkers of targeted inhibitors. More attention should be paid to detection of predictive biomarkers of therapeutic targets and immune checkpoints referring to histological subtype of iCCA to allow refined personalized treatment with improved treatment efficacy, and to help to establish the molecular-pathological basis for individualized iCCA treatment (high-quality evidence and strong recommendation).
Summary of key points of the pathological diagnosis of iCCA
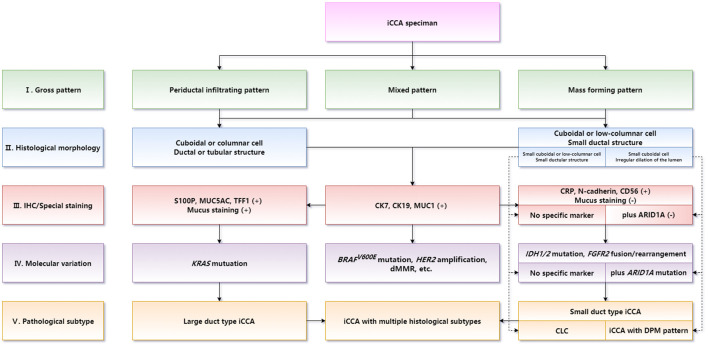
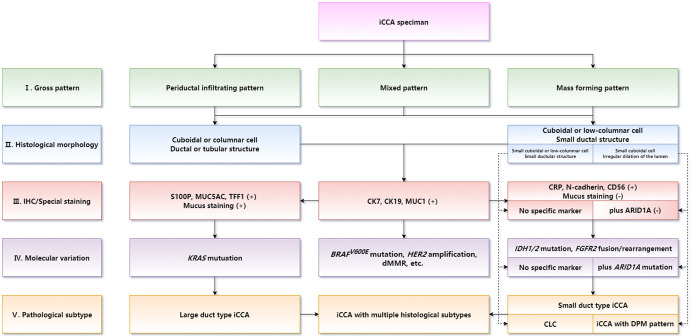
Diagnosis of histological subtypes should be supposed as the core in iCCA routine pathological diagnosis. Differential diagnosis between large duct type and small duct type iCCAs is the key of iCCA routine pathology. Optimizing a combination of IHC marker panels should be used as a reference for histological subtyping. Molecular targets and immunotherapy biomarkers should be detected consulting the histological subtype of iCCA. The main pathobiological characteristics of iCCA should be illustrated in the pathological report form. The diagnostic term intrahepatic cholangiocellular carcinoma is not recommended to be used to refer to iCCA. The correspondence between intrahepatic bile duct groups and iCCA subtype is shown in Figure 8. The flowchart of pathological diagnosis of iCCA is shown in Figure 9.
Fig. 8. Correspondence between the intrahepatic bile duct groups and iCCA subtypes.
iCCA, intrahepatic cholangiocarcinoma.
Fig. 9. Flowchart of the pathological diagnosis of iCCA.
iCCA, intrahepatic cholangiocarcinoma.
Recommendation 9: In the pathological report of iCCA, it is important to illustrate the pathological characteristics associated with the risks of postoperative recurrence and metastasis to assist in the clinical treatment planning.13,44,90 These should include the gross type, histological subtype, immunophenotype, differentiation grade, microvascular invasion, biological behavior, status of surgical margin, pathological tumor–node–metastasis stage, background of liver disease, and other important relevant information. To improve the homogeneity of pathological diagnosis of iCCA, the use of a template for pathological diagnostic reporting should be encouraged (moderate-quality evidence and strong recommendation).
Disclaimer
Translated in full with permission from the Chinese Medical Association by the Journal of Clinical and Translational Hepatology of the Xia & He Publishing Inc., USA. Sole responsibility of the translation rests with the translator. This translation, Copyright © (2022) by the Chinese Medical Association. The article entitled Expert consensus on pathological diagnosis of intrahepatic cholangiocarcinoma (2022 version), DOI: 10.3760/cma.j.cn112151-20220517-00423, Copyright © (2022) was prepared by Chinese Journal of Pathology, Expert consensus on pathological diagnosis of intrahepatic cholangiocarcinoma (2022 version), Chinese Medical Journals Publishing House Company. All content is protected by copyright and may not be reproduced in any manner without written permission from Chinese Medical Association.
The Chinese Medical Association takes no responsibility for the accuracy of the translation from the published Chinese original and is not liable for any errors which may occur. No responsibility is assumed, and responsibility is hereby disclaimed, by the Chinese Medical Association for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of methods, products, instructions or ideas presented in this translation. Independent verification of diagnosis and drug dosages should be made. Discussions, views, and recommendations as to medical procedures, choice of drugs and drug dosages are the responsibility of the authors.
Supporting information
The GRADE system.
Abbreviations
- AB-PAS
Alcian blue and Periodic acid Schiff
- CD
cluster of differentiation
- CK
cytokeratin
- CLC
cholangiolocarcinoma
- CoH
canals of Hering
- CRP
C-reactive protein
- dMMR
mismatch repair deficiency
- DPM
ductal plate malformation
- GRADE
Grading of Recommendations Assessment, Development, and Evaluations
- HCC
hepatocellular carcinoma
- iCCA
intrahepatic cholangiocarcioma
- IHC
immunohistochemistry
- MSI-H
microsatellite instability-high
- MUC
mucin
- S100P
S100 calcium-binding protein P
- TFF1
trefoil factor 1
- TMB
tumor mutational burden
- WHO
World Health Organization
References
- 1.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vijgen S, Terris B, Rubbia-Brandt L. Pathology of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6(1):22–34. doi: 10.21037/hbsn.2016.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kendall T, Verheij J, Gaudio E, Evert M, Guido M, Goeppert B, et al. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):7–18. doi: 10.1111/liv.14093. [DOI] [PubMed] [Google Scholar]
- 5.Ao JY, Cheng QB, Liu C, Jiang XQ. Treatment Difficulties and Strategies of Intrahepatic Cholangiocarcinoma. Journal of Hepatopancreatobiliary Surgery. 2020;32(06):321–325. doi: 10.11952/j.issn.1007-1954.2020.06.001. [DOI] [Google Scholar]
- 6.Gupta A, Dixon E. Epidemiology and risk factors: intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6(2):101–104. doi: 10.21037/hbsn.2017.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, Li Q, Fu J, Jin Z, Su J, Zhang J, et al. Comprehensive analysis of genomic mutation signature and tumor mutation burden for prognosis of intrahepatic cholangiocarcinoma. BMC Cancer. 2021;21(1):112. doi: 10.1186/s12885-021-07788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moeini A, Haber PK, Sia D. Cell of origin in biliary tract cancers and clinical implications. JHEP Rep. 2021;3(2):100226. doi: 10.1016/j.jhepr.2021.100226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cong WM, Dong H, Tan L, Sun XX, Wu MC. Surgicopathological classification of hepatic space-occupying lesions: a single-center experience with literature review. World J Gastroenterol. 2011;17(19):2372–2378. doi: 10.3748/wjg.v17.i19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An L, Zheng R, Zhang S, Chen R, Wang S, Sun K, et al. Hepatocellular carcinoma and intrahepatic cholangiocarcinoma incidence between 2006 and 2015 in China: estimates based on data from 188 population-based cancer registries. Hepatobiliary Surg Nutr. 2023;12(1):45–55. doi: 10.21037/hbsn-21-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian M, Liu W, Tao C, Tang Z, Zhou Y, Song S, et al. Prediction of overall survival in resectable intrahepatic cholangiocarcinoma: IS(ICC) -applied prediction model. Cancer Sci. 2020;111(4):1084–1092. doi: 10.1111/cas.14315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamarca A, Santos-Laso A, Utpatel K, La Casta A, Stock S, Forner A, et al. Liver Metastases of Intrahepatic Cholangiocarcinoma: Implications for an Updated Staging System. Hepatology. 2021;73(6):2311–2325. doi: 10.1002/hep.31598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.General Office of National Health Commission Standard for diagnosis and treatment of primary liver cancer (2022 edition) Journal of Clinical Hepatology. 2022;38(02):288–303. doi: 10.3969/j.issn.1001-5256.2022.02.009. [DOI] [Google Scholar]
- 14.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardinale V, Carpino G, Reid L, Gaudio E, Alvaro D. Multiple cells of origin in cholangiocarcinoma underlie biological, epidemiological and clinical heterogeneity. World J Gastrointest Oncol. 2012;4(5):94–102. doi: 10.4251/wjgo.v4.i5.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39(6):1739–1745. doi: 10.1002/HEP.20130. [DOI] [PubMed] [Google Scholar]
- 17.Liu QY, Ma XY, Yu ZY. Advances in Research of Segmental Anatomy of the Liver. World Chinese Journal of Digestology. 2013;21(27):2780–2786. doi: 10.11569/wcjd.v21.i27.2780. [DOI] [Google Scholar]
- 18.Yamada M, Yamamoto Y, Sugiura T, Kakuda Y, Ashida R, Tamura S, et al. Comparison of the Clinicopathological Features in Small Bile Duct and Bile Ductular Type Intrahepatic Cholangiocarcinoma. Anticancer Res. 2019;39(4):2121–2127. doi: 10.21873/anticanres.13325. [DOI] [PubMed] [Google Scholar]
- 19.Terada T. Combined hepatocellular-cholangiocarcinoma with stem cell features, ductal plate malformation subtype: a case report and proposal of a new subtype. Int J Clin Exp Pathol. 2013;6(4):737–748. [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo F, Fukusato T. Pathogenesis of Cholangiolocellular Carcinoma: Possibility of an Interlobular Duct Origin. Intern Med. 2015;54(14):1685–1694. doi: 10.2169/internalmedicine.54.3540. [DOI] [PubMed] [Google Scholar]
- 21.Maeno S, Kondo F, Sano K, Takada T, Asano T. Morphometric and immunohistochemical study of cholangiolocellular carcinoma: comparison with non-neoplastic cholangiole, interlobular duct and septal duct. J Hepatobiliary Pancreat Sci. 2012;19(3):289–296. doi: 10.1007/s00534-011-0483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KB. Histopathology of a benign bile duct lesion in the liver: Morphologic mimicker or precursor of intrahepatic cholangiocarcinoma. Clin Mol Hepatol. 2016;22(3):400–405. doi: 10.3350/cmh.2016.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guglielmi A, Ruzzenente A, Campagnaro T, Pachera S, Valdegamberi A, Nicoli P, et al. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg. 2009;33(6):1247–1254. doi: 10.1007/s00268-009-9970-0. [DOI] [PubMed] [Google Scholar]
- 24.Nakanuma Y, Klimstra D, Komuta M, Zen Y. WHO classification of tumours, Digestive system tumours. 5th edition. Lyon: IARC Press; 2019. Intrahepatic cholangiocarcinoma; pp. 254–259. [Google Scholar]
- 25.Bagante F, Spolverato G, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, et al. Impact of Morphological Status on Long-Term Outcome Among Patients Undergoing Liver Surgery for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2017;24(9):2491–2501. doi: 10.1245/s10434-017-5870-y. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto Y, Kokudo N, Matsuyama Y, Sakamoto M, Izumi N, Kadoya M, et al. Proposal of a new staging system for intrahepatic cholangiocarcinoma: Analysis of surgical patients from a nationwide survey of the Liver Cancer Study Group of Japan. Cancer. 2016;122(1):61–70. doi: 10.1002/cncr.29686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung T, Rhee H, Nahm JH, Jeon Y, Yoo JE, Kim YJ, et al. Clinicopathological characteristics of intrahepatic cholangiocarcinoma according to gross morphologic type: cholangiolocellular differentiation traits and inflammation- and proliferation-phenotypes. HPB (Oxford) 2020;22(6):864–873. doi: 10.1016/j.hpb.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Aoki Y, Mizuma M, Hata T, Aoki T, Omori Y, Ono Y, et al. Intraductal papillary neoplasms of the bile duct consist of two distinct types specifically associated with clinicopathological features and molecular phenotypes. J Pathol. 2020;251(1):38–48. doi: 10.1002/path.5398. [DOI] [PubMed] [Google Scholar]
- 29.Nakanuma Y, Basturk O, Esposito I, Klimstra D, Komuta M, Zen Y. Intraductal papillary neoplasm of the bile ducts. In: WHO Classification of Tumours Editorial Board, editor. WHO classification of tumours, Digestive system tumours. 5th edition. Lyon: IARC Press; 2019. pp. 279–282. [Google Scholar]
- 30.Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou WP, Jiang BG. Characteristics of lymph node metastasis and prognostic significance of lymphadenectomy in intrahepatic cholangiocarcinoma. Chinese Journal of Practical Surgery. 2020;40(06):669–673. doi: 10.19538/j.cjps.issn1005-2208.2020.06.10. [DOI] [Google Scholar]
- 32.Yin L, Zhao S, Zhu H, Ji G, Zhang X. Primary tumor resection improves survival in patients with multifocal intrahepatic cholangiocarcinoma based on a population study. Sci Rep. 2021;11(1):12166. doi: 10.1038/s41598-021-91823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen XP, Shen F. Chinese Expert Consensus on the Surgical Management of Intrahepatic Cholangiocarcinoma (2020 Edition) Chinese Journal of Digestive Surgery. 2021;20(01):1–15. doi: 10.3760/cma.j.cn115610-20201211-00777. [DOI] [Google Scholar]
- 34.Aloia T, Pawlik TM, Taouli B, Rubbia-Brandt L, Vauthey J. Intrahepatic bile ducts. In: American Joint Committee on Cancer, editor. AJCC cancer staging manual. 8th edition. New York: Springer; 2017. pp. 120–127. [DOI] [Google Scholar]
- 35.Chinese Society of Liver Cancer, Chinese Anti-Cancer Association, Liver Cancer Study Group, Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Pathology Evidence-based Practice Guidelines for the Standardized Pathological Diagnosis of Primary Liver Cancer (2015 Update) Chinese Journal of Hepatobiliary Surgery. 2015;21(3):145–151. doi: 10.3760/cma.j.issn.1007-8118.2015.03.001. [DOI] [Google Scholar]
- 36.Wang K, Zhang H, Xia Y, Liu J, Shen F. Surgical options for intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6(2):79–90. doi: 10.21037/hbsn.2017.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan KM, Tsai CY, Yeh CN, Yeh TS, Lee WC, Jan YY, et al. Characterization of intrahepatic cholangiocarcinoma after curative resection: outcome, prognostic factor, and recurrence. BMC Gastroenterol. 2018;18(1):180. doi: 10.1186/s12876-018-0912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheng X, Ji Y, Ren GP, Lu CL, Yun JP, Chen LH, et al. A standardized pathological proposal for evaluating microvascular invasion of hepatocellular carcinoma: a multicenter study by LCPGC. Hepatol Int. 2020;14(6):1034–1047. doi: 10.1007/s12072-020-10111-4. [DOI] [PubMed] [Google Scholar]
- 39.Thelen A, Scholz A, Weichert W, Wiedenmann B, Neuhaus P, Gessner R, et al. Tumor-associated angiogenesis and lymphangiogenesis correlate with progression of intrahepatic cholangiocarcinoma. Am J Gastroenterol. 2010;105(5):1123–1132. doi: 10.1038/ajg.2009.674. [DOI] [PubMed] [Google Scholar]
- 40.Cadamuro M, Brivio S, Mertens J, Vismara M, Moncsek A, Milani C, et al. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J Hepatol. 2019;70(4):700–709. doi: 10.1016/j.jhep.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esnaola NF, Meyer JE, Karachristos A, Maranki JL, Camp ER, Denlinger CS. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer. 2016;122(9):1349–1369. doi: 10.1002/cncr.29692. [DOI] [PubMed] [Google Scholar]
- 42.Huang YH, Zhang CZ, Huang QS, Yeong J, Wang F, Yang X, et al. Clinicopathologic features, tumor immune microenvironment and genomic landscape of Epstein-Barr virus-associated intrahepatic cholangiocarcinoma. J Hepatol. 2021;74(4):838–849. doi: 10.1016/j.jhep.2020.10.037. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Bai Q, Xu Y, Wang W, Chen L, Han J, et al. Molecular profiling of the biphasic components of hepatic carcinosarcoma by the use of targeted next-generation sequencing. Histopathology. 2019;74(6):944–958. doi: 10.1111/his.13822. [DOI] [PubMed] [Google Scholar]
- 44.Cong WM. Surgical pathology of hepatobiliary tumors. Beijing: People’s Medical Publishing House; 2015. Cholangiocytic malignancy; pp. 320–339. [Google Scholar]
- 45.Liau JY, Tsai JH, Yuan RH, Chang CN, Lee HJ, Jeng YM. Morphological subclassification of intrahepatic cholangiocarcinoma: etiological, clinicopathological, and molecular features. Mod Pathol. 2014;27(8):1163–1173. doi: 10.1038/modpathol.2013.241. [DOI] [PubMed] [Google Scholar]
- 46.Hayashi A, Misumi K, Shibahara J, Arita J, Sakamoto Y, Hasegawa K, et al. Distinct Clinicopathologic and Genetic Features of 2 Histologic Subtypes of Intrahepatic Cholangiocarcinoma. Am J Surg Pathol. 2016;40(8):1021–1030. doi: 10.1097/PAS.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 47.Sirica AE, Gores GJ, Groopman JD, Selaru FM, Strazzabosco M, Wei Wang X, et al. Intrahepatic Cholangiocarcinoma: Continuing Challenges and Translational Advances. Hepatology. 2019;69(4):1803–1815. doi: 10.1002/hep.30289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fabris L, Sato K, Alpini G, Strazzabosco M. The Tumor Microenvironment in Cholangiocarcinoma Progression. Hepatology. 2021;73(Suppl 1):75–85. doi: 10.1002/hep.31410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akita M, Fujikura K, Ajiki T, Fukumoto T, Otani K, Azuma T, et al. Dichotomy in intrahepatic cholangiocarcinomas based on histologic similarities to hilar cholangiocarcinomas. Mod Pathol. 2017;30(7):986–997. doi: 10.1038/modpathol.2017.22. [DOI] [PubMed] [Google Scholar]
- 50.Akita M, Sawada R, Komatsu M, Suleman N, Itoh T, Ajiki T, et al. An immunostaining panel of C-reactive protein, N-cadherin, and S100 calcium binding protein P is useful for intrahepatic cholangiocarcinoma subtyping. Hum Pathol. 2021;109:45–52. doi: 10.1016/j.humpath.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Aishima S, Fujita N, Mano Y, Kubo Y, Tanaka Y, Taketomi A, et al. Different roles of S100P overexpression in intrahepatic cholangiocarcinoma: carcinogenesis of perihilar type and aggressive behavior of peripheral type. Am J Surg Pathol. 2011;35(4):590–598. doi: 10.1097/PAS.0b013e31820ffdf1. [DOI] [PubMed] [Google Scholar]
- 52.Song G, Shi Y, Meng L, Ma J, Huang S, Zhang J, et al. Single-cell transcriptomic analysis suggests two molecularly subtypes of intrahepatic cholangiocarcinoma. Nat Commun. 2022;13(1):1642. doi: 10.1038/s41467-022-29164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakanuma Y, Tsutsui A, Ren XS, Harada K, Sato Y, Sasaki M. What are the precursor and early lesions of peripheral intrahepatic cholangiocarcinoma? Int J Hepatol. 2014;2014:805973. doi: 10.1155/2014/805973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aishima S, Oda Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: different characters of perihilar large duct type versus peripheral small duct type. J Hepatobiliary Pancreat Sci. 2015;22(2):94–100. doi: 10.1002/jhbp.154. [DOI] [PubMed] [Google Scholar]
- 55.Kwon AY, Lee HJ, An HJ, Kang H, Heo JH, Kim G. Intrahepatic Cholangiocarcinoma with Ductal Plate Malformation-like Feature Associated with Bile Duct Adenoma. J Pathol Transl Med. 2015;49(6):531–534. doi: 10.4132/jptm.2015.06.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sasaki M, Sato Y, Nakanuma Y. Bile duct adenoma may be a precursor lesion of small duct type intrahepatic cholangiocarcinoma. Histopathology. 2021;78(2):310–320. doi: 10.1111/his.14222. [DOI] [PubMed] [Google Scholar]
- 57.Rhee H, Ko JE, Chung T, Jee BA, Kwon SM, Nahm JH, et al. Transcriptomic and histopathological analysis of cholangiolocellular differentiation trait in intrahepatic cholangiocarcinoma. Liver Int. 2018;38(1):113–124. doi: 10.1111/liv.13492. [DOI] [PubMed] [Google Scholar]
- 58.Ma B, Meng H, Shen A, Ma Y, Zhao D, Liu G, et al. Prognostic Value of Inflammatory and Tumour Markers in Small-Duct Subtype Intrahepatic Cholangiocarcinoma after Curative-Intent Resection. Gastroenterol Res Pract. 2021;2021:6616062. doi: 10.1155/2021/6616062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma B, Meng H, Tian Y, Wang Y, Song T, Zhang T, et al. Distinct clinical and prognostic implication of IDH1/2 mutation and other most frequent mutations in large duct and small duct subtypes of intrahepatic cholangiocarcinoma. BMC Cancer. 2020;20(1):318. doi: 10.1186/s12885-020-06804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moeini A, Sia D, Zhang Z, Camprecios G, Stueck A, Dong H, et al. Mixed hepatocellular cholangiocarcinoma tumors: Cholangiolocellular carcinoma is a distinct molecular entity. J Hepatol. 2017;66(5):952–961. doi: 10.1016/j.jhep.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 61.Yamamoto M, Oshita A, Nishisaka T, Nakahara H, Itamoto T. Synchronous double primary hepatic cancer consisting of hepatocellular carcinoma and cholangiolocellular carcinoma: a case report. J Med Case Rep. 2018;12(1):224. doi: 10.1186/s13256-018-1762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen J, He J, Deng M, Wu HY, Shi J, Mao L, et al. Clinicopathological, radiologic, and molecular study of 23 combined hepatocellular-cholangiocarcinomas with stem cell features, cholangiolocellular type. Hum Pathol. 2017;64:118–127. doi: 10.1016/j.humpath.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen Canh H, Takahashi K, Yamamura M, Li Z, Sato Y, Yoshimura K, et al. Diversity in cell differentiation, histology, phenotype and vasculature of mass-forming intrahepatic cholangiocarcinomas. Histopathology. 2021;79(5):731–750. doi: 10.1111/his.14417. [DOI] [PubMed] [Google Scholar]
- 64.Chen J, He J, Qiu YD. Research advances on cholangiolocellular carcinoma. Chinese Journal of Hepatobiliary Surgery. 2016;22(11):784–787. doi: 10.3760/cma.j.issn.1007-8118.2016.11.018. [DOI] [Google Scholar]
- 65.Kondo F, Fukusato T, Tokairin T, Saito K, Soejima Y. Cholangiolocellular carcinoma: Is it a subtype of cholangiocarcinoma or combined hepatocellular cholangiocarcinoma? In: Zhang YJ, Cong WM, editors. Pathology of the bile duct. Shanghai: Shanghai Scientific & Technical Publishers; 2019. pp. 120–127. [Google Scholar]
- 66.Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47(5):1544–1556. doi: 10.1002/hep.22238. [DOI] [PubMed] [Google Scholar]
- 67.Sempoux C, Kakar S, Kondo F, Schirmacher P. Combined hepatocellular-cholangiocarcinoma and undifferentiated primary liver carcinoma. In: WHO Classification of Tumours Editorial Board, editor. WHO classification of tumours, Digestive system tumours. 5th edition. Lyon: IARC Press; 2019. pp. 260–262. [Google Scholar]
- 68.Nagata K, Einama T, Kimura A, Murayama M, Takeo H, Nishikawa M, et al. A case of intrahepatic cholangiocarcinoma that was difficult to diagnose prior to surgery: A case report. Oncol Lett. 2019;17(1):823–830. doi: 10.3892/ol.2018.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vij M, Puri Y, Rammohan A, G G, Rajalingam R, Kaliamoorthy I, et al. Pathological, molecular, and clinical characteristics of cholangiocarcinoma: A comprehensive review. World J Gastrointest Oncol. 2022;14(3):607–627. doi: 10.4251/wjgo.v14.i3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Komuta M, Govaere O, Vandecaveye V, Akiba J, Van Steenbergen W, Verslype C, et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012;55(6):1876–1888. doi: 10.1002/hep.25595. [DOI] [PubMed] [Google Scholar]
- 71.Nakanuma Y, Sato Y, Ikeda H, Harada K, Kobayashi M, Sano K, et al. Intrahepatic cholangiocarcinoma with predominant “ductal plate malformation” pattern: a new subtype. Am J Surg Pathol. 2012;36(11):1629–1635. doi: 10.1097/PAS.0b013e31826e0249. [DOI] [PubMed] [Google Scholar]
- 72.Song JS, Lee YJ, Kim KW, Huh J, Jang SJ, Yu E. Cholangiocarcinoma arising in von Meyenburg complexes: report of four cases. Pathol Int. 2008;58(8):503–512. doi: 10.1111/j.1440-1827.2008.02264.x. [DOI] [PubMed] [Google Scholar]
- 73.Terada T. What are ductal plate and ductal plate malformations of human livers? Liver Int. 2013;33(8):1294. doi: 10.1111/liv.12223. [DOI] [PubMed] [Google Scholar]
- 74.Li MJ, Feng JX. Biliary plate malformation and congenital biliary dysplasia. Journal of Hepatobiliary Surgery. 2001;04:316–318. doi: 10.3969/j.issn.1006-4761.2001.04.035. [DOI] [Google Scholar]
- 75.Chung T, Rhee H, Shim HS, Yoo JE, Choi GH, Kim H, et al. Genetic, Clinicopathological, and Radiological Features of Intrahepatic Cholangiocarcinoma with Ductal Plate Malformation Pattern. Gut Liver. 2022;16(4):613–624. doi: 10.5009/gnl210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choe JY, Kim H. Intrahepatic cholangiocarcinoma with predominant ductal plate malformation pattern. Clin Mol Hepatol. 2014;20(2):214–217. doi: 10.3350/cmh.2014.20.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sasaki M, Sato Y, Nakanuma Y. Cholangiolocellular Carcinoma With “Ductal Plate Malformation” Pattern May Be Characterized by ARID1A Genetic Alterations. Am J Surg Pathol. 2019;43(3):352–360. doi: 10.1097/PAS.0000000000001201. [DOI] [PubMed] [Google Scholar]
- 78.Wang XF, Chen Y, Liu MJ, Lin ZH, Chen LH, Wang B. Intrahepatic cholangiocarcinoma characterized by ductal plate malformation: a case report and literature review. Chinese Journal of Clinical and Experimental Pathology. 2021;37(11):1364–1366. doi: 10.13315/j.cnki.cjcep.2021.11.020. [DOI] [Google Scholar]
- 79.Chung T, Park YN. Up-to-Date Pathologic Classification and Molecular Characteristics of Intrahepatic Cholangiocarcinoma. Front Med (Lausanne) 2022;9:857140. doi: 10.3389/fmed.2022.857140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guidelines Committee of Chinese Society of Clinical Oncology . Second Line Treatment of Advanced biliary Cancer. In: Guidelines Committee of Chinese Society of Clinical Oncology, editor. Guidelines of the Chinese Society of Clinical Oncology (CSCO) Biliary Tract Cancer. Beijing: People’s Medical Publishing House; 2021. pp. 63–67. [Google Scholar]
- 81.Benson AB, D’Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(5):541–565. doi: 10.6004/jnccn.2021.0022. [DOI] [PubMed] [Google Scholar]
- 82.Cao J, Hu J, Liu S, Meric-Bernstam F, Abdel-Wahab R, Xu J, et al. Intrahepatic Cholangiocarcinoma: Genomic Heterogeneity Between Eastern and Western Patients. JCO Precis Oncol. 2020;4:557–569. doi: 10.1200/PO.18.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang L, Zhu H, Zhao Y, Pan Q, Mao A, Zhu W, et al. Comprehensive molecular profiling of intrahepatic cholangiocarcinoma in the Chinese population and therapeutic experience. J Transl Med. 2020;18(1):273. doi: 10.1186/s12967-020-02437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov. 2017;7(9):943–962. doi: 10.1158/2159-8290.CD-17-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72(2):353–363. doi: 10.1016/j.jhep.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 86.Munugala N, Maithel SK, Shroff RT. Novel biomarkers and the future of targeted therapies in cholangiocarcinoma: a narrative review. Hepatobiliary Surg Nutr. 2022;11(2):253–266. doi: 10.21037/hbsn-20-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gutiérrez-Larrañaga M, González-López E, Roa-Bautista A, Rodrigues PM, Díaz-González Á, Banales JM, et al. Immune Checkpoint Inhibitors: The Emerging Cornerstone in Cholangiocarcinoma Therapy? Liver Cancer. 2021;10(6):545–560. doi: 10.1159/000518104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng Y, Wang S, Cai J, Ke A, Fan J. The progress of immune checkpoint therapy in primary liver cancer. Biochim Biophys Acta Rev Cancer. 2021;1876(2):188638. doi: 10.1016/j.bbcan.2021.188638. [DOI] [PubMed] [Google Scholar]
- 89.Zhou J, Sun Y, Zhang W, Yuan J, Peng Z, Wang W, et al. Phase Ib study of anlotinib combined with TQB2450 in pretreated advanced biliary tract cancer and biomarker analysis. Hepatology. 2023;77(1):65–76. doi: 10.1002/hep.32548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi JY, Gao Q, Zhou J, Fan J. Interpretation and validation of 8th edition American Joint Commission on Cancer TNM staging system for intrahepatic cholangiocarcinoma. Journal of Surgery Concepts & Practice. 2018;23(03):221–226. doi: 10.16139/j.1007-9610.2018.03.009. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The GRADE system.