Abstract
Recent works indicate that the lipid composition of extracellular vesicles (EVs) can modify their biological functions and their incorporation into recipient cells. In particular high‐fat diets affect EV biogenesis, EV lipid composition, EV targeting and consequently the cross‐talk between tissues. This review connects different research topics to show that a vicious circle is established during the development of high‐fat diet‐induced obesity, connecting the alteration of lipid metabolism, the composition of extracellular vesicles and the spread of deleterious lipids between tissues, which participates in NAFLD/NASH and diabetes development. According to the studies described in this review, it is urgent to take an interest in this question as the modulation of EV lipid composition could be an important factor to take into account during the therapeutic management of patients suffering from metabolic syndrome and related pathologies such as obesity and diabetes. Furthermore, as lipid modification of EVs is a strategy currently being tested to enable better integration into their target tissue or cell, it is important to consider the impact of these lipid modifications on the homeostasis of these targets.
Keywords: diabetes, extracellular vesicles, high‐fat diets, lipids, metabolic syndrome, NAFLD, NASH, obesity
1. INTRODUCTION
Extracellular vesicles (EVs) are small lipid‐derived nanovesicles that are released by cells into the extracellular space. They contain various bioactive molecules from the releasing cells such as proteins, lipids, nucleic acids, and other. These vesicles play important roles in intercellular communication as they can transfer their cargo into recipient cells, influencing their function and behaviour. Among these molecules, lipids do play a significant role in the composition of EVs as they contribute both to the structure and the biological function of EVs. Lipids form the lipid bilayer membrane that encapsulates the content of EVs and contribute to the export of toxic intracellular lipids (e.g., cholesterol, ceramides, palmitate) and to the exchange of lipids between cells. As EVs have different cellular origin, they have different lipid composition. Presently, our knowledge on EV biogenesis is still partial and the link between dietary lipids and the composition of EVs is just beginning to be investigated. Lipids for EVs are derived from various sources within the cell. They are synthesized through a series of enzymatic reactions in different cellular compartments, primarily in the endoplasmic reticulum (ER) (Figure 1). These lipids are then incorporated into the membrane of EVs during their biogenesis process.
FIGURE 1.

Summary the intracellular origin of the lipids that make up extracellular vesicles, and the diseases associated with lipid variations in EVs in response to consumption of high‐fat diets. CER: ceramides; SM: sphingomyelin; PA: Phosphatidic acid; PC: phosphatidylcholine, PE: phosphatidylethanolamine, PI: phosphatidylinositol; PS: phosphatidylserine, LPE: lysophosphatidyl ethanolamine; LPC: lysophosphatidyl choline; CHOL: cholesterol.
There is limited research on how lipids specifically affect the different subpopulations of EVs, so it is difficult to make definitive conclusions, but it was demonstrated that there is a direct correlation between changes in lipid composition of the EVs with the metabolic state of cells (Lam et al., 2021). Recent data, mentioned in the present review indicate that the lipids from the diet (quantity and composition) can impact EVs in several ways: they can impact the composition of EVs by becoming incorporated into their lipid bilayer, or by being included as cargo within the vesicles, or by acting as signalling molecules, or by influencing cellular lipid metabolism in the recipient cells. Particularly, the consumption of a high‐fat diet, which is associated with obesity, insulin resistance, and various metabolic disorders, can increase the number of circulating EVs in the obese state (Eguchi et al., 2016). The increased release of EVs from cells in response to a high‐fat diet suggests that they may play a role in mediating the intercellular communication related to metabolic disturbances. Furthermore, high‐fat diets can alter the lipid composition of EVs. Certain types of lipids, such as ceramides and cholesterol, are more abundant in EVs derived from animals on a high‐fat diet (Zietzer et al., 2021). These lipids have been implicated in the development of insulin resistance and other metabolic dysfunctions. For example, EVs from adipocytes exposed to a high‐fat diets have been shown to induce insulin resistance in insulin‐sensitive recipient cells (Le Lay et al., 2021).
Overall, the relationship between high‐fat diets and EVs is an area of active research and the present review tries to connect different research topics to demonstrate that a vicious circle is established during the development of HFD‐induced obesity, which links the alterations of lipid metabolism, the altered composition and release of EVs, and the spread of deleterious lipids between tissues.
2. DIFFERENT TYPES OF EXTRACELLULAR VESICLES WITH SPECIFIC LIPID COMPOSITIONS
We will focus this review only on the two main types of EVs released from metabolically active cells that have been best described in the literature and for which sufficient data on the role of lipids in their biosynthesis are becoming available, that is, exosomes and microvesicles (Figure 1). In addition, dying cells release >1 µm apoptotic bodies which are cleared by patrolling immune cells. Previous reviews on the genesis of EVs have already been published and have described in detail the signalling pathways and the proteins involved in EV generation and release (Buzas, 2023; Hurley, 2015). Thus, in this review, we will specifically focus on the lipids involved in EV biogenesis in order to determine how modulations of the lipid composition of the diet may impact on this process. However, one must keep in mind that few lipidomic studies have been performed, and thus we do not have yet a general picture of the lipids specifically contained in each EV subtypes. In addition, mainly lipidomic studies were performed on cancer cells, which have very specific lipid and glucose metabolisms. Thus, lipids in cancer cell‐released EVs may be somewhat different from those contained in EVs released from healthy tissues. Also, for many studies, it is difficult to know which type of EVs the authors are working on, and therefore we will mainly use ‘EVs’ as the generic term but strongly recommend the readers to pay attention to the techniques used to isolate EVs. Indeed, impure EV pellets can lead to conflicting results, for example, the presence phosphatidylserine (PS) on all exosomes (Jakubec et al., 2020; Llorente et al., 2013) or only on a subtype of exosomes (Durcin et al., 2017; Matsumoto et al., 2021), or not detected in exosomes (Lai & Breakefield, 2012); or about the distribution of each lipid species inside exosomes, microvesicles and apoptotic bodies isolated from different types of cells (Durcin et al., 2017; Pollet et al., 2018; Skotland et al., 2020). In addition, as EV lipid composition is somehow correlated to the lipid composition of the releasing cell (even among the same cellular model (Royo et al., 2019)) or tissues (adipose tissue (Blandin et al., 2023) skeletal muscle (Jalabert et al., 2021)), the metabolic state of the cell is also an important parameter to consider. Indeed, it is easy to imagine that in starving conditions, cells prevent the export of lipids and proteins and activate their degradations to recycle these compounds for cellular homeostasis. It is therefore important to look carefully at the cellular metabolic state in which the EVs were isolated in order to compare the different studies on lipidomic analyses from EVs.
2.1. Extracellular vesicles from endosomal origin: the exosomes
Exosomes are a subtype of EVs (30−100 nm) released from viable cells (Figure 1). They are formed during the inward budding of the limiting membrane of the late endosomes. During this endosomal vacuolization, cytosolic proteins, nucleic acids, and lipids are incorporated into intraluminal vacuoles (ILVs) inside specific late endosomes, which are called ‘multivesicular bodies’ (MVBs) (Figure 2a). Depending on the cell metabolic state (starved vs. fed) ILV cargoes will be either targeted to lysosomes for degradation, recycled to the endoplasmic reticulum or the Golgi apparatus, or transported to the plasma membrane and secreted (Fader et al., 2008). Indeed, some subsets of MVBs can fuse with the plasma membrane and release ILVs outside the cell (Sotelo & Porter, 1959). Extracellular ILVs are then called ‘exosomes’. Compared to ILVs, exosomes have increased membrane rigidity suggesting a membrane reorganization between the acidic MVB compartment and the neutral pH outside the cells. This could give them a greater stability in the external environment than the plasma membrane‐derived microvesicles (Skotland et al., 2019).
FIGURE 2.

(a) Main lipids and cellular pathways affected by high‐fat diets and consequences on the biogenesis and the lipid composition of extracellular vesicles. (b) Cellular lipids play a crucial role on the curvature of cell membranes. Certain types of lipids can spontaneously form positive or negative curved structures depending on their specific shape. CER: ceramides; SM: sphingomyelin; PA: Phosphatidic acid; PC: phosphatidylcholine, PE: phosphatidylethanolamine, PI: phosphatidylinositol; PS: phosphatidylserine; LPC: lysophosphatidyl choline; LPA: lysophosphatidyl acid; OA: oleic acid; PA: Palmitic acid; BMP: Bis(monoacylglycerol)phosphate.
The lipid composition of the exosomal membrane has similar features of plasma membrane raft domains but is enriched in cholesterol, sphingomyelin (SM), ether‐linked phospholipids and lysoglycerophospholipids and have higher level of saturated phospholipids and saturated fatty acids, when compared to the cellular lipid composition (Buratta et al., 2017; Llorente et al., 2013; Skotland et al., 2023). Conversely, exosomes contain less phosphatidylcholine (PC) than the releasing cells (Laulagnier et al., 2004). However, the specific lipid of MVBs and lysosomes (i.e., Bis(monoacylglycerol)phosphate (BMP)) is not enriched in exosomes (Laulagnier et al., 2004). Although considered as the only lipid able to discriminate between exosomes and microvesicles, it is in practice barely detectable in EVs.
ILV formation inside MVBs combines lipids and proteins from the endosomal sorting complex required for transport (ESCRT machinery (well‐described in (Hurley, 2015)). The presence of cholesterol in exosomes correlates with the fact that MVBs contain most of the cholesterol found in the endocytic pathway, the main entrance point and delivery site of low‐density lipoprotein (LDL)‐derived cholesterol (Möbius et al., 2003) (Figure 2a). Cholesterol regulates both the volume and vacuolization of MVBs (Huotari & Helenius, 2011), and to MVB migration along microtubules (e.g., low cellular cholesterol inhibits MVB migration (Rocha et al., 2009), but increased cholesterol content induces exosomes release (Zhao et al., 2020)). After MVB formation, MVB migration and exosome release require components of the cytoskeleton but also fusogenic lipids such as BMP (Luquain‐Costaz et al., 2020) and phosphatidic acid (PA) (Laulagnier et al., 2004) (Figure 2a).
SM is the most abundant lipids from plasma membrane and is a ceramide precursor. Ceramides can induce negative curvature of membranes (Stancevic & Kolesnick, 2010) (Figure 2b). Consequently, increasing the level of the neutral sphingomyelinase 2 produces ceramides and forces the formation of ILVs inside MVBs triggering the release of more exosomes (Trajkovic et al., 2008). Of note, data on the cancer cells PC‐3 contradict this connection between high levels of ceramides and increased EV release, suggesting that the role of ceramides in exosome release is cell‐dependent (Phuyal et al., 2014). One possible explanation is that in cancer cells ceramides are tumour suppressor lipids and induce cell death by inducing apoptosis (Dany & Ogretmen, 2015). This specific situation likely blunts the release of EVs.
2.2. Extracellular vesicles from the budding of the plasma membrane: the microvesicles
Microvesicles (100–500 nm) are formed during membrane repair in response to a rapid Ca2+ influx (Figure 1). Like for the formation of ILVs, some of the ESCRT proteins are required in addition to lipids (Hurley, 2015) (Figure 2a). Until now, the microvesicle lipid composition has been poorly studied. For adipocyte‐derived EVs, it was described that exosomes have higher content of cholesterol compared to microvesicles (relative to the total EV protein content) (Durcin et al., 2017). For EVs derived from pleural effusion, microvesicles contained more ceramides with long carbon acyl chains, phosphatidylethanolamine (PE), PA, phosphatidylinositol (PI) and less SM, PC, LysoPE (LPE), and LysoPC (LPC) than exosomes (Luo et al., 2020). For Blood‐derived EVs, both exosomes and microvesicles had similar PC and LysoPC levels, but microvesicles were enriched in PA (Paolino et al., 2022) (Figure 1).
Like for exosomes, ceramides are also involved in the release of microvesicles. For example in the brain, the formation of membrane protrusions can occur from lipid rafts, where accumulation of ceramides by local translocation of the acid sphingomyelinase can facilitate membrane blebbing (Bianco et al., 2009). In addition to the release of exosomes, cholesterol also participates in the generation of cholesterol‐rich microvesicles from the plasma membrane through the action of the ATP binding cassette A1 (ABCA1) transporter (Nandi et al., 2009). The main function of ABCA1 is to facilitate the transport of cholesterol and phospholipids from cells to nascent high‐density lipoprotein (HDL) particles, which are composed of apolipoprotein A‐I (apoA‐I). This process helps in the formation of mature HDL particles. Therefore, ABCA1 plays a crucial role in the reverse cholesterol transport pathway, which is important for maintaining cholesterol homeostasis in the body and at the cellular level. Interestingly, in the absence of apoA‐I, ABCA1 can also trigger the release of cholesterol/phospholipid‐rich microvesicles (Nandi et al., 2009) which contain plasma membrane raft domains (e.g., ganglioside (GM1) (Duong et al., 2006)). Undoubtedly due to its action on the local concentration of cholesterol in membranes, ABCA1 can modulate the fluidity of the plasma membrane and can generate membrane curvatures (Nandi et al., 2009) (Figure 2b). In baby hamster kidney (BHK) cells and RAW macrophages, the release of cholesterol in microvesicles accounts for approximately 30% of the total cholesterol released into the medium (Nandi et al., 2009), but it is much lower for fibroblasts (Duong et al., 2006) in agreement with the lower level of plasma membrane vesicle trafficking in fibroblasts compared to phagocytic macrophages.
At the plasma membrane PC and SM are located on the external layer, while PS and PE are found on the inner side. The preservation of this asymmetry is important and is maintained with a complex transmembrane enzymatic balance. During the formation of microvesicles, the loss of plasma membrane asymmetric in phospholipid distribution regulates membrane flippase, floppase and scramblase activities, leading to PS and PE exposition on the outer membrane leaflet, and the activation of contractile proteins involved in microvesicle release (Muralidharan‐Chari et al., 2010). Transmembrane migration of PS also involves ABCA1. It was demonstrated that the overexpression of ABCA1 induced elevated redistribution of PS and PE (but not of PC) from the cytoplasmic to the external leaflet of the plasma membrane (Alder‐Baerens et al., 2005). Consequently, as PS is a negatively charged phospholipid, it confers to microvesicles a higher clotting capacity than exosomes (Lentz, 2003). PS has also been proposed as an “eat‐me” signal for PS receptor T‐cell immunoglobulin mucin protein 4 (TIM4) at the surface of macrophages facilitating microvesicle clearance from body fluids (Naeini et al., 2020).
2.3. Extracellular vesicles from dying cells: the apoptotic bodies
Apoptotic bodies (AP) are another type of EVs specific to eukaryotes, which are formed by phospholipid scrambling and membrane blebbing induced during cell death. They are not usually considered as formal ‘EVs’ but their size and composition can affect the characterization and the isolation of exosomes and microvesicles (Figure 1). In particular, AP are formed during apoptosis by outward budding from the cell surface and as a result they share some features with microvesicles as they also express PS at their surface (Hengartner, 2001). Thus, similarly to microvesicles, they are cleared from biofluids by macrophages and can actively participate in tissue regeneration (Li et al., 2020). In that context it is important to consider that the use of Annexin V for its high PS binding affinity to purify apoptotic bodies might result in the co‐precipitation with microvesicles (Subiros‐Funosas et al., 2017). In addition, the use of a TIM4‐affinity isolation method for EV purification might also result in the co‐precipitation of microvesicles and apoptotic bodies and in the depletion of PS+ exosomes (Yoshida & Hanayama, 2022).
Apoptotic bodies release in response to apoptotic stimuli depends on the breakdown of plasma membrane SM. SM has a high affinity for cholesterol and both lipids participate in membrane fluidity and structural integrity. SM hydrolysis by sphingomyelinases, results in increased cholesterol efflux and increased membrane fluidity (Slotte et al., 1989), thus inducing membrane destabilization and blebbing.
Overall, EV lipid composition is strongly dependent on the cell lipid metabolism, as it reflects the dynamic interplay between lipid synthesis, degradation, and modification within a cell. Therefore, the composition and the type of fat in the diet will modify the lipid membrane composition at the cellular level thus compromising EV composition (Blandin et al., 2023). In that context, the amount of saturated fat in our diet has continuously increased during the last 3 decades. This change in the composition of fatty acids combined with increased caloric intake parallels a significant increase in the prevalence of metabolic syndrome and obesity. Understanding how high‐fat diets modulate the function of EVs is crucial for elucidating their role in metabolic disorders and developing therapeutic approaches targeting EVs for the treatment of obesity and diabetes.
3. HOW HIGH‐FAT DIETS AFFECT EXTRACELLULAR VESICLE LIPID COMPOSITION, RELEASE AND BIOLOGICAL FUNCTIONS?
Among the lipids modified by the diet, SM, ceramides, cholesterol and some phospholipids (Blandin et al., 2023; Shah et al., 2008) are involved in EV biogenesis, release and biological functions (Choi & Snider, 2015). In this review, we will not discuss how the composition of the HFD in terms of quantities of fat and sugar can affect EV composition and fate, as there are no comparative data available. But it might affect the data presented below. Also, the ‘ketogenic’ diet (KD) which is a special high‐fat diet containing very little sugar and protein is not considered here. KD mimics the metabolic state of starvation, forcing the body to use fat as its main source of energy. Therefore, at the cellular level; there is no accumulation of ceramides or cholesterol and no modulation of phospolipids as observed with the HFD diets (Dabke et al., 2020). In addition, as animal model of obesity, we will consider only the diet‐induced obese mice because the relevance of the ob/ob mouse model of obesity as a model for the development of human obesity is uncertain. Ob/ob mice put on weight on a normal chow diet because they are hyperphagic and therefore, the results in terms of lipid composition of their tissues are different from the tissues of diet‐induced obese mice (e.g., adipose tissue (Blandin et al., 2023)). It should also be kept in mind that rodents and humans are distinct with respect to C16‐sphingolipid metabolism (Hornemann et al., 2009), therefore it is important to take precautions in extrapolating lipidomic data obtained from diet‐induced animals to patients suffering from obesity. Finally, all studies mentioned below have been carried out in male mice, as female mice are protected against HFD‐induced metabolic alterations (i.e., absence of adipose tissue inflammation, glucose intolerance, hyperinsulinemia, and islet hypertrophy (Pettersson et al., 2012)). Conversely, in human, female obesity greatly exceed that of males in most countries and female prevalence of obesity is more variable than male prevalence (Garawi et al., 2014). In addition, as a woman transitions to menopause, her risk factors for cardiovascular disease rise, including risk factors such as increasing LDL and decreasing HDL. Therefore, it is important to mention that our view of the consequences of HFD on EV metabolism is still largely biased.
3.1. Extracellular vesicle sphingolipids and high‐fat diets
3.1.1. HFD affect enzymes of sphingolipid metabolism in liver and adipose tissue and thus EV release
Sphingolipidomic analysis of 114 sphingolipids from 21 murine tissues has demonstrated that their levels were increased in many tissues from mice fed with HFD. But contrary to what one might have thought, the sphingolipid composition of adipose tissue from these animals was much less altered than that of the liver or the plasma, showing an immediate effect of HFD on the blood‐liver system (Chocian et al., 2010; Muralidharan et al., 2021). In the liver, ceramide accumulation is associated with the up‐regulation of the neutral sphingomyelinase, which can hydrolyse sphingomyelin from the plasma membrane to produce ceramides (Chocian et al., 2010). As increased concentrations of ceramides are associated with the formation of microvesicles and exosomes, more EVs are expected to be released from the liver of HFD animals. In agreement, the secretion of hepatocyte‐derived EVs was increased after a short period of HFD (Liao et al., 2018; Povero et al., 2020). This increase was correlated with an increased activity of the neutral sphingomyelinase in response to the HFD (Zhao et al., 2020), and with the transcriptional activation of the serine palmitoyl‐transferase (Dasgupta et al., 2020). Hepatocyte‐derived EVs released after a HFD were able to stimulate lipid accumulation in adipocytes (Zhao et al., 2020) and to promote proliferation of β‐cell MIN6 (Camino et al., 2022), in vitro. How the lipid composition of hepatocyte‐derived EVs participate in these biological effects is presently unknown.
Considering the same HFD composition, 8‐week‐old C57BL/6J males fed for 16 weeks with HFD had increased ceramides levels in adipose tissue (i.e., both from de novo synthesis and via the hydrolysis of SM (Shah et al., 2008)). However, 4‐week‐old C57BL/6J males placed for 15 weeks on HFD accumulated dihydroceramides only (intermediary molecules in the ceramide pathway) and not ceramides (Blandin et al., 2023). If we extrapolate these results to humans, they indicate that for the same period of HDF, the age of the subjects at the beginning of HFD diet can impact the lipid composition of EVs released by the adipose tissue. Accumulation of dihydroceramides was associated with an increase in the release of EVs from the adipose tissue of the obese animals (Blandin et al., 2023). This increase was likely due to the induced activity of the dihydroceramide desaturase by the diet known to promote the formation of intraluminal vesicles in MVBs but also to inhibit autophagy to increase exosome production (Hu et al., 2011). At the lipid species level, EVs and the releasing adipose tissue from the 4‐week‐old C57BL/6J males had strong increased concentrations of two specific ceramides (18:0/18:0 and 18:2/18:1) and three specific SM (34:2, 36:1, and 36:2) (Blandin et al., 2023), suggesting that only subtypes of sphingolipids may be involved in EV genesis and sorting from adipose tissue. As these five lipids were similarly increased in adipose tissue and released‐EVs from ob/ob mice, and in isolated adipocytes from HFD and ob/ob mice, we can conclude that they are correlated with the storage of TAG in the adipocytes during the development HFD‐induced obesity (Blandin et al., 2023). However, lipidomic analyses of all metabolic tissues and their respective released‐EVs, during the time course of the development of the diet‐induced obesity are missing to validate this hypothesis.
Taken together, these data indicate that the consumption of HFD affects the release of EVs through the modulation of the intracellular content of sphingolipids. Whether sphingolipids participate in EV biological functions and in organ‐cross talks during the development of metabolic diseases is not known. Nevertheless, in a context of cancer, it was found that SM from tumour‐derived EVs was the main lipid responsible for the effect of EVs on endothelial cell migration and angiogenesis. The authors clearly demonstrated that phospholipids such as PE, PI, PC, and PS also contained in these tumour‐derived EVs showed little effects on endothelial cell migration (Anon, 2023). Similar studies on the role of each sphingolipid contained in EVs and modulated by the HFD are necessary. For instance, it is known that adipose tissue‐released ceramides can regulate vascular redox state and the outcomes of patients with cardiovascular disease (Akawi et al., 2021), but the specific role of each ceramide carried by EVs in this process has never been studied.
3.1.2. HFD perturbate glucagon‐induced EV release from endothelium
Altered sphingolipid levels are also involved in obesity‐induced endothelial dysfunction and atherosclerosis (Choi & Snider, 2015). During the postprandial period, the decrease in the secretion of glucagon by the pancreas is a signal toward the metabolic tissues to store lipids. Conversely, during the starving period, glucagon mobilizes lipids to ensure energy supply. Philipp E. Scherer and his group (Crewe et al., 2018) have demonstrated that this important hormone increases the production of EVs from the endothelial cells located in adipose tissue, at fasted state. At the mechanistic level, they found that glucagon stimulated the neutral sphingomyelinase in endothelial cells leading to the production of endogenous ceramides. Consequently, more EVs were released. Interestingly, this regulation by glucagon was impaired in mice under HFD, likely because the neutral sphingomyelinase is constantly induced in obese mice (Samad et al., 2006; Shah et al., 2008). Of note, the glucagon‐induced ceramide synthesis did not affect the generation of microvesicles from endothelial cells, but only the generation of small exosome‐like vesicles, conversely to what was published for cancer cells (Menck et al., 2017) or human coronary artery endothelial cells (Zietzer et al., 2021). The molecular mechanisms supporting the release of subpopulations of EVs from endothelial cells in response to intracellular increase of ceramides would deserve to be studied. Human coronary artery endothelial cells treated with high concentrations of C16 ceramide released microvesicles enriched in ceramides. It was demonstrated that the transfer of ceramides into endothelial cells induced apoptosis (Zietzer et al., 2021).
3.1.3. HFD perturbate adiponectin‐induced EV release from endothelium
In addition to nutritional hormones like glucagon, cytokines can also participate in EV biogenesis. Adiponectin is a cardioprotective circulating cytokine produced by adipose tissue. Adiponectin can accumulate in heart, vascular endothelium and skeletal muscle through its binding with its receptor T‐cadherin. In vitro, adiponectin accumulates inside MVBs in cells expressing T‐cadherin. The adiponectin/T‐cadherin system enhanced EV biogenesis and secretion, and consequently decreased intracellular ceramide concentrations (Obata et al., 2018). This study has proposed a new mechanism to demonstrated how adiponectin can mediate organ protection against lipotoxicity through EV biogenesis and secretion (Obata et al., 2018). However, it is well‐known that HFD are associated with a decrease in circulating level of adiponectin, which is inversely correlated with the expansion of the adipose tissue. Therefore, it is likely that this tight control of the intracellular ceramide concentration by the adiponectin in its target tissues is altered and might participate in the increase of ceramides in the endothelium of obese subjects and consequently in the release of more EVs from this tissue. In line with this hypothesis, it was found that the number of endothelial cell‐derived microvesicles is increased in the plasma from obese rats (Heinrich et al., 2015), but the connection with adiponectin has not been studied yet.
3.1.4. Sphingolipid enzymatic machinery is exported in extracellular vesicles
The presence of sphingosine 1‐phosphate (S1P) has been revealed in EVs (Gupta et al., 2022; Liao et al., 2018). Sphingosine (SS) is synthesized from ceramides by the action of ceramidase and phosphorylation of sphingosine is then catalysed by the sphingosine kinase (SPHK) to produce S1P (Figure 2a). S1P is released from cells where it works as a bioactive lipid mediator of angiogenesis, vascular maturation, immunity, and chemotaxis in recipient cells. It was described that HFD are associated with increased levels of SS and S1P in several tissues and also in plasma (Choi & Snider, 2015; Guitton et al., 2020). In vitro, palmitate‐treated hepatocytes released EVs enriched in S1P. Gradient of S1P‐enriched EVs generated in a microfluidic gradient generator was chemo attractive for macrophages and reducing the level of S1P in EVs inhibited the chemotaxis of macrophages (Liao et al., 2018). This work clearly demonstrated how EVs could participate in the pro‐inflammatory effect of HFD on the liver and suggest that S1P‐EVs could participate in the development of liver fibrosis.
Inside the liver tissue, EVs can also work as paracrine signals as hepatocyte‐derived EVs can deliver the enzymatic machinery to generate S1P in recipient hepatocytes inducing their proliferation and liver regeneration (Nojima et al., 2016). In addition to its action in the liver, S1P is also a regulator of arterial lesions and it was found that both SPHK1 and S1P are present in EVs released from endothelial cells (Wang et al., 2015). In a context of liver fibrosis, S1P concentration is increased in endothelial cell‐derived EVs and can activate hepatic stellate cells into a myofibroblastic phenotype, responsible for the pathogenesis (Wang et al., 2015).
3.2. Extracellular vesicle phospholipids and high‐fat diets
3.2.1. Phosphatidylcholine enrichment in EVs in response to HFD modulates EV trafficking
Phospholipids are the major component of all cell membranes. They can form lipid bilayers because of their amphiphilic characteristic. It is well‐admitted that the amount and the type of dietary fatty acids significantly altered membrane phospholipid distribution (Thi‐Dinh et al., 1990). Recently, it was found that HFD induced the release of EVs from the intestine (Kumar et al., 2021). The authors demonstrated that conversely the other tissues (e.g., adipose tissue, liver) this increase was correlated with the intestinal content of PC, and not with the levels of ceramides. They demonstrated that this result was due to the effect of HFD on the induction of the Phosphatidylethanolamine N‐methyl transferase (PEMT) which converts PE to PC in the intestine. In addition, HFD induced changes in the lipid profile of intestinal‐released EVs, from predominantly PE to PC. As HFD mice and patients suffering from type 2 diabetes both had higher PC on circulating plasma EVs, the authors further compared the biological effect of control EVs (EV‐C) versus EVs released from the intestine of HFD animals (EV‐HFD), on glucose homeostasis (Kumar et al., 2021). Injection of EV‐HFD in control mice inhibited insulin‐induced phosphorylation of IRS‐2, PI3K and AKT/PKB in insulin sensitive tissue. Remarkably, the majority of EV‐C was taken up by hepatocytes. By contrast, the majority of EV‐HFD enriched in PC were taken up by macrophages. This result demonstrated for the first time that the lipid composition of EVs not only modifies their integration into target tissues but also can change their fate. Therefore, EV‐HFD cleared by macrophages would induce the development of a low grade of inflammation. In line with this hypothesis, PEMT (−/−) mice which are unable to induce PEMT in intestine are protected from HFD‐induced obesity and insulin‐resistance (Vance, 2013).
In addition to the recognition with macrophages, the phospholipid composition seems to play a role in the resistance of EVs in the extracellular environment. A recent study on hepatocyte‐derived EVs isolated from primary hepatocytes coupled with atomic force spectroscopy showed that they were less stiff and less resistant to mechanical failure when compared to hepatocyte‐derived EVs isolated from hepatocyte progenitor cell line MPL29, which are almost as resistant as the influenza virus (Royo et al., 2019). This was attributed to the differential abundance of PI, PE, and LPC lipids between these EVs. The authors speculated that the higher flexibility of hepatocyte‐derived EVs would modulate their interactions with target cells.
3.2.2. LPC induced EV release from hepatocytes and mediated monocyte adhesion
In addition to phospholipids, lysophospholipid levels in plasma and tissues are also regulated by the diet. In particular the inverted‐cone‐shaped LPC (Figure 2b) is increased in plasma of nonobese individuals during the chronic consumption of HFD (Kim et al., 2014), or in the plasma of mice fed with HFD (Blandin et al., 2023). As accumulation of this lipid in membranes can induce positive membrane curvature, LPC‐treated hepatocytes secreted three times more EVs, and their mean size were slightly increased (Hirsova et al., 2016). This new population of hepatocyte‐derived EVs was strongly enriched in CXCL10, a protein involved in macrophage chemotaxis (Ibrahim et al., 2016). This result suggests that the level of LPC in hepatocyte‐derived EVs might participate in the development of liver inflammation associated with HFD consumption. Furthermore, LPC treatment of hepatocytes activated integrin β1 processing and loading into hepatocyte‐derived EVs. As LPC‐hepatocyte‐derived EVs can mediated monocyte adhesion through the presence of integrin β1 (Guo et al., 2019), LPC‐hepatocyte‐derived EVs likely participate in diet‐induced NASH in mice.
A generalized decrease in circulating lysophospholipids has been found after weight loss in human with metabolic syndrome and liver steatosis. This decrease was associated with liver metabolism improvement (Cantero et al., 2018). It could be interesting to determine whether weight loss is also associated with modification of LPC concentrations in hepatocyte‐derived EVs.
3.3. Fatty acids and HFD
EVs are carriers of FFAs. Fibroblast‐, muscle‐ and Adipocyte‐derived EVs have high levels of palmitic (C16:0) and stearic (C18:0) acids compared to other FFAs (Aswad et al., 2014; Blandin et al., 2023; Buratta et al., 2017).
3.3.1. Palmitate affect EV release
Palmitate, the most abundant saturated fatty acid in human serum, is the preferred saturated FFA for de novo synthesis of ceramides, SM and is a precursor for the synthesis of phospholipids (Figure 3). Therefore, indirectly palmitate induces EVs release (Aswad et al., 2014; Hirsova et al., 2016; Ibrahim et al., 2016; Phuyal et al., 2015; Zietzer et al., 2021). Indeed, a high‐palmitate diet in mice triggered the release of EVs highly enriched in palmitate from skeletal muscle cells (Aswad et al., 2014). Palmitate‐enriched EVs affected recipient muscle cell homeostasis by reproducing the same transcriptional alterations as those obtained with muscle cells treated directly with palmitate (Aswad et al., 2014). It was also shown that EV released from palmitate‐treated adipocytes altered insulin signalling in recipient muscle cells (Yu et al., 2018). These data have demonstrated that FFA in EVs are highly involved in the biological action of EVs. Interestingly, the protein content of adipocyte‐released EV was slightly different when EVs were released from adipocytes treated with oleate compared when treated with palmitate suggesting that the nature of FFAs in the diet might have a strong impact on the protein composition of EVs also (Camino et al., 2020).
FIGURE 3.

Intracellular pathways involved in phospholipids and sphingolipids synthesis (red) derived from glycolysis and palmitoyl‐CoA, or synthesis at the plasma membrane (PM). Endoplasmic Reticulum (ER). Yellow boxes = enzymes. CLS: Cardiolipin Synthase; TAZ: Tafazzin, Phospholipid‐Lysophospholipid Transacylase; PSD: Phosphatidylserine Decarboxylase; PGPS: Phosphatidylglycerophosphate Synthase; PSS 1/2: Phosphatidylserine Synthase 1 and 2; PIPK: Phosphatidylinositol‐5‐Phosphate 4‐Kinase Type 2 Alpha; CDS: CDP‐Diacylglycerol Synthase; DGK: Diacylglycerol Kinase Beta; LPAP: Acid Phosphatase 6, Lysophosphatidic; GPAT: Glycero‐3‐Phosphate acyltransferase; LPAT: 1‐Acylglycerol‐3‐Phosphate O‐Acyltransferase 1; DGAT: Diacylglycerol O‐Acyltransferase; SPT: Serine Palmitoyltransferase; ET: Ethanolamine Phosphotransferase; CT: Choline Phosphotransferase; CEPT: CDP‐Cho:DAG choline‐Phosphotransferase; CK: Choline Kinase; PEMT: Phosphatidylethanolamine N‐Methyltransferase; EPT: CDP‐ethanolamine 1,2‐diacylglycerol ethanolaminephosphotransferase; EK: ethanolamine kinase; CERS: Ceramide Synthase; SPHK: Sphingosine Kinase; CERase: Ceraminidase; CERK: Ceramide Kinase; SMS: Sphingomyelin Synthase; SMase: Sphingomyelinase; or transporter CD36: Fatty acid translocase.
In a context of cancer, it was demonstrated that adipocyte‐derived EVs provide a local supply of FFAs that serve as energy source for melanoma cells. Melanoma aggressiveness were increased when melanoma cells were treated with EVs released from adipocytes from obese animals. These ‘obese adipocytes’ secreted larger amount of EVs compared to adipocytes from lean mice (Clement et al., 2020). This study has demonstrated for the first time the importance of the metabolic cross‐talk between the fat and the cancer cells through the EV route and how the diet could impact on it.
3.3.2. The fatty acid receptor CD36/FAT is exported in EVs and participate in EV targeting and internalization
Long chain FFA uptake, including palmitate, is mediated by the CD36/FAT fatty acid translocase at the plasma membrane of numerous cell types. CD36 activity and its cell compartment are regulated at the post‐translational level. It was shown that HFD induced CD36 palmitoylation and consequently its translocation at the plasma membranes of hepatocytes (Zhao et al., 2018) and skeletal muscle cells (Chorner et al., 2016). Interestingly, in addition to its role as fatty acid receptor, CD36 also functions as a PS receptor on macrophages. Consequently, CD36 can bind PS at the surface of circulating EVs, participating in EV phagocytosis in macrophages. Of note CD36 binding site for PS vesicles is highly specific and does not bind other phospholipids (Tait & Smith, 1999).
Almost 20 years ago, a cell‐free form of CD36 was identified and its concentration was found higher in plasma of patients suffering from obesity compared to lean subjects. It was further demonstrated that this free form of CD36 was inside microvesicles derived from platelets activated by FFAs (Alkhatatbeh et al., 2011). EVs derived from adipocytes also contain CD36, and palmitate induces its sorting into EVs (Yan et al., 2021). Consequently, adipocyte‐derived CD36‐enriched EVs are better internalized in recipient hepatocytes, in which they induce lipid accumulation and apoptosis. These data suggest that CD36 in EVs might be an inducer of NAFLD due to its high concentration in adipocyte‐derived EVs from patients suffering from obesity, or from diet‐induced obese animals. The role of CD36 in EV internalization was also studied in cancer cells and described as a major mediator of the engulfment of pancreatic tumour microvesicles by myeloid immune cells (Pfeiler et al., 2019).
3.4. Cholesterol
The body can produce all the cholesterol that it needs but it also obtains cholesterol from lipoproteins generated during fat digestion, through receptor‐mediated mechanisms at the plasma membrane. After entering into the cells, cholesterol is concentrated mainly in lysosomes (Möbius et al., 2003) (Figure 2a). In lysosomes, lipoproteins release cholesterol esters through the action of acid lipases. Free cholesterol is exported from lysosomes for cellular metabolism or storage. Cholesterol export requires the two Niemann‐Pick type C 1 (NPC1) and two (NPC2) transporters which have a central role in maintaining lipid homeostasis. Their mutations (i.e., Niemann–Pick type C disease) lead to massive lysosomal accumulation of cholesterol and sphingolipids (Wanikawa et al., 2020). Consequently, HFD enrichment with 1% cholesterol is enough to enhance lipid storage and inflammation in liver of HFD obese mice compared with animals fed with a chow diet (Chang et al., 2021; Zhou & Wu, 2022). Indeed, HFD impair lysosomal function and autophagic flux (Yamamoto et al., 2017). Therefore, the enrichment of HFD with cholesterol further aggravates the effects of HFD on liver homeostasis (Chang et al., 2021). To avoid the resulting toxic lysosomal cholesterol accumulation, it was demonstrated by using a model of oligodendroglial cells, that the release of exosomes is increased when the levels of NPC proteins are reduced (Strauss et al., 2010). This study has suggested for the first time that cholesterol secretion through the exosomal route contributes to the regulation of cholesterol homeostasis.
Genome‐wide association studies have highlighted the contribution of common variants in NPC1 in adult‐onset obesity and diabetes. These associations were validated in mice models and showed an important interaction with HFD (Lamri et al., 2018). At the cellular level, it was found that the treatment of HuH7 hepatocytes with cholesterol reduced the number of MVBs co‐localized with lysosomes and thus increased exosome release. This new population of exosomes could induce M1 polarization of THP‐1 macrophages (Zhao et al., 2020). Taken together, it appears that the endo‐lysosomal dysfunction in the pathogenesis of NAFLD induces the release of cholesterol‐enriched EVs to reduce intracellular lipotoxicity, and this export participates in liver inflammation. It would be interesting to determine whether cholesterol enrichment of liver‐released EVs can be used as an early marker of deterioration in hepatic lipid metabolism, compared to the dosage of circulating thrombospondin 2 which permits to diagnose only advance liver steatosis (Lee et al., 2021).
4. CONCLUSIONS AND PERSPECTIVES
Taken together, these works highlight the fundamental role of the lipid composition of EVs in their biological functions (Figure 4) and demonstrate that HFD modulate EV lipid composition and change EV fate (Figure 5). HFD alter intracellular levels of lipids which are important for membrane configuration, stiffness, and composition. Consequently, EVs can change of target cells, and the amount of EVs incorporated into a tissue can also be affected. Of note, outside the context of metabolic diseases, the studies mentioned here could help to design functionalized vesicles for biomedical applications. New strategies are currently being developed to take account of EV lipids which include either a hybrid liposome‐EV approach or a decoration of EVs with lipid motifs (see reviewed in (Ghadami & Dellinger, 2023)). Better targeting and incorporation of EVs into their target cells also means more lipid entry by EVs, which can have dramatic metabolic consequences. It is therefore important, for every change of EV lipid concentration to also test the fate of the recipient cell.
FIGURE 4.
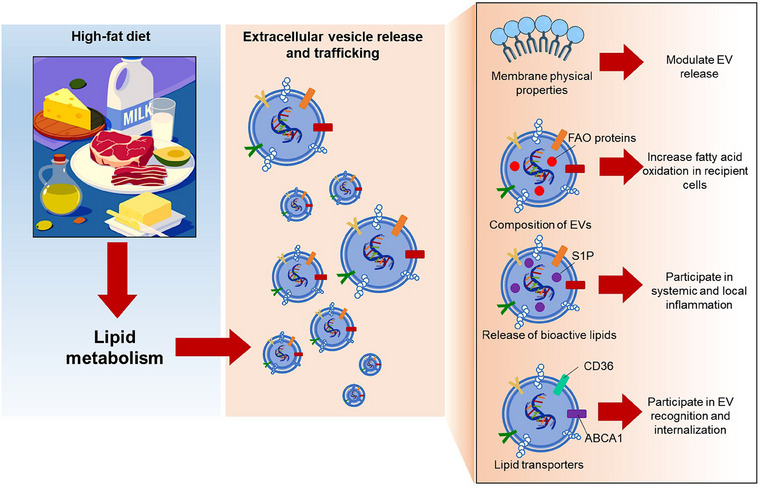
Through the alterations of lipid metabolism and fatty acid oxidation, high‐fat diets impact directly on the lipid composition and curvatures of membranes, inducing the release of extracellular vesicles (EVs). In addition, the lipid and protein compositions of extracellular vesicles are affected. These new cargoes disseminate bioactive lipids (S1P) and proteins for fatty‐acid oxidation (FAO) in recipient cells, or are lipid transporters (CD36, ABCA1) which participate in EV recognition and internalization in target tissues.
FIGURE 5.

Summary of the studies mentioned in this review. They demonstrated how alterations of the lipid metabolism occurring in the liver, skeletal muscle, adipose tissue and endothelium of patients suffering from metabolic diseases and inflammation increase the release of extracellular vesicles (EV), and change their lipid composition. Also indicated the consequences of this altered EV lipid composition on the homeostasis of the recipient cells or tissues.
When obesity is established and inflammation is important, EVs have mostly a deleterious role by carrying lipids and FFAs from tissues to tissues thus amplifying the harmful effect of HFD. At the cellular level, this can have either beneficial consequence: i.e.; by integrating the metabolism of the surrounding cells, recipient cells can adapt their own metabolism to maintain the whole tissue homeostasis; or deleterious consequences, that is, by receiving EVs from cells in bad conditions containing bioactive lipids such as S1P, recipient cells can deregulate their metabolism in an irreparable way. Generally speaking, it appears that HFD induce EV release and thus the flow of EV between tissues. Therefore, we can expect that reducing these EV flows by using metformin or ceramide inhibitors could be beneficial for maintaining tissue homeostasis and could reduce the development of obesity‐associated cancers.
Although systemic inflammation is recognized as an important consequence of HFD, the role of eicosanoids which are shuttled in EVs along with the enzymes in charge of their synthesis has not been studied (Boilard, 2018; Sagini et al., 2018). But data showing that HFD contribute to induce serum pro‐inflammatory eicosanoids in obese or overweight patients (Schweitzer et al., 2021) strongly suggest that eicosanoids in EVs might have an unsuspected role in the inflammatory aetiology of obesity‐associated diseases such as NAFLD, diabetes and cardiovascular diseases. This hypothesis deserves validation.
Another important issue that is given little or no consideration is how the lipid composition of EVs affects the composition and concentration of other EV components such as proteins and nucleic acids. Indeed, the modifications of the content of the EVs in these 3 components is always studied separately, but all EV components are always affected together (Jalabert et al., 2021; Phuyal et al., 2015; Yu et al., 2018). It could even be that the lipids of the EVs interact and select the nucleic acids that are exported into the EVs. In line with this hypothesis, it was described that lipid membranes can act as RNA organization platforms and that these interactions depend on RNA nucleotide content, base pairing, and length (Czerniak & Saenz, 2022). Also, RNA can interact with phospholipids (Vlasov & Yarus, 2002). This could explain why only a subset of miRNAs is found in EVs and the fact that some miRNAs are never exported (Mańka et al., 2021). It is tempting to speculate that the variations of membrane lipid composition associated with the HFD could modify these lipid‐RNA interactions thus resulting in a change of EV RNA composition. This hypothesis deserves validation, but this year at the ISEV2023 meeting, the group of Alissa Weaver demonstrated that intracellular cholesterol cellular enrichment induced the sorting of specific miRNAs into EVs (Czerniak & Saenz, 2022) demonstrating a direct link between cholesterol metabolism and the loading of RNA into EVs.
Working on metabolism is very challenging and a lot of work is done in vitro, or on animal models that only partially reproduce human physiology. In this context, work on human is now urgently needed to understand the impact of diet lipid quality on EV composition, trafficking and cross‐talk between organs to validate these data obtained with animal models of obesity.
AUTHOR CONTRIBUTIONS
Sophie Rome: Supervision; writing—original draft; writing—review and editing. Stefano Tacconi: Conceptualization; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest.
ACKNOWLEDGEMENT
FRENCH AGENCY OF RESEARCH (# ANR‐MEXID‐21‐CE14‐0081).
Rome, S. , & Tacconi, S. (2024). High‐fat diets: You are what you eat….your extracellular vesicles too!. Journal of Extracellular Vesicles, 13, e12382. 10.1002/jev2.12382
REFERENCES
- Akawi, N. , Checa, A. , Antonopoulos, A. S. , Akoumianakis, I. , Daskalaki, E. , Kotanidis, C. P. , Kondo, H. , Lee, K. , Yesilyurt, D. , Badi, I. , Polkinghorne, M. , Akbar, N. , Lundgren, J. , Chuaiphichai, S. , Choudhury, R. , Neubauer, S. , Channon, K. M. , Torekov, S. S. , Wheelock, C. E. , & Antoniades, C. (2021). Fat‐secreted ceramides regulate vascular redox state and influence outcomes in patients with cardiovascular disease. Journal of the American College of Cardiology, 77(20), 2494–2513. 10.1016/j.jacc.2021.03.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder‐Baerens, N. , Müller, P. , Pohl, A. , Korte, T. , Hamon, Y. , Chimini, G. , Pomorski, T. , & Herrmann, A. (2005). Headgroup‐specific exposure of phospholipids in ABCA1‐expressing cells. The Journal of biological chemistry, 280(28), 26321–26329. 10.1074/jbc.M413993200 [DOI] [PubMed] [Google Scholar]
- Alkhatatbeh, M. J. , Mhaidat, N. M. , Enjeti, A. K. , Lincz, L. F. , & Thorne, R. F. (2011). The putative diabetic plasma marker, soluble CD36, is non‐cleaved, non‐soluble and entirely associated with microparticles. Journal of Thrombosis and Haemostasis: JTH, 9(4), 844–851. 10.1111/j.1538-7836.2011.04220.x [DOI] [PubMed] [Google Scholar]
- Anon . (2023). Extracellular Membrane Vesicles from Tumor Cells Promote Angiogenesis via Sphingomyelin1 | Cancer Research | American Association for Cancer Research. https://aacrjournals.org/cancerres/article/62/21/6312/509459/Extracellular‐Membrane‐Vesicles‐from‐Tumor‐Cells [PubMed]
- Aswad, H. , Forterre, A. , Wiklander, O. P. , Vial, G. , Danty‐Berger, E. , Jalabert, A. , Lamazière, A. , Meugnier, E. , Pesenti, S. , Ott, C. , Chikh, K. , El‐Andaloussi, S. , Vidal, H. , Lefai, E. , Rieusset, J. , & Rome, S. (2014). Exosomes participate in the alteration of muscle homeostasis during lipid‐induced insulin resistance in mice. Diabetologia, 57(10), 2155–2164. 10.1007/s00125-014-3337-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco, F. , Perrotta, C. , Novellino, L. , Francolini, M. , Riganti, L. , Menna, E. , Saglietti, L. , Schuchman, E. H. , Furlan, R. , Clementi, E. , Matteoli, M. , & Verderio, C. (2009). Acid sphingomyelinase activity triggers microparticle release from glial cells. The EMBO Journal, 28(8), 1043–1054. 10.1038/emboj.2009.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandin, A. , Dugail, I. , Hilairet, G. , Ponnaiah, M. , Ghesquière, V. , Froger, J. , Ducheix, S. , Fizanne, L. , Boursier, J. , Cariou, B. , Lhomme, M. , & Le Lay, S. (2023). Lipidomic analysis of adipose‐derived extracellular vesicles reveals specific EV lipid sorting informative of the obesity metabolic state. Cell Reports, 42(3), 112169. 10.1016/j.celrep.2023.112169 [DOI] [PubMed] [Google Scholar]
- Boilard, E. (2018). Extracellular vesicles and their content in bioactive lipid mediators: More than a sack of microRNA. Journal of Lipid Research, 59(11), 2037–2046. 10.1194/jlr.R084640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratta, S. , Shimanaka, Y. , Costanzi, E. , Ni, S. , Urbanelli, L. , Kono, N. , Morena, F. , Sagini, K. , Giovagnoli, S. , Romani, R. , Gargaro, M. , Arai, H. , & Emiliani, C. (2021). Lipotoxic stress alters the membrane lipid profile of extracellular vesicles released by Huh‐7 hepatocarcinoma cells. Scientific Reports, 11(1), 4613. 10.1038/s41598-021-84268-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratta, S. , Urbanelli, L. , Sagini, K. , Giovagnoli, S. , Caponi, S. , Fioretto, D. , Mitro, N. , Caruso, D. , & Emiliani, C. (2017). Extracellular vesicles released by fibroblasts undergoing H‐Ras induced senescence show changes in lipid profile. PloS One, 12(11), e0188840. 10.1371/journal.pone.0188840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzas, E. I. (2023). The roles of extracellular vesicles in the immune system. Nature Reviews. Immunology, 23(4), 236–250. 10.1038/s41577-022-00763-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camino, T. , Lago‐Baameiro, N. , Bravo, S. B. , Sueiro, A. , Couto, I. , Santos, F. , Baltar, J. , Casanueva, F. F. , & Pardo, M. (2020). Vesicles shed by pathological murine adipocytes spread pathology: Characterization and functional role of insulin resistant/hypertrophied adiposomes. International Journal of Molecular Sciences, 21(6), 2252. 10.3390/ijms21062252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camino, T. , Lago‐Baameiro, N. , Sueiro, A. , Bravo, S. B. , Couto, I. , Santos, F. F. , Baltar, J. , Casanueva, F. F. , & Pardo, M. (2022). Brown adipose tissue sheds extracellular vesicles that carry potential biomarkers of metabolic and thermogenesis activity which are affected by high fat diet intervention. International Journal of Molecular Sciences, 23(18), 10826. 10.3390/ijms231810826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero, I. , Abete, I. , Del Bas, J. M. , Caimari, A. , Arola, L. , Zulet, M. A. , & Martinez, J. A. (2018). Changes in lysophospholipids and liver status after weight loss: The RESMENA study. Nutrition & Metabolism, 15, 51. 10.1186/s12986-018-0288-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, J. , Koseki, M. , Saga, A. , Kanno, K. , Higo, T. , Okuzaki, D. , Okada, T. , Inui, H. , Tanaka, K. , Asaji, M. , Zhu, Y. , Kamada, Y. , Ono, M. , Saibara, T. , Ichi, I. , Ohama, T. , Nishida, M. , Yamashita, S. , & Sakata, Y. (2021). Dietary oxysterol, 7‐ketocholesterol accelerates hepatic lipid accumulation and macrophage infiltration in obese mice. Frontiers in Endocrinology, 11, 614692. 10.3389/fendo.2020.614692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chocian, G. , Chabowski, A. , Zendzian‐Piotrowska, M. , Harasim, E. , Łukaszuk, B. , & Górski, J. (2010). High fat diet induces ceramide and sphingomyelin formation in rat's liver nuclei. Molecular and Cellular Biochemistry, 340(1‐2), 125–131. 10.1007/s11010-010-0409-6 [DOI] [PubMed] [Google Scholar]
- Choi, S. , & Snider, A. J. (2015). Sphingolipids in high fat diet and obesity‐related diseases. Mediators of Inflammation, 2015, 520618. 10.1155/2015/520618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorner, Z. , Barbeau, P. A. , Castellani, L. , Wright, D. C. , Chabowski, A. , & Holloway, G. P. (2016). Dietary α‐linolenic acid supplementation alters skeletal muscle plasma membrane lipid composition, sarcolemmal FAT/CD36 abundance, and palmitate transport rates. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 311(6), R1234–R1242. 10.1152/ajpregu.00346.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement, E. , Lazar, I. , Attané, C. , Carrié, L. , Dauvillier, S. , Ducoux‐Petit, M. , Esteve, D. , Menneteau, T. , Moutahir, M. , Le Gonidec, S. , Dalle, S. , Valet, P. , Burlet‐Schiltz, O. , Muller, C. , & Nieto, L. (2020). Adipocyte extracellular vesicles carry enzymes and fatty acids that stimulate mitochondrial metabolism and remodeling in tumor cells. The EMBO Journal, 39(3), e102525. 10.15252/embj.2019102525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo, M. , Raposo, G. , & Théry, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell and Developmental Biology, 30, 255–289. 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- Crewe, C. , Joffin, N. , Rutkowski, J. M. , Kim, M. , Zhang, F. , Towler, D. A. , Gordillo, R. , & Scherer, P. E. (2018). An endothelial‐to‐adipocyte extracellular vesicle axis governed by metabolic state. Cell, 175(3), 695–708.e13. 10.1016/j.cell.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniak, T. , & Saenz, J. P. (2022). Lipid membranes modulate the activity of RNA through sequence‐dependent interactions. Proceedings of the National Academy of Sciences of the United States of America, 119(4), e2119235119. 10.1073/pnas.2119235119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabke, P. , Brogden, G. , Naim, H. Y. , & Das, A. M. (2020). Ketogenic diet: Impact on cellular lipids in hippocampal murine neurons. Nutrients, 12(12), 3870. 10.3390/nu12123870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dany, M. , & Ogretmen, B. (2015). Ceramide induced mitophagy and tumor suppression. Biochimica et Biophysica Acta, 1853(10Pt B), 2834–2845. 10.1016/j.bbamcr.2014.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta, D. , Nakao, Y. , Mauer, A. S. , Thompson, J. M. , Sehrawat, T. S. , Liao, C. Y. , Krishnan, A. , Lucien, F. , Guo, Q. , Liu, M. , Xue, F. , Fukushima, M. , Katsumi, T. , Bansal, A. , Pandey, M. K. , Maiers, J. L. , DeGrado, T. , Ibrahim, S. H. , Revzin, A. , … Malhi, H. (2020). IRE1A stimulates hepatocyte‐derived extracellular vesicles that promote inflammation in mice with steatohepatitis. Gastroenterology, 159(4), 1487–1503.e17. 10.1053/j.gastro.2020.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong, P. T. , Collins, H. L. , Nickel, M. , Lund‐Katz, S. , Rothblat, G. H. , & Phillips, M. C. (2006). Characterization of nascent HDL particles and microparticles formed by ABCA1‐mediated efflux of cellular lipids to apoA‐I. Journal of Lipid Research, 47(4), 832–843. 10.1194/jlr.M500531-JLR200 [DOI] [PubMed] [Google Scholar]
- Durcin, M. , Fleury, A. , Taillebois, E. , Hilairet, G. , Krupova, Z. , Henry, C. , Truchet, S. , Trötzmüller, M. , Köfeler, H. , Mabilleau, G. , Hue, O. , Andriantsitohaina, R. , Martin, P. , & Le Lay, S. (2017). Characterisation of adipocyte‐derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. Journal of Extracellular Vesicles, 6(1), 1305677. 10.1080/20013078.2017.1305677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi, A. , Lazic, M. , Armando, A. M. , Phillips, S. A. , Katebian, R. , Maraka, S. , Quehenberger, O. , Sears, D. D. , & Feldstein, A. E. (2016). Circulating adipocyte‐derived extracellular vesicles are novel markers of metabolic stress. Journal of Molecular Medicine (Berlin, Germany), 94(11), 1241–1253. 10.1007/s00109-016-1446-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader, C. M. , Sánchez, D. , Furlán, M. , & Colombo, M. I. (2008). Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in k562 cells. Traffic (Copenhagen, Denmark), 9(2), 230–250. 10.1111/j.1600-0854.2007.00677.x [DOI] [PubMed] [Google Scholar]
- Garawi, F. , Devries, K. , Thorogood, N. , & Uauy, R. (2014). Global differences between women and men in the prevalence of obesity: Is there an association with gender inequality? European Journal of Clinical Nutrition, 68(10), 1101–1106. 10.1038/ejcn.2014.86 [DOI] [PubMed] [Google Scholar]
- Ghadami, S. , & Dellinger, K. (2023). The lipid composition of extracellular vesicles: applications in diagnostics and therapeutic delivery. Frontiers in Molecular Biosciences, 10, 1198044. 10.3389/fmolb.2023.1198044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitton, J. , Bandet, C. L. , Mariko, M. L. , Tan‐Chen, S. , Bourron, O. , Benomar, Y. , Hajduch, E. , & Le Stunff, H. (2020). Sphingosine‐1‐phosphate metabolism in the regulation of obesity/type 2 diabetes. Cells, 9(7), 1682. 10.3390/cells9071682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Q. , Furuta, K. , Lucien, F. , Gutierrez Sanchez, L. H. , Hirsova, P. , Krishnan, A. , Kabashima, A. , Pavelko, K. D. , Madden, B. , Alhuwaish, H. , Gao, Y. , Revzin, A. , & Ibrahim, S. H. (2019). Integrin β1‐enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. Journal of Hepatology, 71(6), 1193–1205. 10.1016/j.jhep.2019.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, P. , Kadamberi, I. P. , Mittal, S. , Tsaih, S. W. , George, J. , Kumar, S. , Vijayan, D. K. , Geethadevi, A. , Parashar, D. , Topchyan, P. , McAlarnen, L. , Volkman, B. F. , Cui, W. , Zhang, K. Y. J. , Di Vizio, D. , Chaluvally‐Raghavan, P. , & Pradeep, S. (2022). Tumor derived extracellular vesicles drive T cell exhaustion in tumor microenvironment through sphingosine mediated signaling and impacting immunotherapy outcomes in ovarian cancer. Advanced Science (Weinheim, Baden‐Wurttemberg, Germany), 9(14), e2104452. 10.1002/advs.202104452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich, L. F. , Andersen, D. K. , Cleasby, M. E. , & Lawson, C. (2015). Long‐term high fat feeding of rats results in increased numbers of circulating microvesicles with pro‐inflammatory effects on endothelial cells. The British Journal of Nutrition, 113(11), 1704–1711. 10.1017/S0007114515001117 [DOI] [PubMed] [Google Scholar]
- Hengartner, M. O. (2001). Apoptosis: Corralling the corpses. Cell, 104(3), 325–328. 10.1016/s0092-8674(01)00219-7 [DOI] [PubMed] [Google Scholar]
- Hirsova, P. , Ibrahim, S. H. , Krishnan, A. , Verma, V. K. , Bronk, S. F. , Werneburg, N. W. , Charlton, M. R. , Shah, V. H. , Malhi, H. , & Gores, G. J. (2016). Lipid‐induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology, 150(4), 956–967. 10.1053/j.gastro.2015.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornemann, T. , Penno, A. , Rütti, M. F. , Ernst, D. , Kivrak‐Pfiffner, F. , Rohrer, L. , & von Eckardstein, A. (2009). The SPTLC3 subunit of serine palmitoyltransferase generates short chain sphingoid bases. The Journal of Biological Chemistry, 284(39), 26322–26330. 10.1074/jbc.M109.023192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, W. , Ross, J. , Geng, T. , Brice, S. E. , & Cowart, L. A. (2011). Differential regulation of dihydroceramide desaturase by palmitate versus monounsaturated fatty acids: implications for insulin resistance. The Journal of Biological Chemistry, 286(19), 16596–16605. 10.1074/jbc.M110.186916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotari, J. , & Helenius, A. (2011). Endosome maturation. The EMBO Journal, 30(17), 3481–3500. 10.1038/emboj.2011.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley, J. H. (2015). ESCRTs are everywhere. The EMBO Journal, 34(19), 2398–2407. 10.15252/embj.201592484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim, S. H. , Hirsova, P. , Tomita, K. , Bronk, S. F. , Werneburg, N. W. , Harrison, S. A. , Goodfellow, V. S. , Malhi, H. , & Gores, G. J. (2016). Mixed lineage kinase 3 mediates release of C‐X‐C motif ligand 10‐bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology (Baltimore, Md.), 63(3), 731–744. 10.1002/hep.28252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubec, M. , Maple‐Grødem, J. , Akbari, S. , Nesse, S. , Halskau, Ø. , & Mork‐Jansson, A. E. (2020). Plasma‐derived exosome‐like vesicles are enriched in lyso‐phospholipids and pass the blood‐brain barrier. PloS One, 15(9), e0232442. 10.1371/journal.pone.0232442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalabert, A. , Reininger, L. , Berger, E. , Coute, Y. , Meugnier, E. , Forterre, A. , Errazuriz‐Cerda, E. , Geloen, A. , Aouadi, M. , Bouzakri, K. , Rieusset, J. , & Rome, S. (2021). Profiling of ob/ob mice skeletal muscle exosome‐like vesicles demonstrates combined action of miRNAs, proteins and lipids to modulate lipid homeostasis in recipient cells. Scientific Reports, 11(1), 21626. 10.1038/s41598-021-00983-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuu Thi‐Dinh, K. L. , Demarne, Y. , Nicolas, C. , & Lhuillery, C. (1990). Effect of dietary fat on phospholipid class distribution and fatty acid composition in rat fat cell plasma membrane. Lipids, 25(5), 278–283. 10.1007/BF02544388 [DOI] [PubMed] [Google Scholar]
- Kim, H. Y. , Kim, M. , Park, H. M. , Kim, J. , Kim, E. J. , Lee, C. H. , & Park, J. H. (2014). Lysophospholipid profile in serum and liver by high‐fat diet and tumor induction in obesity‐resistant BALB/c mice. Nutrition (Burbank, Los Angeles County, Calif.), 30(11‐12), 1433–1441. 10.1016/j.nut.2014.04.013 [DOI] [PubMed] [Google Scholar]
- Kumar, A. , Sundaram, K. , Mu, J. , Dryden, G. W. , Sriwastva, M. K. , Lei, C. , Zhang, L. , Qiu, X. , Xu, F. , Yan, J. , Zhang, X. , Park, J. W. , Merchant, M. L. , Bohler, H. C. L. , Wang, B. , Zhang, S. , Qin, C. , Xu, Z. , Han, X. , … Zhang, H. G. (2021). High‐fat diet‐induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nature Communications, 12(1), 213. 10.1038/s41467-020-20500-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, C. P. , & Breakefield, X. O. (2012). Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Frontiers in Physiology, 3, 228. 10.3389/fphys.2012.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, S. M. , Zhang, C. , Wang, Z. , Ni, Z. , Zhang, S. , Yang, S. , Huang, X. , Mo, L. , Li, J. , Lee, B. , Mei, M. , Huang, L. , Shi, M. , Xu, Z. , Meng, F. P. , Cao, W. J. , Zhou, M. J. , Shi, L. , Chua, G. H. , … Shui, G. (2021). A multi‐omics investigation of the composition and function of extracellular vesicles along the temporal trajectory of COVID‐19. Nature Metabolism, 3(7), 909–922. 10.1038/s42255-021-00425-4 [DOI] [PubMed] [Google Scholar]
- Lamri, A. , Pigeyre, M. , Garver, W. S. , & Meyre, D. (2018). The extending spectrum of NPC1‐related human disorders: From Niemann‐Pick C1 disease to obesity. Endocrine Reviews, 39(2), 192–220. 10.1210/er.2017-00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laulagnier, K. , Grand, D. , Dujardin, A. , Hamdi, S. , Vincent‐Schneider, H. , Lankar, D. , Salles, J. P. , Bonnerot, C. , Perret, B. , & Record, M. (2004). PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Letters, 572(1–3), 11–14. 10.1016/j.febslet.2004.06.082 [DOI] [PubMed] [Google Scholar]
- Laulagnier, K. , Motta, C. , Hamdi, S. , Roy, S. , Fauvelle, F. , Pageaux, J. F. , Kobayashi, T. , Salles, J. P. , Perret, B. , Bonnerot, C. , & Record, M. (2004). Mast cell‐ and dendritic cell‐derived exosomes display a specific lipid composition and an unusual membrane organization. The Biochemical Journal, 380(Pt (1)), 161–171. 10.1042/BJ20031594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. H. , Seto, W. K. , Lui, D. T. , Fong, C. H. , Wan, H. Y. , Cheung, C. Y. , Chow, W. S. , Woo, Y. C. , Yuen, M. F. , Xu, A. , & Lam, K. S. (2021). Circulating thrombospondin‐2 as a novel fibrosis biomarker of nonalcoholic fatty liver disease in type 2 diabetes. Diabetes Care, 44(9), 2089–2097. 10.2337/dc21-0131 [DOI] [PubMed] [Google Scholar]
- Le Lay, S. , Rome, S. , Loyer, X. , & Nieto, L. (2021). Adipocyte‐derived extracellular vesicles in health and diseases: Nano‐packages with vast biological properties. FASEB BioAdvances, 3(6), 407–419. 10.1096/fba.2020-00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz, B. R. (2003). Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Progress in Lipid Research, 42(5), 423–438. 10.1016/s0163-7827(03)00025-0 [DOI] [PubMed] [Google Scholar]
- Li, M. , Liao, L. , & Tian, W. (2020). Extracellular vesicles derived from apoptotic cells: An essential link between death and regeneration. Frontiers in Cell and Developmental Biology, 8, 573511. 10.3389/fcell.2020.573511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, C. Y. , Song, M. J. , Gao, Y. , Mauer, A. S. , Revzin, A. , & Malhi, H. (2018). Hepatocyte‐derived lipotoxic extracellular vesicle sphingosine 1‐phosphate induces macrophage chemotaxis. Frontiers in Immunology, 9, 2980. 10.3389/fimmu.2018.02980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, C. Y. , Song, M. J. , Gao, Y. , Mauer, A. S. , Revzin, A. , & Malhi, H. (2018). Hepatocyte‐derived lipotoxic extracellular vesicle sphingosine 1‐phosphate induces macrophage chemotaxis. Frontiers in Immunology, 9, 2980. 10.3389/fimmu.2018.02980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente, A. , Skotland, T. , Sylvänne, T. , Kauhanen, D. , Róg, T. , Orłowski, A. , Vattulainen, I. , Ekroos, K. , & Sandvig, K. (2013). Molecular lipidomics of exosomes released by PC‐3 prostate cancer cells. Biochimicaet Biophysica Acta, 1831(7), 1302–1309. 10.1016/j.bbalip.2013.04.011 [DOI] [PubMed] [Google Scholar]
- Luo, P. , Mao, K. , Xu, J. , Wu, F. , Wang, X. , Wang, S. , Zhou, M. , Duan, L. , Tan, Q. , Ma, G. , Yang, G. , Du, R. , Huang, H. , Huang, Q. , Li, Y. , Guo, M. , & Jin, Y. (2020). Metabolic characteristics of large and small extracellular vesicles from pleural effusion reveal biomarker candidates for the diagnosis of tuberculosis and malignancy. Journal of Extracellular Vesicles, 9(1), 1790158. 10.1080/20013078.2020.1790158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquain‐Costaz, C. , Rabia, M. , Hullin‐Matsuda, F. , & Delton, I. (2020). Bis(monoacylglycero)phosphate, an important actor in the host endocytic machinery hijacked by SARS‐CoV‐2 and related viruses. Biochimie, 179, 247–256. 10.1016/j.biochi.2020.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydic, T. A. , Townsend, S. , Adda, C. G. , Collins, C. , Mathivanan, S. , & Reid, G. E. (2015). Rapid and comprehensive ‘shotgun’ lipidome profiling of colorectal cancer cell derived exosomes. Methods (San Diego, Calif.), 87, 83–95. 10.1016/j.ymeth.2015.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mańka, R. , Janas, P. , Sapoń, K. , Janas, T. , & Janas, T. (2021). Role of RNA motifs in RNA interaction with membrane lipid rafts: Implications for therapeutic applications of exosomal RNAs. International Journal of Molecular Sciences, 22(17), 9416. 10.3390/ijms22179416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto, A. , Takahashi, Y. , Ogata, K. , Kitamura, S. , Nakagawa, N. , Yamamoto, A. , Ishihama, Y. , & Takakura, Y. (2021). Phosphatidylserine‐deficient small extracellular vesicle is a major somatic cell‐derived sEV subpopulation in blood. iScience, 24(8), 102839. 10.1016/j.isci.2021.102839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menck, K. , Sönmezer, C. , Worst, T. S. , Schulz, M. , Dihazi, G. H. , Streit, F. , Erdmann, G. , Kling, S. , Boutros, M. , Binder, C. , & Gross, J. C. (2017). Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. Journal of Extracellular Vesicles, 6(1), 1378056. 10.1080/20013078.2017.1378056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möbius, W. , van Donselaar, E. , Ohno‐Iwashita, Y. , Shimada, Y. , Heijnen, H. F. , Slot, J. W. , & Geuze, H. J. (2003). Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic (Copenhagen, Denmark), 4(4), 222–231. 10.1034/j.1600-0854.2003.00072.x [DOI] [PubMed] [Google Scholar]
- Muralidharan, S. , Shimobayashi, M. , Ji, S. , Burla, B. , Hall, M. N. , Wenk, M. R. , & Torta, F. (2021). A reference map of sphingolipids in murine tissues. Cell Reports, 35(11), 109250. 10.1016/j.celrep.2021.109250 [DOI] [PubMed] [Google Scholar]
- Muralidharan‐Chari, V. , Clancy, J. W. , Sedgwick, A. , & D'Souza‐Schorey, C. (2010). Microvesicles: mediators of extracellular communication during cancer progression. Journal of Cell Science, 123(Pt 10), 1603–1611. 10.1242/jcs.064386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeini, M. B. , Bianconi, V. , Pirro, M. , & Sahebkar, A. (2020). The role of phosphatidylserine recognition receptors in multiple biological functions. Cellular & Molecular Biology Letters, 25, 23. 10.1186/s11658-020-00214-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi, S. , Ma, L. , Denis, M. , Karwatsky, J. , Li, Z. , Jiang, X. C. , & Zha, X. (2009). ABCA1‐mediated cholesterol efflux generates microparticles in addition to HDL through processes governed by membrane rigidity. Journal of Lipid Research, 50(3), 456–466. 10.1194/jlr.M800345-JLR200 [DOI] [PubMed] [Google Scholar]
- Nojima, H. , Freeman, C. M. , Schuster, R. M. , Japtok, L. , Kleuser, B. , Edwards, M. J. , Gulbins, E. , & Lentsch, A. B. (2016). Hepatocyte exosomes mediate liver repair and regeneration via sphingosine‐1‐phosphate. Journal of Hepatology, 64(1), 60–68. 10.1016/j.jhep.2015.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata, Y. , Kita, S. , Koyama, Y. , Fukuda, S. , Takeda, H. , Takahashi, M. , Fujishima, Y. , Nagao, H. , Masuda, S. , Tanaka, Y. , Nakamura, Y. , Nishizawa, H. , Funahashi, T. , Ranscht, B. , Izumi, Y. , Bamba, T. , Fukusaki, E. , Hanayama, R. , Shimada, S. , … Shimomura, I. (2018). Adiponectin/T‐cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight, 3(8), e99680. 10.1172/jci.insight.99680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolino, G. , Buratta, S. , Mercuri, S. R. , Pellegrino, R. M. , Urbanelli, L. , Emiliani, C. , Bertuccini, L. , Iosi, F. , Huber, V. , Brianti, P. , Prezioso, C. , Di Nicola, M. R. , Federici, C. , & Lugini, L. (2022). Lipidic profile changes in exosomes and microvesicles derived from plasma of monoclonal antibody‐treated psoriatic patients. Frontiers in Cell and Developmental Biology, 10, 923769. 10.3389/fcell.2022.923769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson, U. S. , Waldén, T. B. , Carlsson, P. O. , Jansson, L. , & Phillipson, M. (2012). Female mice are protected against high‐fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PloS One, 7(9), e46057. 10.1371/journal.pone.0046057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiler, S. , Thakur, M. , Grünauer, P. , Megens, R. T. A. , Joshi, U. , Coletti, R. , Samara, V. , Müller‐Stoy, G. , Ishikawa‐Ankerhold, H. , Stark, K. , Klingl, A. , Fröhlich, T. , Arnold, G. J. , Wörmann, S. , Bruns, C. J. , Algül, H. , Weber, C. , Massberg, S. , & Engelmann, B. (2019). CD36‐triggered cell invasion and persistent tissue colonization by tumor microvesicles during metastasis. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 33(2), 1860–1872. 10.1096/fj.201800985R [DOI] [PubMed] [Google Scholar]
- Phuyal, S. , Hessvik, N. P. , Skotland, T. , Sandvig, K. , & Llorente, A. (2014). Regulation of exosome release by glycosphingolipids and flotillins. The FEBS Journal, 281(9), 2214–2227. 10.1111/febs.12775 [DOI] [PubMed] [Google Scholar]
- Phuyal, S. , Skotland, T. , Hessvik, N. P. , Simolin, H. , Øverbye, A. , Brech, A. , Parton, R. G. , Ekroos, K. , Sandvig, K. , & Llorente, A. (2015). The ether lipid precursor hexadecylglycerol stimulates the release and changes the composition of exosomes derived from PC‐3 cells. The Journal of Biological Chemistry, 290(7), 4225–4237. 10.1074/jbc.M114.593962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollet, H. , Conrard, L. , Cloos, A. S. , & Tyteca, D. (2018). Plasma membrane lipid domains as platforms for vesicle biogenesis and shedding? Biomolecules, 8(3), 94. 10.3390/biom8030094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povero, D. , Yamashita, H. , Ren, W. , Subramanian, M. G. , Myers, R. P. , Eguchi, A. , Simonetto, D. A. , Goodman, Z. D. , Harrison, S. A. , Sanyal, A. J. , Bosch, J. , & Feldstein, A. E. (2020). Characterization and proteome of circulating extracellular vesicles as potential biomarkers for NASH. Hepatology Communications, 4(9), 1263–1278. 10.1002/hep4.1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha, N. , Kuijl, C. , van der Kant, R. , Janssen, L. , Houben, D. , Janssen, H. , Zwart, W. , & Neefjes, J. (2009). Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7‐RILP‐p150 Glued and late endosome positioning. The Journal of Cell Biology, 185(7), 1209–1225. 10.1083/jcb.200811005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo, F. , Gil‐Carton, D. , Gonzalez, E. , Mleczko, J. , Palomo, L. , Perez‐Cormenzana, M. , Mayo, R. , Alonso, C. , & Falcon‐Perez, J. M. (2019). Differences in the metabolite composition and mechanical properties of extracellular vesicles secreted by hepatic cellular models. Journal of Extracellular Vesicles, 8(1), 1575678. 10.1080/20013078.2019.1575678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagini, K. , Costanzi, E. , Emiliani, C. , Buratta, S. , & Urbanelli, L. (2018). Extracellular vesicles as conveyors of membrane‐derived bioactive lipids in immune system. International Journal of Molecular Sciences, 19(4), 1227. 10.3390/ijms19041227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagini, K. , Urbanelli, L. , Costanzi, E. , Mitro, N. , Caruso, D. , Emiliani, C. , & Buratta, S. (2018). Oncogenic H‐Ras expression induces fatty acid profile changes in human fibroblasts and extracellular vesicles. International Journal of Molecular Sciences, 19(11), 3515. 10.3390/ijms19113515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad, F. , Hester, K. D. , Yang, G. , Hannun, Y. A. , & Bielawski, J. (2006). Altered adipose and plasma sphingolipid metabolism in obesity: A potential mechanism for cardiovascular and metabolic risk. Diabetes, 55(9), 2579–2587. 10.2337/db06-0330 [DOI] [PubMed] [Google Scholar]
- Schweitzer, G. R. B. , Rios, I. N. M. S. , Gonçalves, V. S. S. , Magalhães, K. G. , & Pizato, N. (2021). Effect of n‐3 long‐chain polyunsaturated fatty acid intake on the eicosanoid profile in individuals with obesity and overweight: a systematic review and meta‐analysis of clinical trials. Journal of Nutritional Science, 10, e53. 10.1017/jns.2021.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, C. , Yang, G. , Lee, I. , Bielawski, J. , Hannun, Y. A. , & Samad, F. (2008). Protection from high fat diet‐induced increase in ceramide in mice lacking plasminogen activator inhibitor 1. The Journal of Biological Chemistry, 283(20), 13538–13548. 10.1074/jbc.M709950200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotland, T. , Hessvik, N. P. , Sandvig, K. , & Llorente, A. (2019). Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. Journal of Lipid Research, 60(1), 9–18. 10.1194/jlr.R084343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotland, T. , Llorente, A. , & Sandvig, K. (2023). Lipids in extracellular vesicles: What can be learned about membrane structure and function? Cold Spring Harbor Perspectives in Biology, 15(8), a041415. 10.1101/cshperspect.a041415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotland, T. , Sagini, K. , Sandvig, K. , & Llorente, A. (2020). An emerging focus on lipids in extracellular vesicles. Advanced Drug Delivery Reviews, 159, 308–321. 10.1016/j.addr.2020.03.002 [DOI] [PubMed] [Google Scholar]
- Slotte, J. P. , Hedström, G. , Rannström, S. , & Ekman, S. (1989). Effects of sphingomyelin degradation on cell cholesterol oxidizability and steady‐state distribution between the cell surface and the cell interior. Biochimica et Biophysica Acta, 985(1), 90–96. 10.1016/0005-2736(89)90108-9 [DOI] [PubMed] [Google Scholar]
- Sotelo, J. R. , & Porter, K. R. (1959). An electron microscope study of the rat ovum. The Journal of Biophysical and Biochemical Cytology, 5(2), 327–342. 10.1083/jcb.5.2.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancevic, B. , & Kolesnick, R. (2010). Ceramide‐rich platforms in transmembrane signaling. FEBS Letters, 584(9), 1728–1740. 10.1016/j.febslet.2010.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss, K. , Goebel, C. , Runz, H. , Möbius, W. , Weiss, S. , Feussner, I. , Simons, M. , & Schneider, A. (2010). Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann‐Pick type C disease. The Journal of Biological Chemistry, 285(34), 26279–26288. 10.1074/jbc.M110.134775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subiros‐Funosas, R. , Mendive‐Tapia, L. , Sot, J. , Pound, J. D. , Barth, N. , Varela, Y. , Goñi, F. M. , Paterson, M. , Gregory, C. D. , Albericio, F. , Dransfield, I. , Lavilla, R. , & Vendrell, M. (2017). A Trp‐BODIPY cyclic peptide for fluorescence labelling of apoptotic bodies. Chemical Communications (Cambridge, England), 53(5), 945–948. 10.1039/c6cc07879f [DOI] [PubMed] [Google Scholar]
- Tait, J. F. , & Smith, C. (1999). Phosphatidylserine receptors: Role of CD36 in binding of anionic phospholipid vesicles to monocytic cells. The Journal of Biological Chemistry, 274(5), 3048–3054. 10.1074/jbc.274.5.3048 [DOI] [PubMed] [Google Scholar]
- Teng, F. , & Fussenegger, M. (2020). Shedding light on extracellular vesicle biogenesis and bioengineering. Advanced Science (Weinheim, Baden‐Wurttemberg, Germany), 8(1), 2003505. 10.1002/advs.202003505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovic, K. , Hsu, C. , Chiantia, S. , Rajendran, L. , Wenzel, D. , Wieland, F. , Schwille, P. , Brügger, B. , & Simons, M. (2008). Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science (New York, N.Y.), 319(5867), 1244–1247. 10.1126/science.1153124 [DOI] [PubMed] [Google Scholar]
- Vance, D. E. (2013). Physiological roles of phosphatidylethanolamine N‐methyltransferase. Biochimica et Biophysica Acta, 1831(3), 626–632. 10.1016/j.bbalip.2012.07.017 [DOI] [PubMed] [Google Scholar]
- Verderio, C. , Gabrielli, M. , & Giussani, P. (2018). Role of sphingolipids in the biogenesis and biological activity of extracellular vesicles. Journal of Lipid Research, 59(8), 1325–1340. 10.1194/jlr.R083915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasov, A. , & Yarus, M. (2002). Vzaimodeĭstvie RNK s fosfolipidnymi membranami [Interaction of RNA with phospholipid membranes]. Molekuliarnaia Biologiia, 36(3), 496–502. [PubMed] [Google Scholar]
- Wang, R. , Ding, Q. , Yaqoob, U. , de Assuncao, T. M. , Verma, V. K. , Hirsova, P. , Cao, S. , Mukhopadhyay, D. , Huebert, R. C. , & Shah, V. H. (2015). Exosome adherence and internalization by hepatic stellate cells triggers sphingosine 1‐phosphate‐dependent migration. The Journal of Biological Chemistry, 290(52), 30684–30696. 10.1074/jbc.M115.671735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanikawa, M. , Nakamura, H. , Emori, S. , Hashimoto, N. , & Murayama, T. (2020). Accumulation of sphingomyelin in Niemann‐Pick disease type C cells disrupts Rab9‐dependent vesicular trafficking of cholesterol. Journal of Cellular Physiology, 235(3), 2300–2309. 10.1002/jcp.29137 [DOI] [PubMed] [Google Scholar]
- Yamamoto, T. , Takabatake, Y. , Takahashi, A. , Kimura, T. , Namba, T. , Matsuda, J. , Minami, S. , Kaimori, J. Y. , Matsui, I. , Matsusaka, T. , Niimura, F. , Yanagita, M. , & Isaka, Y. (2017). High‐fat diet‐induced lysosomal dysfunction and impaired autophagic flux contribute to lipotoxicity in the kidney. Journal of the American Society of Nephrology: JASN, 28(5), 1534–1551. 10.1681/ASN.2016070731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C. , Tian, X. , Li, J. , Liu, D. , Ye, D. , Xie, Z. , Han, Y. , & Zou, M. H. (2021). A high‐fat diet attenuates AMPK α1 in adipocytes to induce exosome shedding and nonalcoholic fatty liver development in vivo. Diabetes, 70(2), 577–588. 10.2337/db20-0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, T. , & Hanayama, R. (2022). TIM4‐affinity methods targeting phosphatidylserine for isolation or detection of extracellular vesicles. Methods in Molecular Biology (Clifton, N.J.), 2466, 23–36. 10.1007/978-1-0716-2176-9_2 [DOI] [PubMed] [Google Scholar]
- Yu, Y. , Du, H. , Wei, S. , Feng, L. , Li, J. , Yao, F. , Zhang, M. , Hatch, G. M. , & Chen, L. (2018). Adipocyte‐derived exosomal MiR‐27a induces insulin resistance in skeletal muscle through repression of PPARγ. Theranostics, 8(8), 2171–2188. 10.7150/thno.22565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L. , Zhang, C. , Luo, X. , Wang, P. , Zhou, W. , Zhong, S. , Xie, Y. , Jiang, Y. , Yang, P. , Tang, R. , Pan, Q. , Hall, A. R. , Luong, T. V. , Fan, J. , Varghese, Z. , Moorhead, J. F. , Pinzani, M. , Chen, Y. , & Ruan, X. Z. (2018). CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non‐alcoholic steatohepatitis. Journal of Hepatology, 69(3), 705–717. 10.1016/j.jhep.2018.04.006 [DOI] [PubMed] [Google Scholar]
- Zhao, Y. , Zhao, M. F. , Jiang, S. , Wu, J. , Liu, J. , Yuan, X. W. , Shen, D. , Zhang, J. Z. , Zhou, N. , He, J. , Fang, L. , Sun, X. T. , Xue, B. , & Li, C. J. (2020). Liver governs adipose remodelling via extracellular vesicles in response to lipid overload. Nature Communications, 11(1), 719. 10.1038/s41467-020-14450-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z. , Zhong, L. , Li, P. , He, K. , Qiu, C. , Zhao, L. , & Gong, J. (2020). Cholesterol impairs hepatocyte lysosomal function causing M1 polarization of macrophages via exosomal miR‐122‐5p. Experimental Cell Research, 387(1), 111738. 10.1016/j.yexcr.2019.111738 [DOI] [PubMed] [Google Scholar]
- Zhou, J. , & Wu, L. (2022). Modified highland barley regulates lipid metabolism and liver injury in high fat and cholesterol diet ICR mice. Foods (Basel, Switzerland), 11(24), 4067. 10.3390/foods11244067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietzer, A. , Jahnel, A. L. , Bulic, M. , Gutbrod, K. , Düsing, P. , Hosen, M. R. , Dörmann, P. , Werner, N. , Nickenig, G. , & Jansen, F. (2021). Activation of neutral sphingomyelinase 2 through hyperglycemia contributes to endothelial apoptosis via vesicle‐bound intercellular transfer of ceramides. Cellular and Molecular Life Sciences: CMLS, 79(1), 48. 10.1007/s00018-021-04049-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietzer, A. , Jahnel, A. L. , Bulic, M. , Gutbrod, K. , Düsing, P. , Hosen, M. R. , Dörmann, P. , Werner, N. , Nickenig, G. , & Jansen, F. (2021). Activation of neutral sphingomyelinase 2 through hyperglycemia contributes to endothelial apoptosis via vesicle‐bound intercellular transfer of ceramides. Cellular and Molecular Life Sciences: CMLS, 79(1), 48. 10.1007/s00018-021-04049-5 [DOI] [PMC free article] [PubMed] [Google Scholar]


