Abstract
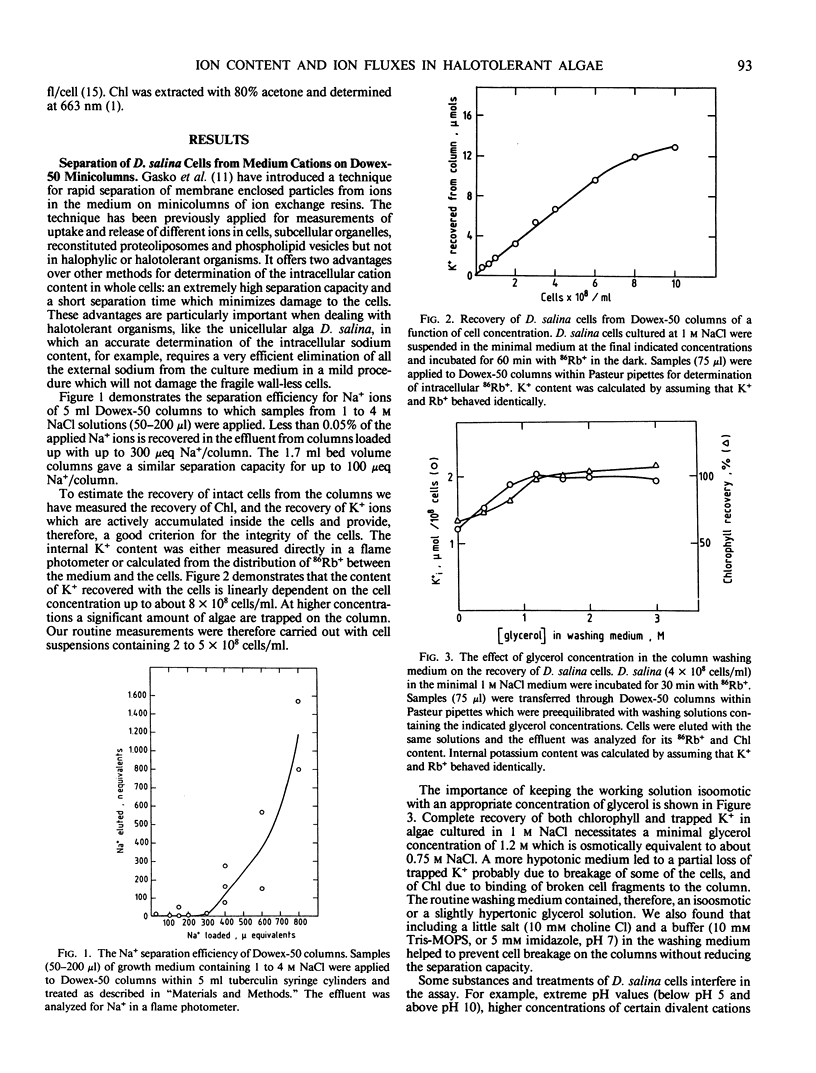
A method to determine intracellular cation contents in Dunaliella by separation on cation-exchange minicolumns is described. The separation efficiency of cells from extracellular cations is over 99.9%; the procedure causes no apparent perturbation to the cells and can be applied to measure both fluxes and internal content of any desired cation. Using this technique it is demonstrated that the intracellular averaged Na+, K+, and Ca2+ concentrations in Dunaliella salina cultured at 1 to 4 molar NaCl, 5 millimolar K+, and 0.3 millimolar Ca2+ are 20 to 100 millimolar, 150 to 250 millimolar, and 1 to 3 millimolar, respectively. The intracellular K+ concentration is maintained constant over a wide range of media K+ concentrations (0.5-10 millimolar), leading to a ratio of K+ in the cells to K+ in the medium of 10 to 1,000. Severe limitation of external K+, induces loss of K+ and increase in Na+ inside the cells. The results suggest that Dunaliella cells possess efficient mechanisms to eliminate Na+ and accumulate K+ and that intracellular Na+ and K+ concentrations are carefully regulated. The contribution of the intracellular Na+ and K+ salts to the total osmotic pressure of cells grown at 1 to 4 molar NaCl, is 5 to 20%.
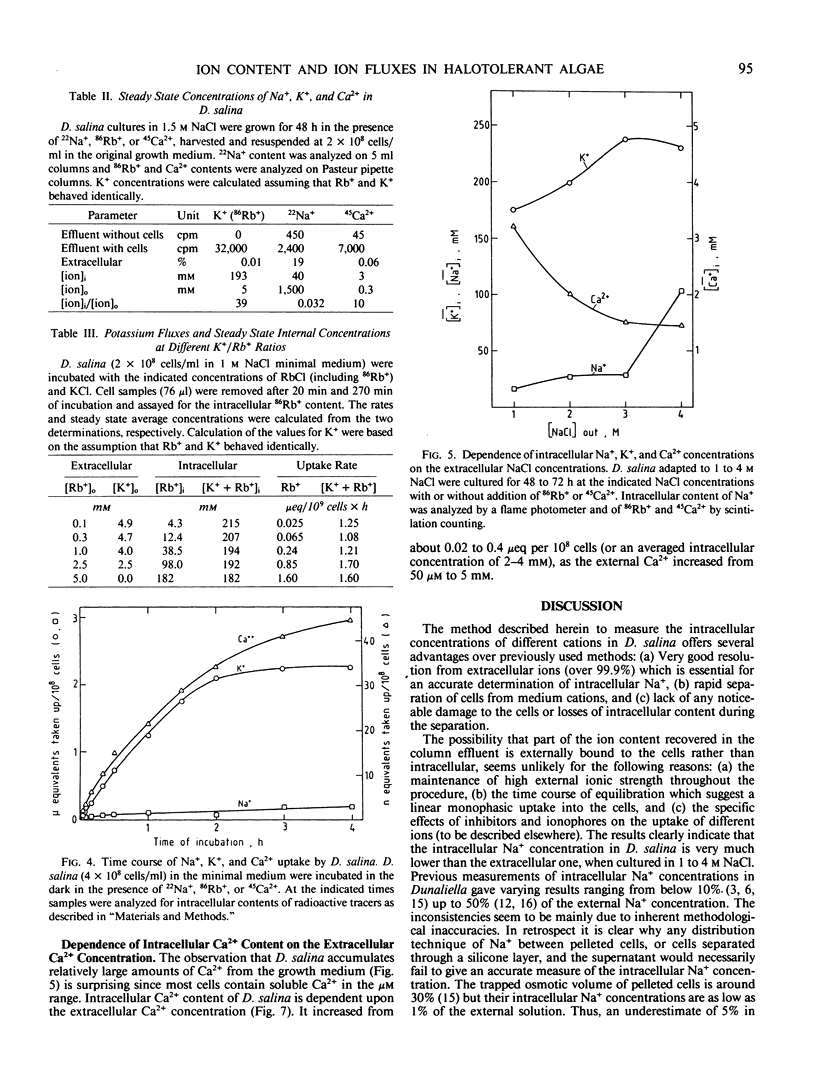
Full text
PDF

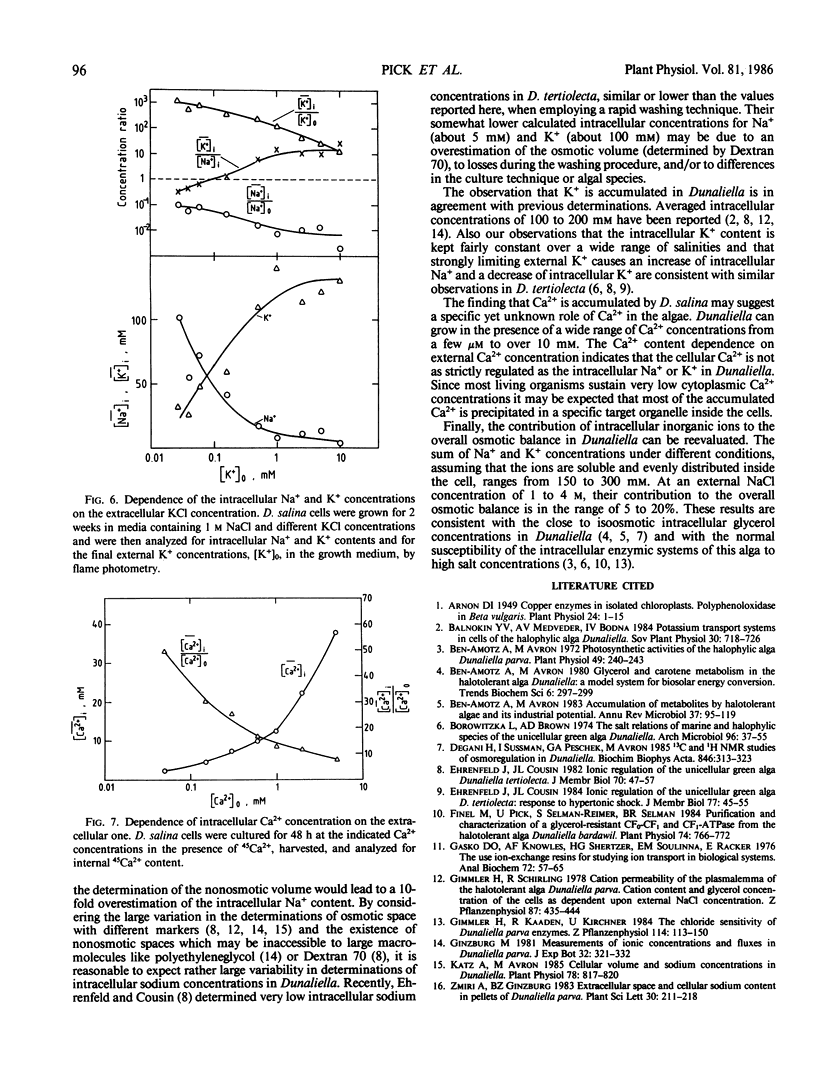
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amotz A., Avron M. Accumulation of metabolites by halotolerant algae and its industrial potential. Annu Rev Microbiol. 1983;37:95–119. doi: 10.1146/annurev.mi.37.100183.000523. [DOI] [PubMed] [Google Scholar]
- Ben-Amotz A., Avron M. Photosynthetic Activities of the Halophilic Alga Dunaliella parva. Plant Physiol. 1972 Feb;49(2):240–243. doi: 10.1104/pp.49.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitzka L. J., Brown A. D. The salt relations of marine and halophilic species of the unicellular green alga, Dunaliella. The role of glycerol as a compatible solute. Arch Mikrobiol. 1974 Mar 1;96(1):37–52. doi: 10.1007/BF00590161. [DOI] [PubMed] [Google Scholar]
- Finel M., Pick U., Selman-Reimer S., Selman B. R. Purification and Characterization of a Glycerol-Resistant CF(0)-CF(1) and CF(1)-ATPase from the Halotolerant Alga Dunaliella bardawil. Plant Physiol. 1984 Apr;74(4):766–772. doi: 10.1104/pp.74.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasko O. D., Knowles A. F., Shertzer H. G., Suolinna E. M., Racker E. The use of ion-exchange resins for studying ion transport in biological systems. Anal Biochem. 1976 May 7;72:57–65. doi: 10.1016/0003-2697(76)90506-6. [DOI] [PubMed] [Google Scholar]
- Katz A., Avron M. Determination of intracellular osmotic volume and sodium concentration in dunaliella. Plant Physiol. 1985 Aug;78(4):817–820. doi: 10.1104/pp.78.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]


