Abstract
The ability of Desulfovibrio fructosovorans MR400 ΔhynABC to express the heterologous cloned [NiFe] hydrogenase of Desulfovibrio gigas was investigated. The [NiFe] hydrogenase operon from D. gigas, hynABCD, was cloned, sequenced, and introduced into D. fructosovorans MR400. A portion of the recombinant heterologous [NiFe] hydrogenase was totally matured, exhibiting catalytic and spectroscopic properties identical to those of the native D. gigas protein. A chimeric operon containing hynAB from D. gigas and hynC from D. fructosovorans placed under the control of the D. fructosovorans hynAp promoter was constructed and expressed in D. fructosovorans MR400. Under these conditions, the same level of activity was obtained as with the D. gigas hydrogenase operon.
Hydrogenase, which catalyzes the reversible oxidation of molecular hydrogen, is involved in many relevant anaerobic processes where hydrogen is oxidized or evolved (1). This enzyme plays a central role in energy-generating mechanisms of sulfate-reducing bacteria that exhibit a strictly anaerobic respiration of sulfate (3, 28, 30). Among the [NiFe] hydrogenases, the Desulfovibrio gigas enzyme is the most studied of its kind. Extensive biochemical and biophysical analyses (4, 7, 16, 38) have shown that it is a heterodimeric, periplasmic protein exhibiting a molecular mass of 89 kDa (28- and 60-kDa subunits) and contains four redox centers: one nickel center, one [3Fe-4S] cluster, and two [4Fe-4S] clusters. The three-dimensional structure of the D. gigas hydrogenase determined at 2.85 Å resolution (43) revealed that the small subunit coordinated the three iron-sulfur clusters and that the active site, composed of an NiFe cluster (12, 44), was buried in the large subunit. Although much is known, progress in understanding the molecular mechanism of the [NiFe] hydrogenase is facilitated by a genetic system that allows the production of a correctly matured and active Desulfovibrio enzyme. As yet, such a system does not exist for D. gigas, and because Desulfovibrio fructosovorans is genetically accessible, it is a candidate for the expression of Desulfovibrio recombinant hydrogenases. Such a system would be especially useful because functional heterologous hydrogenases have not been produced in Escherichia coli (46). The aim of the present study was to explore the possibility of expressing the D. gigas [NiFe] hydrogenase operon in D. fructosovorans. In this paper we report the description of the D. gigas [NiFe] hydrogenase operon and the characterization of the recombinant heterologous enzyme.
Sequence analysis of the D. gigas [NiFe] hydrogenase operon.
The D. gigas genomic library was probed with a PCR-amplified fragment which contained the D. gigas [NiFe] hydrogenase structural genes (45). The hydrogenase operon was sequenced by EUROGENTEC, Seraing, Belgium. The D. gigas hyn operon contains only four genes: hynA encodes the small subunit (reference 45 and this work), hynB encodes the large subunit (reference 45 and this work), and hynC and hynD encode newly identified maturation proteins. No other maturation genes were identified downstream from hynD. The calculated molecular weights of the deduced amino acid sequences were 33,869 for the pre-small subunit, 28,322 for the mature small subunit, 61,397 for the pre-large subunit, and 59,620 for the mature large subunit. Comparison between the deduced amino acid sequences of the hydrogenases from D. fructosovorans (33) and D. gigas revealed that the small subunits shared 64% identity and 81% homology and that the large subunits shared 64% identity and 79% homology. Only the new regions of interest of the D. gigas hydrogenase operon are presented in Fig. 1. The 400-bp region upstream from hynA is likely to contain the ς70-type promoter region since expression of the enzyme was obtained (see below). The product of the hynC gene shares 64% identity with HynC from D. fructosovorans (35). It also shares strong homologies with HyaD from E. coli hydrogenase 1 (24), HybD from E. coli hydrogenase 2 (27), HycI from E. coli hydrogenase 3 (32), HupD from Rhodobacter capsulatus (9), Rhizobium leguminosarum (18), and Bradyrhizobium japonicum (42), and HoxM from Alcaligenes eutrophus (19) and Azotobacter vinelandii (26). Proteins homologous to HynC have been found to be responsible for the C-terminal cleavage of the [NiFe] hydrogenase large subunits (5, 21, 32). The second gene identified was named hynD, and the product of this gene shares strong homologies with HypC/HupC from A. vinelandii (8), R. capsulatus (9), A. eutrophus (10), E. coli (20), B. japonicum (29), R. leguminosarum (31), and Azotobacter chroococcum (40). It is interesting to note that members of the hyp/hup gene family, which have been shown to play a major role in the maturation process of the hydrogenase (5, 11, 27) by interacting with the large subunit (13), are not usually present within the hydrogenase operons but rather are in contiguous regions. Indeed, hynD is not present in the D. fructosovorans operon in spite of the close relationship which exists between D. gigas and D. fructosovorans. Nevertheless, the absence of large numbers of genes encoding maturation proteins seems to be a characteristic of the Desulfovibrio [NiFe] hydrogenase operons.
FIG. 1.
Nucleotide and derived amino acid sequences of the DNA region upstream and downstream from the hynA and hynB structural genes of the D. gigas [NiFe] hydrogenase. The ribosome binding sites (RBS) are overlined. The broken upward arrows indicate the translation start sites, and the broken downward arrows indicate the translation stop sites. The important restriction sites used in pBCG4 construction are designated. The sequences of the oligonucleotides used for PCR amplification are indicated in boldface.
Properties of the recombinant D. gigas [NiFe] hydrogenase.
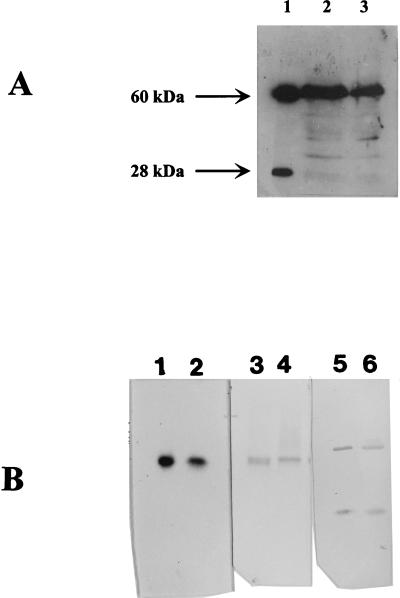
The 3.9-kb BstEII-XbaI fragment (Fig. 1) which contains the hynABCD operon from D. gigas was cloned into pBMC6 (36). The resulting plasmid was called pBCG4 and was introduced into D. fructosovorans MR400 (hyn::npt [Knr] ΔhynABC) (34) by electrotransformation (36). The [NiFe] hydrogenase activity measured in D. fructosovorans MR400 (pBCG4) was 0.15 ± 0.05 U/mg. The soluble extract from D. fructosovorans MR400 (pBCG4) was loaded onto a sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and analyzed by an immunoblotting experiment with antibodies directed against D. gigas [NiFe] hydrogenase (Fig. 2A, lane 2). On the chemoluminogram, the large subunit (60 kDa) appeared as a strong band. Its migration pattern was similar to that of the matured large subunit of the native enzyme (Fig. 2A, lane 1), which suggests the presence of a processed form. The lower-molecular-weight bands are attributed to a nonspecific cross-reaction since they appeared after a long time exposure, which was necessary to detect traces of the small subunit. On the other hand, the small subunit (28 kDa) was barely detectable, and the faint band above it presented a 33-kDa molecular mass which might correspond to the unprocessed form of that subunit. In order to check whether exportation was responsible for the lack of the small subunit in the soluble fraction, the membrane fraction was tested by Western immunoblotting analysis. No accumulation of the precursor form of the D. gigas small subunit was observed (data not shown). The weak detection of the small subunit might therefore be the result of its degradation, which can be due to a maturation failure. Indeed, instability of the small subunit has been reported in the case of large subunit maturation defects in A. eutrophus (11, 39). As a control, the [NiFe] hydrogenase activity was measured in extracts from D. fructosovorans MR400 complemented with its own homologous operon. In that case, an activity of 1.0 ± 0.4 U/mg was measured and no unprocessed forms were identified by Western immunoblotting experiments (data not shown). It is therefore possible to conclude that the small subunit of the heterologous recombinant hydrogenase appears to be mainly unprocessed and that the presence of the genes on a multicopy plasmid is not responsible for this partial maturation. However, further characterization studies were carried out to determine the origin of the hydrogenase activity measured in D. fructosovorans MR400(pBCG4). The soluble protein fraction was enriched up to a specific activity of 1.8 U/mg. This partially purified enzyme exhibited catalytic properties similar to those of the D. gigas native enzyme (14, 16). When the hydrogenase activity was tested in a native PAGE gel the one activity band that was present (Fig. 2B, lane 2) exhibited an Rf of 0.40, which is identical to that of the native enzyme (Fig. 2B, lane 1). Furthermore, in the partially purified fraction, the molecular masses of the subunits of the heterologous recombinant enzyme were found to be identical to those of the native enzyme and no trace of the precursor forms of each subunit was detected (Fig. 2B, lane 6).
FIG. 2.
Analysis of the native and recombinant D. gigas [NiFe] hydrogenases. (A) SDS-PAGE and chemoluminescent Western immunoblotting analysis of soluble extracts from the recombinant D. gigas [NiFe] hydrogenase. Lane 1, purified native hydrogenase (250 ng); lane 2, extract from D. fructosovorans MR400 (pBCG4) (60 μg); lane 3, extract from D. fructosovorans MR400 (pBCFG14) (60 μg). (B) Pairwise comparisons of pure wild-type D. gigas [NiFe] hydrogenase (lanes 1, 3, and 5) and partially purified recombinant D. gigas [NiFe] hydrogenase (lanes 2, 4, and 6). Lanes 1 (140 ng) and 2 (11 μg), hydrogenase activity staining (native PAGE gel); lanes 3 (150 ng) and 4 (10 μg), enzyme detection by Western blotting with D. gigas [NiFe] hydrogenase polyclonal antibodies; lanes 5 and 6, Western blotting (240 ng and 11 μg, respectively, on an SDS-PAGE gel).
EPR spectroscopy of recombinant D. gigas [NiFe] hydrogenases.
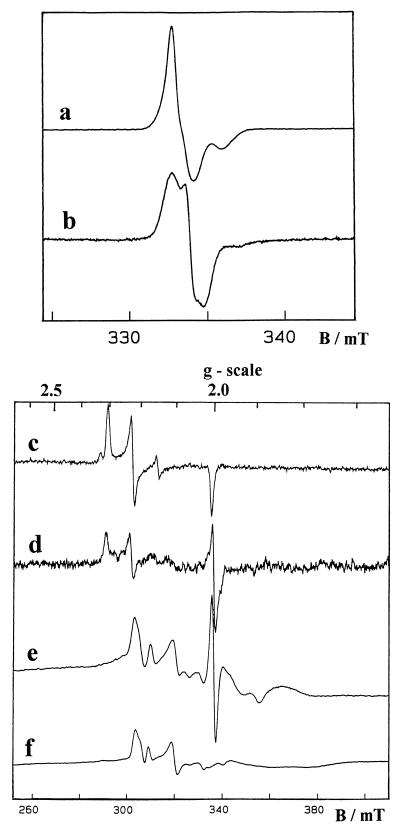
In order to check the correct arrangement of the metal centers, the partially purified D. gigas hydrogenase was studied by electron paramagnetic resonance (EPR) spectroscopy. The D. fructosovorans homologous recombinant enzyme was also analyzed as a control. As isolated in air, the enzymes showed, at low temperature (15 K), nearly isotropic EPR signals centered at g = 2.02, which arise from their oxidized [3Fe-4S]1+ center (Fig. 3, signals a and b). The D. gigas recombinant hydrogenase [3Fe-4S]1+ signal exhibits a distinct line shape which is identical to that previously observed for the native enzyme (Fig. 3, signal b) and which is quite different from the signals observed for the D. fructosovorans hydrogenase (Fig. 3, signal a) (2, 17). Ni(III) EPR signals were also observed for the oxidized recombinant hydrogenases as a mixture of the well-known Ni-A (g = 2.31, 2.23, and 2.01) and Ni-B (g = 2.33, 2.16, and 2.01) signals (Fig. 3, signals c and d) (7).
FIG. 3.
EPR spectra given by recombinant hydrogenases from D. fructosovorans (signals a, c, and e) and D. gigas (signals b, d, and f). Signals a to d are from oxidized enzymes and signals e and f are from H2-reduced enzymes. The experimental conditions used (for the signals indicated in parentheses) were as follows: Temperature 15 K (a and b), 100 K (c and d), and 6.5 K (e and f); microwave frequency, 9.420 GHz; microwave power, 0.04 mW (a and b) and 10 mW (c to f); modulation amplitude, 0.1 mT (a and b) and 1 mT (c to f).
Upon reduction of the recombinant hydrogenases with hydrogen gas, these signals disappeared and were replaced by the Ni-C signal at g = 2.19, 2.14, and 2.01. At very low temperature (<10°K), the broad and fast-relaxing signal given by the reduced [4Fe-4S]1+ clusters was detected, and the magnetic coupling between the proximal [4Fe-4S]1+ center and the Ni-C species led to the complex split Ni-C signal (15) characterized by major features at g = 2.21 and 2.10 (Fig. 3, signals e and f). Thus, all the EPR signals given by the D. gigas recombinant hydrogenase were found to be identical to those observed with the native enzyme (2, 7), which indicates that the four metal centers are present in the same environment. Moreover, as the split Ni-C signal is very sensitive to minor variations in the relative arrangement of the interacting centers, it can be inferred that no structural changes occurred in the cloned enzyme. This suggests that the active fraction of the heterologous recombinant [NiFe] hydrogenase was correctly matured. To our knowledge, this is the first report of heterologous expression of a hydrogenase, and at the present time 64% identity and 80% homology constitute the threshold above which a heterologous recombinant hydrogenase can be expressed in an active form.
Construction of the chimeric [NiFe] hydrogenase operon.
The [NiFe] hydrogenase activity measured in D. fructosovorans MR400 (pBCG4) (0.15 ± 0.05 U/mg) was about six times lower than when D. fructosovorans MR400 was complemented with its own homologous operon (1.0 ± 0.4 U/mg). This strong decrease is assumed to be due to a poor maturation efficiency of the heterologous enzyme, even though a low expression level might also have an additional effect. These two possibilities were tested by inserting D. gigas structural genes in the D. fructosovorans operon. The resulting plasmid, which contained a chimeric operon made up of the D. fructosovorans hydrogenase promoter (35), the D. gigas hydrogenase structural genes hynA and hynB (reference 45 and this work), and the D. fructosovorans hynC gene (35), was called pBCFG14. Surprisingly, similar levels of activity were observed (0.16 ± 0.05 U/mg). This indicates that the presence of the D. fructosovorans hydrogenase promoter did not increase the level of activity. The immunoblotting experiments performed on the soluble extracts from D. fructosovorans MR400 (pBCFG14) (Fig. 2B, lane 3) showed the same pattern as in the case of D. fructosovorans (pBCG4). The biochemical and EPR analyses conducted on the partially purified hydrogenase activity revealed that the heterologous hydrogenase expressed in D. fructosovorans MR400 (pBCFG14) was absolutely identical to the native enzyme, as previously observed in the case of D. fructosovorans MR400 (pBCG4). Considering that the absence of hynC led to the expression of an inactive enzyme (35), it is possible to assume that no other C-terminal protease is at work in D. fructosovorans and therefore that the HynC C-terminal protease from D. fructosovorans was able to cleave the HynB large subunit from D. gigas. This is quite surprising since we know from studies performed on A. eutrophus (5, 39) and E. coli (21, 22, 25, 32, 37) that these types of proteases are so specific that they can distinguish between the different [NiFe] hydrogenases present in these microorganisms. One possible explanation for this discrepancy might lie in the amino acid sequence around the protease cleavage site. The C-terminal regions from the D. gigas and D. fructosovorans hydrogenases are highly conserved while those from the three E. coli hydrogenases (6, 24, 27) and the two A. eutrophus hydrogenases (19, 41) are significantly different from each other. In keeping with the results of previous site-directed mutagenesis experiments (23), it is then possible to assume that the amino acid sequence at the cleavage site makes a specific recognition sequence for the C-terminal protease. In the same way, the fact that the absence of hynD in the chimeric operon did not decrease the level of activity of the recombinant hydrogenase suggests that this function was likely to be supplied in trans by a D. fructosovorans homologous gene. The low yield of D. gigas hydrogenase maturation in D. fructosovorans is an intriguing observation which suggests that the processing might be kinetically limited. All the components necessary for the correct maturation of the heterologous hydrogenase are present but some steps might be too slow, compared to structural gene expression, to allow the structural subunits as a whole to be processed. Our experiments suggest that neither the homologous HynC nor the heterologous HynD protein is responsible for this rate-limiting processing since the presence of either protein did not influence the level of activity.
Nucleotide sequence accession numbers.
The nucleotide sequences described in this study were submitted to the EMBL and GenBank data libraries and were assigned accession numbers AJ223628 and AJ223629.
Acknowledgments
We acknowledge J. D. Wall for critically reading the manuscript and for valuable suggestions. We are indebted to A. Bélaich for her assistance in genetic constructions. We thank Long-Fei Wu for very helpful discussions. We are also grateful to J. Bonicel for determination of the N-terminal amino acid sequences of the subunits of the recombinant hydrogenase and to P. Sauve for the estimation of the molecular mass of the protein by analytical ultracentrifugation.
This work was supported by grant BI02-CT94-2041 from the European Commission Biotechnology Program.
REFERENCES
- 1.Adams M W W, Mortenson L E, Chen J S. Hydrogenase. Biochim Biophys Acta. 1980;594:105–176. doi: 10.1016/0304-4173(80)90007-5. [DOI] [PubMed] [Google Scholar]
- 2.Asso M, Guigliarelli B, Yagi T, Bertrand P. EPR and redox properties of Desulfovibrio vulgaris Miyazaki hydrogenase: comparison with the Ni-Fe enzyme from Desulfovibrio gigas. Biochim Biophys Acta. 1992;1122:50–56. doi: 10.1016/0167-4838(92)90126-x. [DOI] [PubMed] [Google Scholar]
- 3.Badziong W, Thauer R K. Vectorial electron transport in Desulfovibrio vulgaris (Marburg) growing on hydrogen plus sulfate as sole energy source. Arch Microbiol. 1980;125:167–174. [Google Scholar]
- 4.Barondeau D P, Roberts L M, Lindahl P A. Stability of the Ni-C state and oxidative titration of Desulfovibrio gigas hydrogenase monitored by EPR and electronic absorption spectroscopies. J Am Chem Soc. 1994;116:3442–3448. [Google Scholar]
- 5.Bernhard M, Schwartz E, Rietdorf J, Friedrich B. The Alcaligenes eutrophus membrane-bound hydrogenase gene locus encodes functions involved in maturation and electron transport coupling. J Bacteriol. 1996;178:4522–4529. doi: 10.1128/jb.178.15.4522-4529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Böhm R, Sauter M, Böck A. Nucleotide sequence and expression of an operon in Escherichia coli coding for formate hydrogenlyase components. Mol Microbiol. 1990;4:231–243. doi: 10.1111/j.1365-2958.1990.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 7.Cammack R, Patil D S, Hatchikian E C, Fernandez V M. Nickel and iron-sulfur centres in Desulfovibrio gigas hydrogenase: ESR spectra, redox properties and interactions. Biochim Biophys Acta. 1987;912:98–109. [Google Scholar]
- 8.Chen J C, Mortenson L E. Identification of six open reading frames from a region of the Azotobacter vinelandii genome likely involved in dihydrogen metabolism. Biochim Biophys Acta. 1992;1131:199–202. doi: 10.1016/0167-4781(92)90077-d. [DOI] [PubMed] [Google Scholar]
- 9.Colbeau A, Magnin J P, Cauvin B, Champion T, Vignais P. Organisation of the genes necessary for hydrogenase expression in Rhodobacter capsulatus. Sequence analysis and identification of two hyp regulatory mutants. Mol Microbiol. 1993;8:15–29. doi: 10.1111/j.1365-2958.1993.tb01199.x. [DOI] [PubMed] [Google Scholar]
- 10.Dernedde J, Eitinger T, Friedrich B. Analysis of a pleiotropic gene region involved in formation of catalytically active hydrogenase in Alcaligenes eutrophus H16. Arch Microbiol. 1993;159:545–553. doi: 10.1007/BF00249034. [DOI] [PubMed] [Google Scholar]
- 11.Dernedde J, Eitinger T, Patenge N, Friedrich B. hyp gene products in Alcaligenes eutrophus are part of a hydrogenase-maturation system. Eur J Biochem. 1996;235:351–358. doi: 10.1111/j.1432-1033.1996.00351.x. [DOI] [PubMed] [Google Scholar]
- 12.Dole F, Fournel A, Magro V, Hatchikian E C, Bertrand P, Guigliarelli B. Nature and electronic structure of the Ni-X dinuclear center of Desulfovibrio gigas hydrogenase. Implications for the enzymatic mechanism. Biochemistry. 1997;36:7847–7854. doi: 10.1021/bi963171i. [DOI] [PubMed] [Google Scholar]
- 13.Drapal N, Böck A. Interaction of the hydrogenase accessory protein HypC with HycE, the large subunit of Escherichia coli hydrogenase 3 during enzyme maturation. Biochemistry. 1998;37:2941–2948. doi: 10.1021/bi9720078. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez V M, Hatchikian E C, Cammack R. Properties and reactivation of two different deactivated forms of Desulfovibrio gigas hydrogenase. Biochim Biophys Acta. 1985;832:69–79. [Google Scholar]
- 15.Guigliarelli B, More C, Fournel A, Asso M, Hatchikian E C, Williams R, Cammack R, Bertrand P. Structural organization of the Ni and [4Fe-4S] centers in the active form of Desulfovibrio gigas hydrogenase. Analysis of the magnetic interactions by electron paramagnetic resonance spectroscopy. Biochemistry. 1995;34:4781–4790. doi: 10.1021/bi00014a036. [DOI] [PubMed] [Google Scholar]
- 16.Hatchikian E C, Bruschi M, LeGall J. Characterization of the periplasmic hydrogenase from Desulfovibrio gigas. Biochem Biophys Res Commun. 1978;82:451–462. doi: 10.1016/0006-291x(78)90896-3. [DOI] [PubMed] [Google Scholar]
- 17.Hatchikian E C, Traore A S, Fernandez V M, Cammack R. Characterization of the nickel-iron periplasmic hydrogenase from Desulfovibrio fructosovorans. Eur J Biochem. 1990;187:635–643. doi: 10.1111/j.1432-1033.1990.tb15347.x. [DOI] [PubMed] [Google Scholar]
- 18.Hidalgo E, Palacios J M, Murillo J, Ruiz-Argüeso T. Nucleotide sequence and characterization of four additional genes of the hydrogenase structural operon from Rhizobium leguminosarum bv. viciae. J Bacteriol. 1992;174:4130–4139. doi: 10.1128/jb.174.12.4130-4139.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kortlüke C, Horstmann K, Schwartz E, Rohde M, Binsack R, Friedrich B. A gene complex coding for the membrane-bound hydrogenase of Alcaligenes eutrophus. J Bacteriol. 1992;174:6277–6289. doi: 10.1128/jb.174.19.6277-6289.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutz S, Jacobi A, Schlensog V, Böhm R, Sawers G, Böck A. Molecular characterization of an operon (hyp) necessary for the activity of the three hydrogenase isoenzymes in Escherichia coli. Mol Microbiol. 1991;5:123–135. doi: 10.1111/j.1365-2958.1991.tb01833.x. [DOI] [PubMed] [Google Scholar]
- 21.Maier T, Böck A. In: Advances in inorganic biochemistry: mechanisms of metallocenter assembly. Hausinger R P, Eichhorn G L, Marzilli L G, editors. New York, N.Y: VCH Publishers Inc.; 1996. pp. 173–192. [Google Scholar]
- 22.Maier T, Böck A. Generation of active [NiFe] hydrogenase in vitro from a nickel-free precursor form. Biochemistry. 1996;35:10089–10093. doi: 10.1021/bi960567l. [DOI] [PubMed] [Google Scholar]
- 23.Massanz C, Fernandez V M, Friedrich B. C-terminal extension of the H2-activating subunit, HoxH, directs maturation of the NAD-reducing hydrogenase in Alcaligenes eutrophus. Eur J Biochem. 1997;245:441–448. doi: 10.1111/j.1432-1033.1997.t01-3-00441.x. [DOI] [PubMed] [Google Scholar]
- 24.Menon N K, Robbins J, Peck H D, Chatelus C Y, Choi E S, Przybyla A E. Cloning and sequencing of a putative Escherichia coli [NiFe] hydrogenase-1 operon containing six open reading frames. J Bacteriol. 1990;172:1969–1977. doi: 10.1128/jb.172.4.1969-1977.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menon N K, Robbins J, Wendt J C, Shanmugam K T, Przybyla A E. Mutational analysis and characterization of the Escherichia coli hya operon, which encodes [NiFe] hydrogenase 1. J Bacteriol. 1991;173:4851–4861. doi: 10.1128/jb.173.15.4851-4861.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menon A L, Mortenson L E, Robson R L. Nucleotide sequence and genetic analysis of hydrogen oxidation (hox) genes in Azotobacter vinelandii. J Bacteriol. 1992;174:4549–4557. doi: 10.1128/jb.174.14.4549-4557.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menon N K, Chatelus C Y, DerVartanian M, Wendt J C, Shanmugam K T, Peck H D, Przybyla A. Cloning, sequencing, and mutational analysis of the hyb operon encoding Escherichia coli hydrogenase 2. J Bacteriol. 1994;176:4416–4423. doi: 10.1128/jb.176.14.4416-4423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odom J M, Peck H D., Jr Hydrogenase, electron-transfer proteins and energy coupling in the sulfate-reducing bacteria Desulfovibrio. Annu Rev Microbiol. 1984;38:551–592. doi: 10.1146/annurev.mi.38.100184.003003. [DOI] [PubMed] [Google Scholar]
- 29.Olson J W, Maier R J. The sequences of hypF, hypC and hypD complete the hyp gene cluster required for hydrogenase activity in Bradyrhizobium japonicum. Gene. 1997;199:93–99. doi: 10.1016/s0378-1119(97)00352-1. [DOI] [PubMed] [Google Scholar]
- 30.Postgate J R. The sulphate-reducing bacteria. 2nd ed. Cambridge, United Kingdom: Cambridge University Press; 1984. p. 151. [Google Scholar]
- 31.Rey L, Murillo J, Hernando Y, Hidalgo E, Cabrera E, Imperial J, Ruiz-Argüeso T. Molecular analysis of a microaerobically induced operon required for hydrogenase synthesis in Rhizobium leguminosarum bv. Viciae. Mol Microbiol. 1993;8:471–481. doi: 10.1111/j.1365-2958.1993.tb01591.x. [DOI] [PubMed] [Google Scholar]
- 32.Rossman R, Maier T, Lottspeich F, Böck A. Characterization of a protease from Escherichia coli involved in hydrogenase maturation. Eur J Biochem. 1995;227:545–550. doi: 10.1111/j.1432-1033.1995.tb20422.x. [DOI] [PubMed] [Google Scholar]
- 33.Rousset M, Dermoun Z, Hatchikian E C, Bélaich J-P. Cloning and sequencing of the locus encoding the large and small subunit genes of the periplasmic [NiFe] hydrogenase from Desulfovibrio fructosovorans. Gene. 1990;94:95–101. doi: 10.1016/0378-1119(90)90473-5. [DOI] [PubMed] [Google Scholar]
- 34.Rousset M, Dermoun Z, Chippaux M, Bélaich J-P. Marker exchange mutagenesis of the hyn genes in Desulfovibrio fructosovorans. Mol Microbiol. 1991;5:1735–1740. doi: 10.1111/j.1365-2958.1991.tb01922.x. [DOI] [PubMed] [Google Scholar]
- 35.Rousset M, Dermoun Z, Wall J D, Bélaich J-P. Analysis of the periplasmic [NiFe] hydrogenase transcription unit from Desulfovibrio fructosovorans. J Bacteriol. 1993;175:3388–3393. doi: 10.1128/jb.175.11.3388-3393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rousset M, Casalot L, Rapp-Giles B J, Dermoun Z, de Philip P, Bélaich J P, Wall J D. New shuttle vectors for the introduction of cloned DNA in Desulfovibrio. Plasmid. 1997;39:114–122. doi: 10.1006/plas.1997.1321. [DOI] [PubMed] [Google Scholar]
- 37.Sauter M, Bohm R, Böck A. Mutational analysis of the operon (hyc) determining hydrogenase 3 formation in Escherichia coli. Mol Microbiol. 1992;6:1523–1532. doi: 10.1111/j.1365-2958.1992.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 38.Teixeira M, Moura I, Xavier A V, Moura J J G, LeGall J, DerVartanian D V, Peck H D, Jr, Huynh B H. Redox intermediates of Desulfovibrio gigas hydrogenase generated under hydrogen: Mössbauer and EPR characterization of the Fe-S and Ni centers. J Biol Chem. 1989;264:16435–16450. [PubMed] [Google Scholar]
- 39.Thiemermann S, Dernedde J, Bernhard M, Schroeder W, Massanz C, Friedrich B. Carboxyl-terminal processing of the cytoplasmic NAD-reducing hydrogenase of Alcaligenes eutrophus requires the hoxW gene product. J Bacteriol. 1996;178:2368–2374. doi: 10.1128/jb.178.8.2368-2374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tibelius K H, Du L, Tito D, Stejskal F. The Azotobacter chroococcum hydrogenase gene cluster: sequences and genetic analysis of four accessory genes, hupA, hupB, hupY and hupC. Gene. 1993;127:53–61. doi: 10.1016/0378-1119(93)90616-b. [DOI] [PubMed] [Google Scholar]
- 41.Tran-Betcke A, Warnecke U, Böcker C, Zaborosch C, Friedrich B. Cloning and nucleotide sequences of the genes for the subunits of NAD-reducing hydrogenase of Alcaligenes eutrophus H16. J Bacteriol. 1990;172:2920–2929. doi: 10.1128/jb.172.6.2920-2929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.VanSoon C, Browaeys J, Verreth C, Vanderlevden J. Nucleotide sequence analysis of four genes, hupC, hupD, hupF and hupG, downstream of the hydrogenase structural genes in Bradyrhizobium japonicum. J Mol Biol. 1993;234:508–512. doi: 10.1006/jmbi.1993.1605. [DOI] [PubMed] [Google Scholar]
- 43.Volbeda A, Charon M H, Piras C, Hatchikian E C, Frey M, Fontecilla-Camps J C. Crystal structure of the nickel-iron hydrogenase from Desulfovibrio gigas. Nature. 1995;373:580–587. doi: 10.1038/373580a0. [DOI] [PubMed] [Google Scholar]
- 44.Volbeda A, Garcin E, Piras C, de Lacey A L, Fernandez V M, Hatchikian E C, Frey M, Fontecilla-Camps J C. Structure of the [NiFe] hydrogenase active site: evidence for biologically uncommon Fe ligands. J Am Chem Soc. 1996;118:12989–12996. [Google Scholar]
- 45.Voordouw G, Menon N K, LeGall J, Choi E S, Peck H D, Jr, Przybyla A E. Analysis and comparison of the nucleotide sequences encoding [NiFe] and [NiFeSe] hydrogenases from Desulfovibrio gigas and Desulfovibrio baculatus. J Bacteriol. 1989;171:2894–2899. doi: 10.1128/jb.171.5.2894-2899.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voordouw G. Hydrogenase genes in Desulfovibrio. In: Bélaich J-P, Bruschi M, Garcia J-L, editors. Microbiology and biochemistry of strict anaerobes involved in interspecies H2 transfer. FEMS Symposium. New York, N.Y: Plenum Press; 1990. pp. 37–51. [Google Scholar]