Abstract
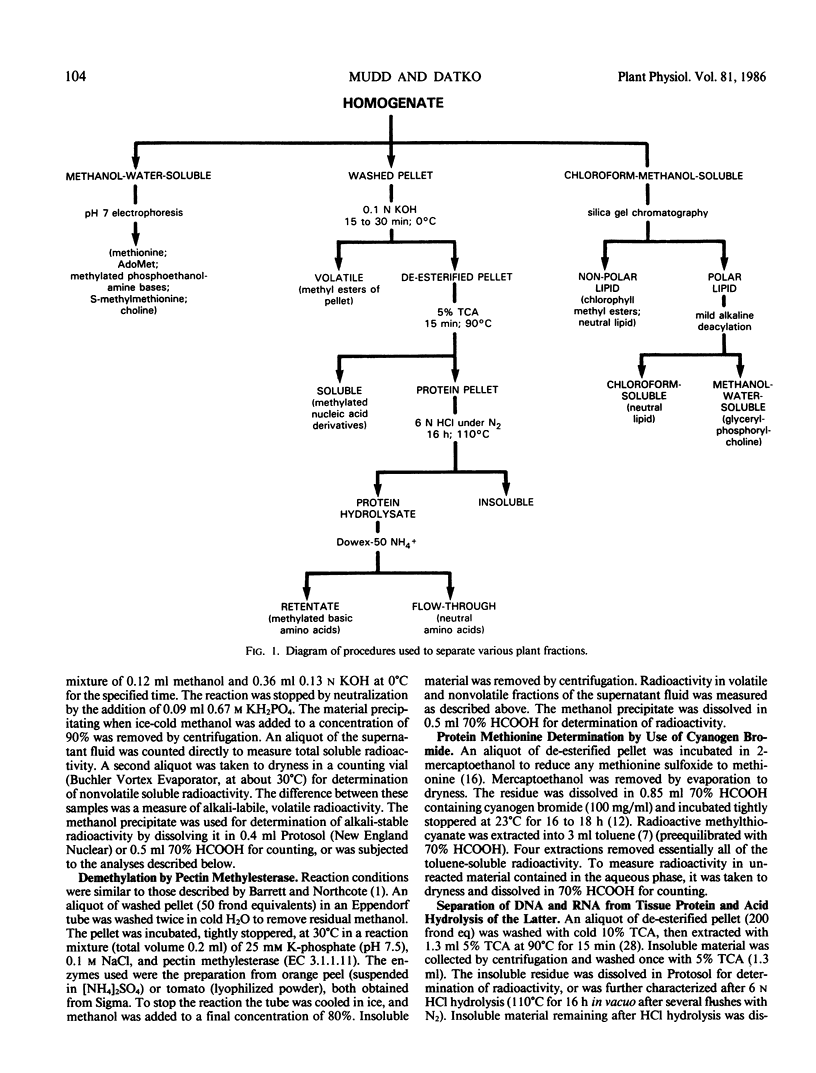
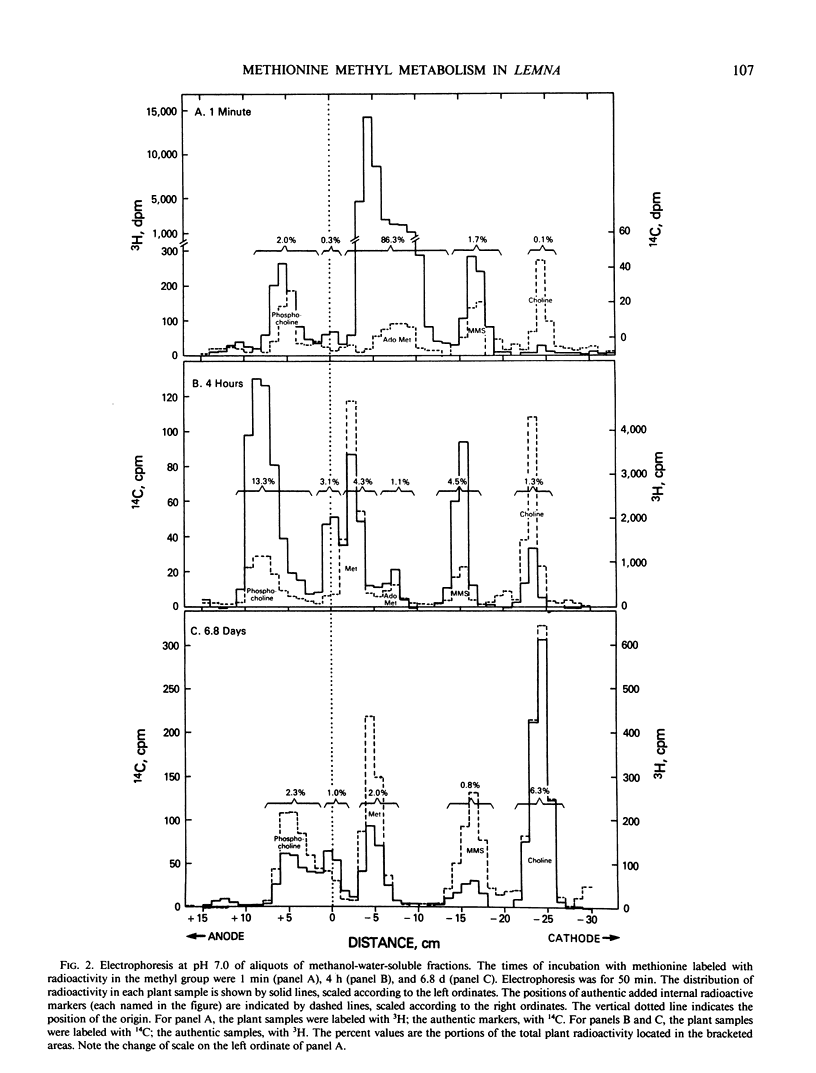
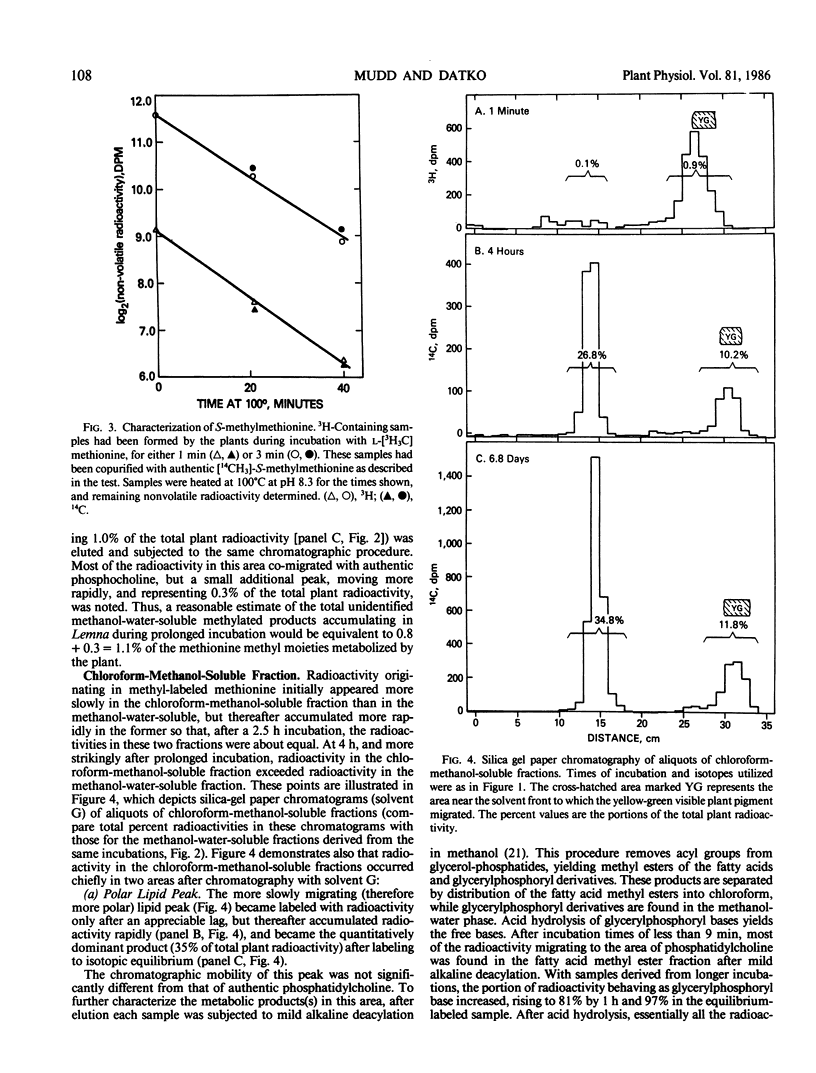
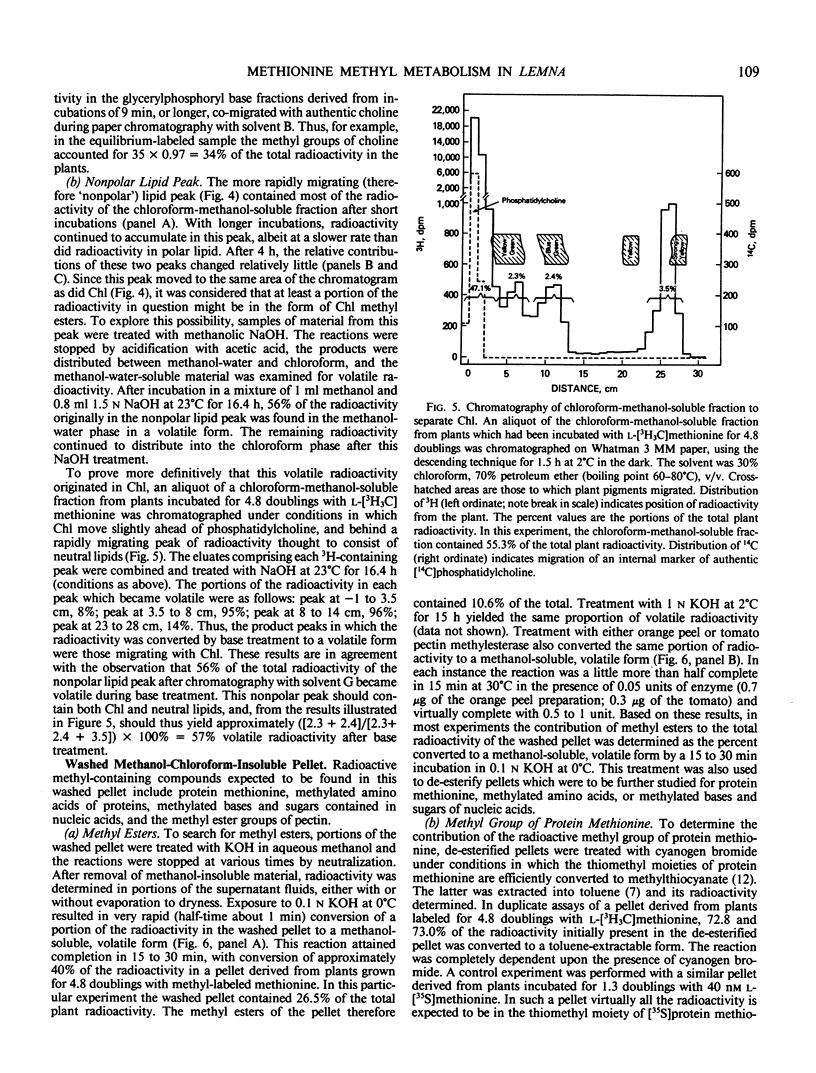
To provide information upon the ways in which Lemna paucicostata uses the methyl group of methionine, plants were grown for various periods (from 1 minute to 6.8 days) in the presence of a tracer dose of radioactive methyl-labeled methionine. Protein methionine accounted for approximately 19% of the accumulated methyl moieties; other methylated products, about 81%. The latter group included (percent of total methyl in parentheses): methylated ethanolamine derivatives (46%); methyl esters of the pellet (chiefly, or solely, pectin methyl esters) (15%); chlorophyll methyl esters (8%); unidentified neutral lipids (6%); nucleic acid derivatives (2-5%); methylated basic amino acids (2%). No other major methylated compounds were observed in any plant fraction. Available evidence suggests that little, if any, oxidation of the methyl group of methionine, directly or indirectly, occurs in Lemna. Our results indicate that S-methyl-methionine sulfonium is formed relatively rapidly, but does not accumulate at a commensurate rate, probably being reconverted to methionine. To our knowledge, this is the first time a reasonably complete accounting of the metabolic fate of methionine methyl has been obtained for any plant. The extent to which the results with Lemna may be representative of the situation for other higher plants is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRETT A. J., NORTHCOTE D. H. APPLE FRUIT PECTIC SUBSTANCES. Biochem J. 1965 Mar;94:617–627. doi: 10.1042/bj0940617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer W. D., Talmadge K. W., Keegstra K., Albersheim P. The Structure of Plant Cell Walls: II. The Hemicellulose of the Walls of Suspension-cultured Sycamore Cells. Plant Physiol. 1973 Jan;51(1):174–187. doi: 10.1104/pp.51.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevenga N. J., Harper A. E. Effect of glycine and serine on methionine metabolism in rats fed diets high in methionine. J Nutr. 1970 Oct;100(10):1205–1214. doi: 10.1093/jn/100.10.1205. [DOI] [PubMed] [Google Scholar]
- Datko A. H., Giovanelli J., Mudd S. H. Homocysteine biosynthesis in green plants. O-Phosphorylhomoserine as the physiological substrate for cystathionine gamma-synthase. J Biol Chem. 1974 Feb 25;249(4):1139–1155. [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Giovanelli J. Lemna paucicostata Hegelm. 6746: DEVELOPMENT OF STANDARDIZED GROWTH CONDITIONS SUITABLE FOR BIOCHEMICAL EXPERIMENTATION. Plant Physiol. 1980 May;65(5):906–912. doi: 10.1104/pp.65.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H. Responses of Sulfur-Containing Compounds in Lemna paucicostata Hegelm. 6746 to Changes in Availability of Sulfur Sources. Plant Physiol. 1984 Jun;75(2):474–479. doi: 10.1104/pp.75.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H. Uptake of Amino Acids and Other Organic Compounds by Lemna paucicostata Hegelm. 6746. Plant Physiol. 1985 Mar;77(3):770–778. doi: 10.1104/pp.77.3.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger G. M., Duncan A. The determination of methionine in proteins by gas-liquid chromatography. Biochem J. 1976 Jun 1;155(3):615–621. doi: 10.1042/bj1550615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Homoserine esterification in green plants. Plant Physiol. 1974 Nov;54(5):725–736. doi: 10.1104/pp.54.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Quantitative analysis of pathways of methionine metabolism and their regulation in lemna. Plant Physiol. 1985 Jul;78(3):555–560. doi: 10.1104/pp.78.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Recycling of methionine sulfur in a higher plant by two pathways characterized by either loss or retention of the 4-carbon moiety. Biochem Biophys Res Commun. 1981 May 29;100(2):831–839. doi: 10.1016/s0006-291x(81)80249-5. [DOI] [PubMed] [Google Scholar]
- Hart D. A., Kindel P. K. Isolation and partial characterization of apiogalacturonans from the cell wall of Lemna minor. Biochem J. 1970 Feb;116(4):569–579. doi: 10.1042/bj1160569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M., Furano A. V. Methylated amino acids in the proteins of bacterial and mammalian cells. Arch Biochem Biophys. 1975 Aug;169(2):529–539. doi: 10.1016/0003-9861(75)90196-4. [DOI] [PubMed] [Google Scholar]
- MacDONNELL L. R., JANG R., JANSEN E. F., LINEWEAVER H. The specificity of pectinesterases from several sources with some notes on purification of orange pectinesterase. Arch Biochem. 1950 Sep;28(2):260–273. [PubMed] [Google Scholar]
- Marshall M. O., Kates M. Biosynthesis of nitrogenous phospholipids in spinach leaves. Can J Biochem. 1974 Jun;52(6):469–482. doi: 10.1139/o74-071. [DOI] [PubMed] [Google Scholar]
- Moran R. Formulae for determination of chlorophyllous pigments extracted with n,n-dimethylformamide. Plant Physiol. 1982 Jun;69(6):1376–1381. doi: 10.1104/pp.69.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran R., Porath D. Chlorophyll determination in intact tissues using n,n-dimethylformamide. Plant Physiol. 1980 Mar;65(3):478–479. doi: 10.1104/pp.65.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]


