Highlights
-
•
Tumor-derived neutrophils (or TANs) promoted the tumorigenic and metastatic potential of lung cancer cells.
-
•
TANs displayed a N2-like status and secreted the cytokine IL-10 to facilitate the activation of c-Met/STAT3 signaling, eventually enhancing distant metastasis.
-
•
STAT3 increased PD-L1 level in tumor cells, which promoted neutrophils polarization towards a N2-like status, resulting in a positive TANs-IL-10-STAT3-PD-L1-TANs feedback loop.
-
•
Blockade of IL-10 additionally eliminated metastatic tumor nodules and enhanced the anticancer effects of chemotherapy.
-
•
The findings suggested a positive feedback loop governing tumor cells and TANs, that controls tumor progression and patient outcome in lung cancer.
Keywords: Tumor associated neutrophils, IL-10, STAT3, PD-L1
Abstract
Tumor-associated neutrophils (TANs) can exist in either a pro-inflammatory or an anti-inflammatory state, known as N1 and N2, respectively. Anti-inflammatory TANs have been shown to correlate with poor prognosis and tumor progression in patients. To explore the role and mechanisms of TANs in lung cancer development, we isolated neutrophils from both peripheral blood and tumor tissues of patients/mice, and assessed their functional interaction with lung cancer cells both in vitro and in vivo. Our results revealed that tumor-derived neutrophils (or TANs) promote the tumorigenic and metastatic potential of lung cancer cells. Upon tumorigenesis, TANs display a N2-like status and secrete the cytokine IL-10 to facilitate the activation of c-Met/STAT3 signaling, which ultimately enhances distant metastasis in vivo. Meanwhile, the transcription factor STAT3 increases PD-L1 level in tumor cells, which promotes neutrophils polarization towards a N2-like status, leading to a positive feedback loop between TANs, IL-10, STAT3, PD-L1, and TANs themselves. Blocking IL-10, we additionally eliminated metastatic tumor nodules and enhanced the anticancer effects of chemotherapy in a Lewis mouse model. Our findings suggest a positive feedback loop between tumor cells and TANs that controls tumor progression and patient outcome in lung cancer.
Introduction
The interaction between malignant cells and inflammatory cells plays crucial roles in various stages at various stages of tumor progression. For example, pro-inflammatory mediators secreted by immune and other protective cells can induce changes in intracellular signaling pathways, leading to increased proliferation, decreased apoptosis, and epithelial-mesenchymal transition in tumor cells [1]. In addition, transformed tumor cells themselves could produce signaling proteins of the innate immune system, such as chemokines (e.g., CCL3 and CXCL1), cytokines (e.g., IL-1b, TNF-a) and their receptors, which contributes to the recruitment and activation of inflammatory cells [2]. Recent evidence has shown that the immune landscape in cancer patients displays spatiotemporal heterogeneity and is a significant prognostic indicator. Assessing the adaptive immune response and the densities of tumor-infiltrating T lymphocytes has been linked to overall survival in patients with cancer [3,4]. While the crucial role of tumor-associated macrophages in promoting immunosuppression, tumor progression, and metastasis is well-defined, the importance of other innate immune cells that infiltrate tumors remains incompletely understood.
Neutrophils, as the most abundant circulating leukocytes, are also among the primary innate immune cells that infiltrate tumor tissues [5]. These infiltrating neutrophils, known as TANs, exhibit significant plasticity and can differentiate into distinct anti-tumorigenic N1 and pro-tumorigenic N2 phenotypes in response to environmental signals [6]. Moreover, neutrophils secrete cytokines and chemokines that alter the tumor microenvironment and impact tumor progression, including factors such as neutrophil elastase, matrix metalloproteinases, and vascular endothelial growth factor [7]. Moreover, the released neutrophil extracellular traps are associated with tumor dormancy and metastasis in vivo [8]. Elevated neutrophil levels and the neutrophil/lymphocyte ratio in peripheral blood have been identified as predictors of poor prognosis in several cancer types, including pancreatic, gastric, breast, and colon cancer [9,10]. Studies have also shown that increased TANs are associated with malignant phenotypes and adverse prognosis in colon cancer, while higher TANs density can lead to a better response to chemotherapy [11,12]. Nevertheless, the impact of TANs on the prognosis of lung cancer patients is still a topic of debate. The varying outcomes could arise from the signaling pathways governing the N1 and N2 phenotypes within the tumor microenvironment. Consequently, the clinical relevance of TANs remains uncertain, and further investigation is required to understand the mechanisms underlying TAN-mediated progression of lung cancer.
This study involved an analysis of the prognostic significance of TANs, revealing a correlation between enriched TANs and tumor metastasis as well as poor prognosis in lung cancer patients. Our results indicated that infiltrated TANs promoted tumorigenesis, migration, and invasiveness of tumor cells through the secretion of IL-10 and the activation of c-Met/STAT3 signaling. Importantly, we have demonstrated that the activated STAT3/PD-L1 axis in tumor cells facilitated the polarization of neutrophils towards the N2 phenotype, thereby promoting the distribution of TANs in tumor tissues. Therefore, the disruption of this positive feedback loop between tumor cells and TANs presents a novel therapeutic approach for the treatment of lung cancer.
Materials and methods
Cell culture and reagents
Murine lung cancer cell line Lewis was obtained from American Type Culture Collection (USA) and maintained in Roswell Park Memorial Institute 1640 culture medium containing 10 % fetal bovine serum in a 5 % CO2 incubator at 37 °C. Recombinant Human/murine IL-10 were purchased from T&L biological technology (China). IL-10 neutralizing antibody (NAs) was purchased from Bioz (USA). STAT3 inhibitor SH-4–54 and PD-1 inhibitor nivolumab was obtained from Selleck (USA).
Clinical specimens and data analysis
Thirty-six human lung adenocarcinoma (LUAD) tissues were procured from Jiangsu Cancer Hospital and categorized into metastatic (M1, n = 18) and non-metastatic (M0, n = 18) groups based on 3-year follow-up data. All patients agreed to participate in the study and informed with written consent. Ethical review was granted by the Ethics Committee of Jiangsu Cancer Hospital & Jiangsu Institute of Cancer Research & The Affiliated Cancer Hospital of Nanjing Medical University. Meanwhile, clinical and transcriptome profile of eighteen LUAD datasets, including TCGA_LUAD (n = 513), GSE68465 (n = 462), GSE72094 (n = 442), GSE31210 (n = 246), LUAD_ONCOSG (n = 169), GSE42127 (n = 133), GSE50081 (n = 127), GSE13213 (n = 117), GSE81089 (n = 108), GSE37745 (n = 106), GSE11969 (n = 90), GSE30219 (n = 85), GSE14814 (n = 71), GSE3141 (n = 58), GSE19188 (n = 45), GSE29016 (n = 38), GSE101929 (n = 32) and GSE29013 (n = 30) were downloaded and utilized to analyze the relationship between the neutrophil infiltration and patient survival. The level of neutrophils in each tumor sample was analyzed based on the mRNA expression data using CIBERSOFT X (https://cibersortx.stanford.edu/).
Neutrophils isolation and co-culture
Neutrophils were isolated by FastStep™ Human Neutrophil Isolation Kit (Creative biolab, USA) and Murine Neutrophil Isolation Kit (Solarbio, China). Subsequently, peripheral blood or tumor tissues-derived neutrophils were co-cultured with tumor cells at a ratio of 1:1 (neutrophil: tumor cell) in Roswell Park Memorial Institute 1640 culture medium containing 10 % fetal bovine serum in a 5 % CO2 incubator at 37 °C. After 72 h, the neutrophils population in supernatant was removed and tumor cell proliferation/migration/colony formation was then determined. All neutrophils were maintained for at most 14 days. Isolated neutrophils were cultured with tumor cells at once after isolation.
Identification the purify of isolated TANs by flow cytometry
FITC anti-mouse CD45 Antibody (1:100, Clone: 30-F11, Cat#103107, Biolegend); APC anti-mouse/human CD11b Antibody (1:100, Clone: M1/70, Cat#101211, Biolegend); BD Horizon™ BV421 Rat Anti-Mouse LY-6 G (1:100, Clone: 1A8, Cat# 562737, BD); BD Pharmingen™ PE-Cy™7 Rat Anti-Mouse Ly-6C (1:100, Clone: AL-21, Cat# 560593, BD) were utilized to identification the purify of isolated TANs. Then, Samples were analyzed by a FACS Aria II (BD Biosciences, San Jose, CA, USA), and data was anazlyed using Flow Jo (Tree Star, Ashland, OR, USA). The results demonstrated a TAN purity of over 95 % (Supplementary Fig. 1).
Cell proliferation and colony formation assay
Cell proliferation was determined by cell counting kit-8 (CCK8, Biyuntian, China) assay. Briefly, pre-treated tumor cells were seeded into a 96-well plate (2 × 103 cells/well). Subsequently, the cells were incubated with a 10 % CCK-8 solution for 4 h at 0, 24, 48, and 72 h time points. The absorbance at 450 nm was detected using a microplate reader (Biorad, USA). Tumorigenic capability was determined by colony formation assay. Briefly, 500 pre-treated tumor cells were seeded in a 6-well plate and cultured with serum-free medium. Following a 10-day incubation, colonies were stained with crystal violet, and the number of colonies was counted.
Transwell assay
Cell migration was measured using Tranwell assay in 24-well Boyden Chambers (8 μm, Corning, USA). Cells that migrated to the underside of the membranes of each insert were stained with crystal violet and counted at under a low magnification microscope (Leica, Germany).
Real-time fluorescent quantitative polymerase chain reaction (qPCR)
Total RNA extracted from Lewis, CP3 and CP5 cells was reversely transcribed into cDNA with a reverse transcription kit (Applied Biosystems, USA). Quantified PCR was performed by applying the SYBR Green Real-Time PCR Master Mixes (Thermo Fisher, USA) on an ABI7500 instrument (Applied Biosystems, USA). The primer sequences (CXCR2: forward primer 5′-CCTGTCTTACTTTTCCGAAGGAC 23–3′ and reverse primer 5′-TTGCTGTATTGTTGCCCATGT-3′) were synthesized by Sangon (China). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control.
Enzyme linked immunosorbent assay (ELISA)
After co-culture with Lewis (neutrophils: Lewis, 1:1) or not, 1 × 105 neutrophils were cultured in 2 ml medium for 48 or 72 h. IL-10 or IL-8 level in supernatant were examined by Human/Murine IL-10 ELISA Kit (Beyotime Biotechnology, China), Human IL-8 (Elabscience, USA) and Mouse CXCL1/KC/N51 ELISA Kit (Beyotime Biotechnology, China).
Western blotting
Proteins were extracted with lysis buffer (Beyotime Biotechnology, China), separated by 10 % polyacrylamide gel electrophoresis (PAGE), and subsequently transferred to a nitrocellulose membrane (Beyotime Biotechnology, China) using a wet transfer apparatus. After blocking the samples with 5 % bovine serum albumin for 30 min at room temperature, they were incubated overnight at 4 °C with the following primary antibodies: anti-total STAT3 (ab68153), anti-phosphorylated STAT3 (ab76315), anti-c-Met (ab216334), and anti-PD-L1 (ab205921), which were all obtained from Abcam, UK. Subsequently, the membrane was incubated with secondary antibodies for 2 h and visualized using a gel imaging system (Thermo Fisher, USA).
Immunostaining
Sections of tumor tissues or primary tumor cells isolated from two cancer patients (CP3 and CP5) colonies (in Matrigel) were treated with 3 % H2O2 and blocked with 5 % bovine serum albumin for 30 min at room temperature. Subsequently, samples were incubated with primary antibodies: anti-phosphorylated STAT3 (ab76315), anti-CD66b (ab197678,), anti-IL-10 (ab133575), anti-c-Met (ab216574, all purchased from Abcam, UK) overnight at 4 °C. Subsequently, samples were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature, visualized and analyzed under optical microscope (Leica, Germany) or confocal microscope (Olympus, Germany). Protein expression was quantified by Image-Pro-Plus 6.0 software (USA). The mean expression intensity in control group (M0 group) was set as 1. The relative expression was calculated in each sample and analyzed.
Animal protocols
C57BL/6 mice (6∼8 weeks) were purchased from Vitalriver (USA) and raised in a specific pathogen-free facility. 2 × 105 Lewis cells were subcutaneously injected into mice (n = 6 in each group). Tumor volume was recorded daily. Mice were intratumor injected with 1 × 105 TANs on day 10 and 15 by intratumor injection. Meanwhile, mice were treated with PBS, IL-10 neutralizing antibodies (NAs, 50 μg/kg, intratumor injection), oxaliplatin (5 mg/kg) or combination on day 12 and 18 by tail vein injection. Mice were sacrificed on day 25 for lung metastatic analysis and protein quantification. The tumor volume (n = 6 in each group) and survival (n = 6 in each group) were recorded daily. The calculation formula of tumor volume: tumor volume = length × width2/2.
Statistical analysis
The experimental data were analyzed using GraphPad Prism 6.0 (GraphPad Software, USA) and are presented as mean ± SD. Group differences were assessed using independent sample t-tests for two-group comparisons and one-way ANOVA followed by Tukey's post hoc test for multiple group comparisons. Survival analysis was conducted using the Kaplan-Meier estimator. Each experiment was independently repeated at least three times. Statistical significance was set at p < 0.05.
Results
Enriched TANs correlated with poor prognosis in lung cancer patients
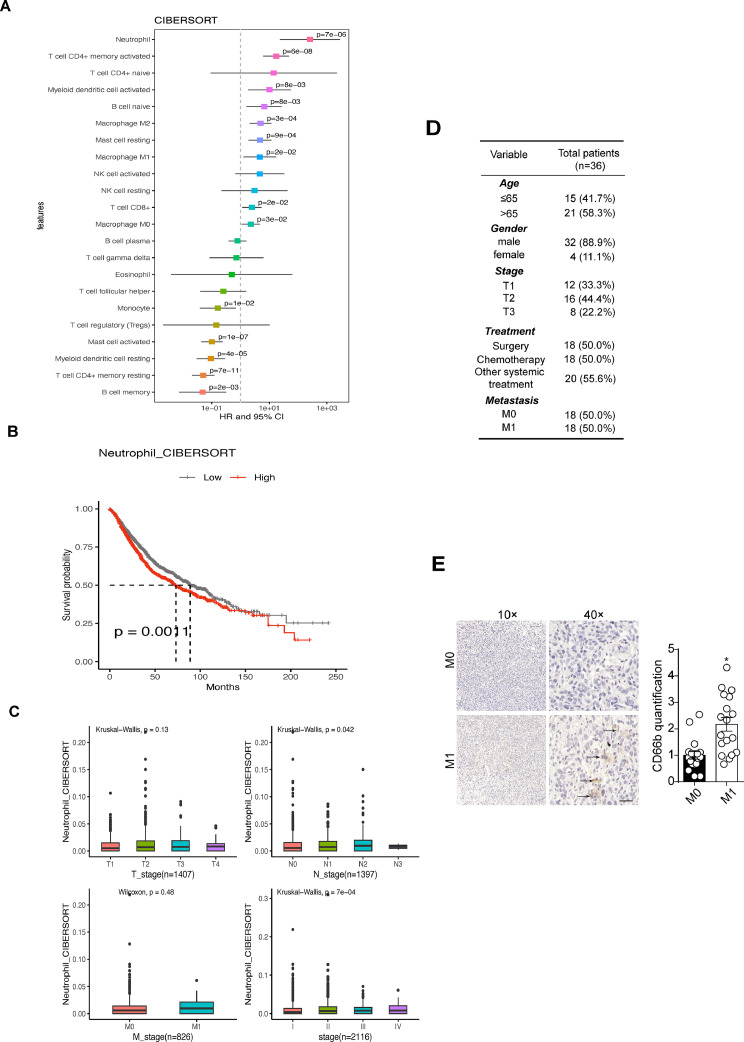
Transcriptomic data from eighteen LUAD datasets revealed a correlation between neutrophil levels and poor prognosis in 2872 LUAD patients (Fig. 1A). Additionally, high TAN levels were associated with significantly reduced survival time compared to low levels (Fig. 1B). Moreover, higher pathological stages in LUAD patients were linked to increased TAN abundance (Fig. 1C). A local cohort of 36 LUAD patients was stratified into metastatic/non-metastatic groups based on post-surgery follow-up visits (3 years, Fig. 1D), showing markedly elevated TAN distribution in metastatic tumor tissues compared to the non-metastatic group (Fig. 1E). These findings collectively suggest a correlation between enriched TANs, tumor metastasis, and poor prognosis in LUAD patients.
Fig. 1.
Enriched TANs correlated with poor prognosis in patients with lung adenocarcinoma (LUAD). A, Analysis the association between different tumor-infiltrated immune cells and overall survivals in 2872 LUAD patients from eighteen different LUAD datasets. The level of neutrophils in each tumor sample was determined by CIBERSORT assay. B, Kaplan-Meier overall survival curve was shown according to high and low neutrophils distribution in 2872 LUAD patients. C, Neutrophils quantification in LUAD patients of different pathological stages. D, the clinical characteristics of the patients. E, immunohistochemistry of CD66b in tumor tissues derived from M0 (n = 18) and M1 (n = 18) lung patients. The scale bar was 50 μm.
TANs promoted tumorigenic and metastatic potential of lung cancer cells
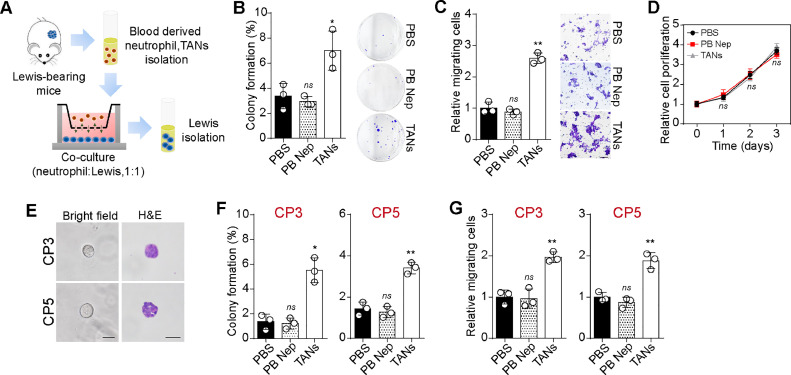
Motivated by the strong association between TANs and poor prognosis in LUAD, we sought to elucidate the mechanism underlying TANs-induced tumor progression. We utilized neutrophils derived from peripheral blood or tumor infiltrating leukocytes (TILs) of subcutaneous Lewis-bearing mice. Subsequently, we co-cultured these neutrophils with Lewis cancer cells to assess their impact on tumor cell proliferation, migration, and colony formation (Fig. 2A). Notably, co-culture with TANs led to increased colony formation potential (Fig. 2B) and migratory phenotypes (Fig. 2C) in tumor cells, compared to groups co-cultured with PBS or peripheral blood-derived neutrophils. However, there was no significant difference in cell proliferation among the groups co-cultured with PBS, TANs, or peripheral blood-derived neutrophils groups (Fig. 2D), indicating that TANs promoted tumorigenic and metastatic potential in lung cancer. To further validate the role of TANs in clinical specimens, we isolated tumor cells from fresh lung tumor tissues and attempted to establish a primary lung cancer cell culture model using 3D Matrigel gels. Subsequently, we added PBS, peripheral blood-derived neutrophils, and TANs (derived from tumor tissue donors) into the supernatant of the 3D culture system, respectively. Primary tumor cells isolated from two cancer patients (CP3 and CP5) formed spherical colonies and exhibited proliferative phenotypes within 72 h (Fig. 2E). Following this, a cell migration and tumor formation assay was performed to examine the influence of neutrophils on CP3 and CP5. Consistently, TANs co-culture significantly enhanced the capability of colony formation (Fig. 2F) and migration (Fig. 2G) in primary tumor cells. Taken together, these findings indicate that TANs play a pro-tumor role in facilitating lung cancer development.
Fig. 2.
TANs promoted tumorigenic and metastatic potential. A, schematic diagram of tumor cells/neutrophils co-culture system. Neutrophils were isolated from peripheral blood or tumor tissues, then co-cultured with Lewis cells (neutrophils: Lewis, 1:1). 48 h later, tumor cells were isolated for further assay. B, colony formation capability of Lewis pre-cultured with PBS, peripheral blood derived neutrophils and TANs respectively. C, cell migration of Lewis pre-cultured with PBS, peripheral blood derived neutrophils and TANs respectively. The scale bar was 50 μm. D, cell proliferation of Lewis pre-cultured with PBS, peripheral blood derived neutrophils and TANs respectively. E, representative images of tumor colonies (day 3) derived from CP3 and CP5. The scale bar was 50 μm. F, colony formation capability of CP3/CP5 pre-cultured with PBS, peripheral blood derived neutrophils and TANs respectively. G, cell migration of CP3/CP5 pre-cultured with PBS, peripheral blood derived neutrophils and TANs respectively.
IL-10 blockade suppressed tumorigenic and metastatic potential-induced by TANs
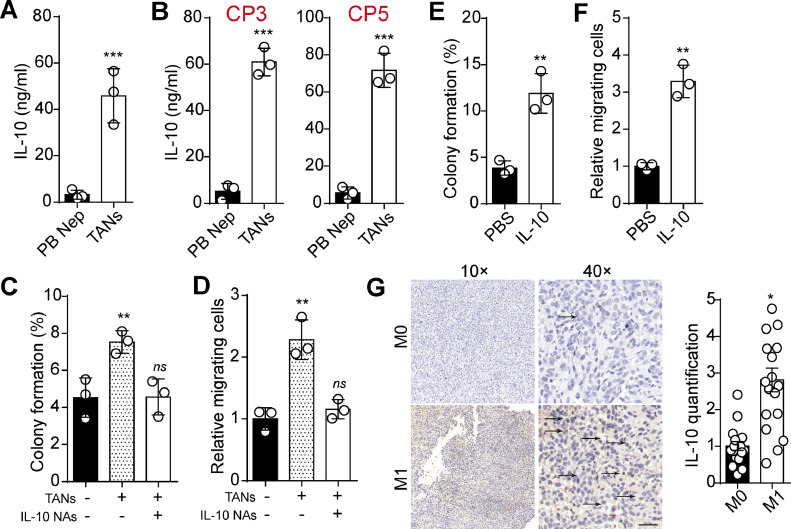
TANs are a source of IL-10 within the inflammatory and suppressive tumor microenvironment [13], and IL-10 has been previously reported to act as tumor promoter to drive tumor stemness or metastasis [14,15]. Consequently, we investigated whether TANs play a role in promoting lung cancer development through IL-10. As anticipated, we observed increased IL-10 production in the supernatant of TANs compared to peripheral blood-derived neutrophils (Fig. 3A). Similar results were found in TANs derived from LUAD patients (Fig. 3B). Additionally, IL-10 NAs suppressed colony formation and cell migration-induced by TANs (Fig. 3C and D). Those results suggested an essential role of IL-10 in mediating TANs-induced tumor progression. To further substantiate our hypothesis, we treated Lewis cells with IL-10 and assessed colony formation and cell migration. Consistently, IL-10 significantly promoted colony formation and cell migration of Lewis cells (Fig. 3E and F). Immunostaining revealed increased IL-10 expression in tumor tissues from M1 patients (Fig. 3G), which was in line with the observed changes in TANs distribution. These results indicated that TANs could promote LUAD progression through IL-10.
Fig. 3.
IL-10 blockade suppressed tumorigenic and metastatic potential. A, IL-10 quantification in supernatant of TANs and peripheral blood-derived neutrophils (48 h, 105 cells in 2 ml medium) using ELISA. B, IL-10 quantification in supernatant of TANs and peripheral blood-derived neutrophils from PC3 and PC5 patients. C, colony formation capability of Lewis cells pre-cultured with TANs, IL-10 NAs (0.1 μg/ml in culture system) or not. D, cell migration of Lewis cells pre-cultured with TANs, IL-10 NAs (0.1 μg/ml in culture system) or not. E, colony formation capability of Lewis cells pre-cultured with PBS or IL-10 (0.1 μg/ml in culture system). F, cell migration of Leiws cells pre-cultured with PBS or IL-10 (0.1 μg/ml in culture system). G, immunohistochemistry of IL-10 in tumor tissues derived from M0 (n = 18) and M1 (n = 18) lung cancer patients. The scale bar was 50 μm. NAs: Neutralizing antibodies.
IL-10 activated c-Met/STAT3 pathways to promote tumorigenesis and metastasis
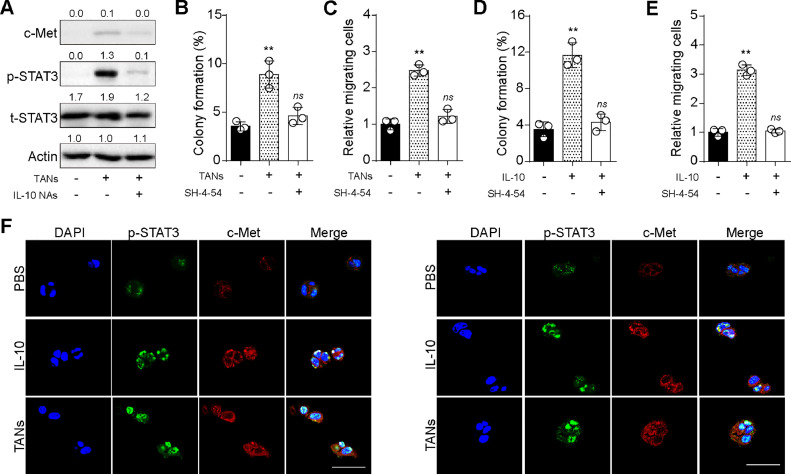
Considering the crucial role of IL-10 in TANs-induced tumor progression, we aimed to elucidate the mechanism underlying IL-10-associated cancer development. Given previous findings of the correlation between IL-10-promoted tumor invasion and c-Met/STAT3 signaling in gastric cancer [16], we examined the expression level of c-Met-and STAT3 in TANs co-cultured Lewis cells. Consequently, we observed elevated expression of c-Met-and phosphorylated STAT3 in Lewis cells co-cultured with TANs, while IL-10 neutralizing antibodies suppressed the upregulation of c-Met/STAT3 induced by TANs (Fig. 4A). Subsequently, to further validate the role of the c-Met/STAT3 axis, Lewis cells were co-cultured with TANs and treated with SH-4–54 (a STAT3 inhibitor). Notably, inhibition of STAT3 hindered Lewis colony formation (Fig. 4B) and cell migration (Fig. 4C) induced by TANs. Similar results were observed in IL-10-treated Lewis cells (Fig. 4D and E), indicating that TANs/IL-10 promoted lung cancer cell migration and tumorigenic potential through c-Met/STAT3 signaling. Furthermore, to assess the role of the c-Met/STAT3 axis in a clinical context, CP3 and CP5 cells in Matrigel gels were prepared for paraffin embedding, followed by immunostaining of c-Met-and phosphorylated STAT3. Consistent with the in vitro results with Lewis cells, activation of c-Met/STAT3 signaling was observed in TANs or IL-10 treated CP3/CP5 cells (Fig. 4F). Taken together, these findings reveal that TANs/IL-10 activate c-Met/STAT3 pathways to promote LUAD progression.
Fig. 4.
IL-10 activated c-Met/STAT3 pathways. A, western blotting of c-Met, p-STAT3 and t-STAT3 in Lewis pre-cultured with TANs, IL-10 NAs (0.1 μg/ml) or not. B, colony formation capability of Lewis pre-cultured with TANs, SH-4–54 (20 nM) or not. C, cell migration of Lewis pre-cultured with TANs, SH-4–54 (20 nM) or not. D, colony formation capability of Lewis pre-cultured with IL-10 (100 ng/ml), SH-4–54 (20 nM) or not. E, cell migration of Lewis pre-cultured with IL-10 (100 ng/ml), SH-4–54 (20 nM) or not. F, immunofluorescence of c-Met-and p-STAT3 in CP3 (left) and CP5 (right) pre-cultured with TANs, IL-10 or not. The scale bar was 50 μm.
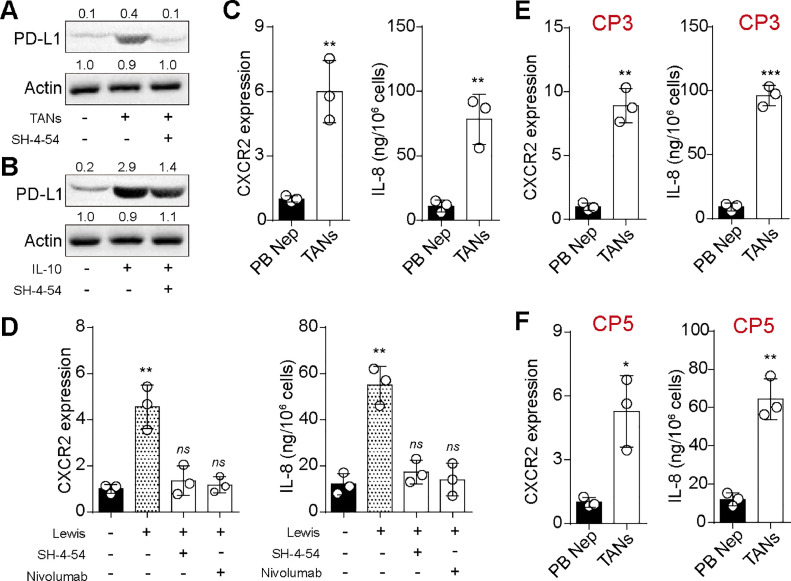
STAT3/PD-L1 axis in tumor cells promoted polarization of neutrophils towards N2-like status
Considering the tumorigenic role of TANs, we aimed to investigate the influence of tumor cells on neutrophils and their polarization. Substantial evidence indicates that the PD-L1/PD-1 axis impedes the cytotoxic activity of neutrophils, while STAT3 significantly contributes to tumor-associated immunosuppression by upregulating PD-L1 signaling [17,18]. Consequently, we hypothesized that tumor cells may promote the polarization of neutrophils toward an N2-like phenotype through the STAT3/PD-L1 axis. Initially, we assessed the expression of PD-L1 in TANs or IL-10-treated Lewis cells. Western blot analysis revealed that both TANs co-culture and IL-10 treatment upregulated PD-L1 expression in Lewis cells, and inhibition of STAT3 signaling by SH-4–54 suppressed PD-L1 upregulation (Fig. 5A and B). These findings suggested that TANs/IL-10 promoted PD-L1 expression through STAT3 signaling. Subsequently, we performed qPCR and ELISA assays to evaluate the expression of CXCR2 and IL-8, known markers of N2-like neutrophils, in peripheral blood-derived neutrophils and TANs. Significantly elevated expression of CXCR2 and IL-8 was observed in TANs compared to peripheral blood-derived neutrophils (Fig. 5C), indicating an N2-like status of TANs. We then co-cultured peripheral blood-derived neutrophils with Lewis cells for 72 h and examined the expression of CXCL1 (KC) and CXCR2. Remarkably, increased CXCR2 and CXCL1/KC levels were detected in Lewis co-cultured neutrophils, while treatment with SH-4–54 or nivolumab suppressed the upregulation of CXCR2 and CXCL1 (KC) induced by Lewis (Fig. 5D). Similar results were observed in neutrophils from CP3 and CP5 (Fig. 5E and F). Moreover, the expression of PD-1 in TANs was upregulated compared with peripheral blood-derived neutrophils (Supplementary figure 2). Collectively, these data illustrate that the STAT3/PD-L1 axis in tumor cells promotes the polarization of neutrophils toward an N2-like status, thereby facilitating the distribution of TANs in tumor tissues.
Fig. 5.
STAT3/PD-L1 axis in tumor cells promoted polarization of neutrophils towards N2-like status. A, western blotting of PD-L1 in Lewis pre-cultured with TANs, SH-4–54 (20 nM) or not. B, western blotting of PD-L1 in Lewis pre-cultured with IL-10 (100 ng/ml), SH-4–54 (20 nM) or not. C, quantification of CXCR2 and CXCL1 (KC) in TANs and peripheral blood-derived neutrophils using qPCR and ELISA. D, quantification of CXCR2 and CXCL1 (KC) in peripheral blood-derived neutrophils pre-cultured with Lewis, SH-4–54 (20 nM), nivolumab (10 nM) or not. E and F, quantification of CXCR2 and IL-8 in TANs and peripheral blood-derived neutrophils, isolated from CP3 (E) and CP5 (F) patient.
Interruption of IL-10 signaling improved outcome of chemotherapy in vivo
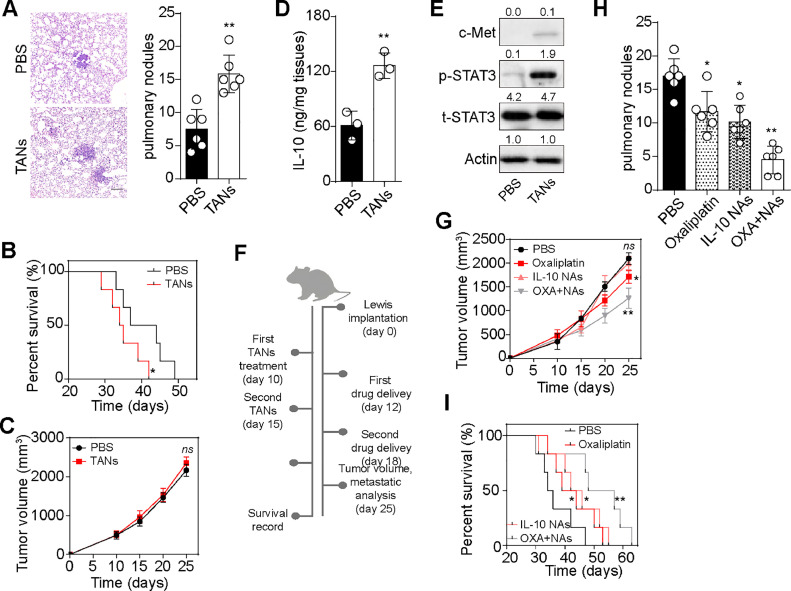
To investigate the role of TANs in vivo, we injected Lewis cells into C56BL/6 mice to establish a subcutaneous tumor-bearing mouse model. TANs isolated from tumor tissues were then reintroduced into Lewis-bearing mice through intratumor injection. Intriguingly, we observed enhanced pulmonary metastasis and poorer overall survival in mice treated with TANs compared to the PBS-treated group (Fig. 6A and B). However, we found minimal differences in tumor growth between the PBS and TANs groups, consistent with our in vitro findings (Fig. 6C). Subsequently, we assessed the activation of IL-10/c-Met/STAT3/PD-L1 signaling in tumor tissues. As expected, mice treated with TANs exhibited elevated IL-10 secretion and c-Met/STAT3/PD-L1 expression (Fig. 6D and E), indicating that TANs promoted lung cancer development through the IL-10/STAT3 axis in vivo. Recognizing the crucial role of TANs/IL-10/STAT3 signaling in promoting lung cancer development, we investigated whether blocking TANs/IL-10/STAT3 signaling could improve outcomes in TANs-enriched lung cancer. Therefore, Lewis-bearing mice were treated with TANs on day 10 after tumor implantation, followed by IL-10 NAs, the chemotherapeutic agent oxaliplatin, or a combination treatment (Fig. 6F). Remarkably, we observed limited tumor-suppressive effects in the oxaliplatin group, which might be associated with STAT3 signaling-induced chemoresistance. However, the combination of IL-10 NAs and oxaliplatin significantly enhanced tumor effects in TANs-treated Lewis-bearing mice (Fig. 6G). Similarly, inhibition of STAT3 signals attenuated the chemoresistance induced by TANs in vitro. Furthermore, the combination of IL-10 and oxaliplatin effectively suppressed lung metastasis (Fig. 6H) and prolonged survival time (Fig. 6I), indicating that IL-10 NAs improved the outcome of oxaliplatin treatment. Collectively, these results suggest that the TANs/IL-10/STAT3 axis promotes lung cancer development in vivo, and blockade of IL-10 signaling suppresses tumor metastasis and improves the outcome of chemotherapy.
Fig. 6.
Interruption of IL-10 signaling improved outcome of chemotherapy. A, H&E staining and metastatic pulmonary tumor nodules counting in subcutaneous Lewis-bearing mice treated with PBS or TANs. The scale bar was 100 μm. B, overall survival of subcutaneous Lewis-bearing mice treated with PBS or TANs. C, tumor volume of subcutaneous Lewis-bearing mice treated with PBS or TANs. D, IL-10 quantification in tumor tissues isolated from subcutaneous Lewis-bearing mice treated with PBS or TANs. E, western blotting of c-Met, p-STAT3 and t-STAT3 in tumor cells isolated from subcutaneous Lewis-bearing mice treated with PBS or TANs. F, schematic diagram of Lewis-bearing mice treatment (treated with TANs, following with IL-10 NAs, chemotherapeutic oxaliplatin or combining treatment). G, tumor volume of Lewis-bearing mice treated with PBS, oxaliplatin, IL-10 NAs or combination. H, metastatic pulmonary tumor nodules counting in subcutaneous Lewis-bearing mice treated with PBS, oxaliplatin, IL-10 NAs or combination. I, overall survival of subcutaneous Lewis-bearing mice treated with PBS, oxaliplatin, IL-10 NAs or combination. J. TANs could promote the malignancy potential of lung cancer cells through paracrine IL-10 signaling, which stimulated the activation of c-Met/STAT3 signaling in tumor cells. In turn, the transcription factor STAT3 increased PD-L1 level in tumor cells, which facilitated neutrophils polarization towards a N2-like status, forming a positive TANs-IL-10-STAT3-PD-L1-TANs feedback loop to stimulate tumor progression. Tumor-derived neutrophils: TANs.
Discussion
Inflammatory cells constitute a significant component of the tumor microenvironment [19,20], and their interactions with cancer cells and stromal inflammatory cells greatly influence tumor initiation and progression [21]. Neutrophils have been reported to display direct cytotoxicity toward specific tumor cells and to be involved in matrix degradation and promotion of angiogenesis in various experimental tumor progression models [22]. However, the role of neutrophils in lung cancer progression and the prognostic significance of infiltrating neutrophils in lung cancer patients remain contentious. Early studies have shown that tumor-associated neutrophils (TANs) enhance antitumor immunity by stimulating the proliferation and responsiveness of CD8+ T cells, thereby improving survival in cancer patients [23]. Conversely, some studies have indicated that TANs in a tumor microenvironment rich in TGF-β may exhibit immunosuppressive mechanisms [24]. Additionally, several studies have reported that TANs promote tumor metastasis of cancer cells through various molecular mechanisms in both in vitro and in vivo settings [25,26]. These findings collectively suggest that the pro- or anti-tumor functions of TANs depend on the local microenvironment and the interplay between tumor cells and TANs. Our study not only revealed an association between elevated TANs in tumor tissues, distant metastasis, and poor survival in LUAD patients but also demonstrated for the first time that the direct interaction between TANs and tumor cells governs tumor progression in LUAD. Furthermore, our results indicated that TANs enhance the tumorigenic and migratory capacities of LUAD cells in vitro, which correlates with poor survival in vivo. These findings underscore the potential of TANs as a meaningful clinical prognostic biomarker in LUAD.
Previous research has indicated that TANs promote tumor progression by secreting various cytokines, such as TNFα, IL-6, EGF, FGF, VEGF, and TGF-β [27]. TANs have been reported to induce epithelial-mesenchymal transition (EMT) and facilitate migration and invasion in tumor cells [28]. Additionally, Shao et al. demonstrated that TANs could also secrete bone morphogenetic protein 2 and TGF-β2, enhancing stem cell characteristics in HCC cells [29]. This study provides the initial evidence, to the best of our knowledge, that IL-10 secreted by TANs enhances the tumorigenic and metastatic potential of LUAD cells. Neutrophils have been reported to suppress cytotoxic cell function through the secretion of IL-10 [30]. Although IL-10 is commonly known as an immunosuppressive cytokine that regulates T cell infiltration and dysfunction, its direct pro-tumor effect has also been observed. For example, IL-10 derived from M2 macrophages was found to regulate proliferation and invasion in gastric cancer cells [14]. It has also been reported that increased IL-10 mRNA expression in tumor-associated macrophage correlated with the progression of lung cancer [30]. Our study investigates the direct pro-tumor effect of TANs-derived IL-10 in LUAD for the first time and demonstrates that blocking IL-10 significantly inhibits the tumorigenic and metastatic potential induced by TANs in LUAD, both in vitro and in vivo. Furthermore, our study reveals that the combination of IL-10 NAs and chemotherapeutic drugs effectively reverses chemotherapeutic resistance, suppresses tumor metastasis, and prolongs survival in mouse models of lung cancer, offering a promising therapeutic strategy for clinical lung cancer treatment.
Accumulating evidence indicates that STAT3 is significantly upregulated upon IL-10 stimulation, and the IL-10/STAT3 axis plays a crucial role in various disease developments, including inflammatory diseases and cancers [31,32]. As an upstream regulator of STAT3, c-Met-has been identified as an oncogene and a potential therapeutic target [33,34]. Our findings confirm that the cell culture supernatant of TANs induces a notable increase in c-Met-protein expression levels and STAT3 phosphorylation in lung cells. This effect can be reversed by IL-10 NAs. Previous studies have demonstrated that activated STAT3 regulates multiple genes involved in proliferation, survival, and metastasis, such as cyclin D1, p53, p21, Bcl-2, and MMP9 [35]. Consistently, our study also established that STAT3 activation contributed to tumorigenesis and metastasis in LUAD. Moreover, we demonstrate for the first time that STAT3 activation upregulates PD-L1 expression in TANs/IL-10-treated LUAD cells. It has been documented that neutrophils in the tumor microenvironment and peritumoral tissues exhibit high levels of PD-L1. Importantly, previous research has indicated that PD-L1 expression on tumor cells effectively inhibits neutrophil cytotoxicity and promotes tumor growth through the PD-L1/PD-1 axis in cancers [17], suggesting the involvement of the PD-L1/PD-1 axis in regulating direct neutrophil/tumor cell interactions. However, the mechanism by which PD-1/PD-L1 interaction reduces neutrophil cytotoxicity remains unclear. Our results demonstrate that PD-L1-upregulated LUAD cells promote the polarization of neutrophils towards an N2-like state through the PD-1/PD-L1 axis. N2 neutrophils have been reported as a significant source of IL-10 [36], which can further activate the c-Met/STAT3/PD-L1 signaling in tumor cells. These findings highlight a positive feedback loop between lung cancer cells and TANs that plays a crucial role in lung cancer progression. Overall, our observations shed light on the interaction between TANs and tumor cells, emphasizing the therapeutic potential of disrupting this positive feedback loop in clinical lung cancer treatment. However, the study has certain limitations. The short lifespan and sensitivity of TANs to extraction enzymes hindered more extensive functional studies. The clinical significance of neutrophil infiltration in LUAD remains controversial due to additional factors influencing prognostic relevance, such as immune microenvironment and gut microbiome composition. Furthermore, PD-1-expressing neutrophils not only interact with PD-L1-expressing tumor cells but also play a role in regulating anti-tumor immunity, including the suppression of cytotoxic T-cell functions. Thus, further investigation is needed to understand the interaction between LUAD cells and PD-L1-expressing TANs and its impact on T cell-associated antitumor immunity in the tumor microenvironment. Overall, this study may inspire future research on the network of interactions among tumor cells, TANs, and adaptive immune cells in the lung tumor microenvironment.
In conclusion, the enrichment of TANs is strongly linked to an unfavorable prognosis in LUAD patients. IL-10, which is secreted by TANs, promotes LUAD tumorigenesis and metastasis via the c-Met/STAT3/PD-L1 signaling pathway (Fig. 6J). Additionally, upregulation of PD-L1 in LUAD cells enhances the conversion of neutrophils into N2-like TANs. in vivo experiments have shown that inhibiting IL-10 signaling can reverse TANs-induced tumor progression and improve chemotherapy resistance. These findings indicate that targeting IL-10 signaling could offer a promising therapeutic approach for LUAD patients.
Disclosure
Ethics statement
This research has been approved by the Ethics Committee of Jiangsu Cancer Hospital & Jiangsu Institute of Cancer Research & The Affiliated Cancer Hospital of Nanjing Medical University (Approval No. 2021-018). All participants have provided written informed consents. All animal experiments are performed according to the guidelines, and all experiments were approved by the Ethics Committee of Jiangsu Cancer Hospital & Jiangsu Institute of Cancer Research & The Affiliated Cancer Hospital of Nanjing Medical University.
Consent for publication
Not applicable.
Data availability
Not applicable.
Funding
This study is supported by Jiangsu Cancer Hospital & Jiangsu Institute of Cancer Research & The Affiliated Cancer Hospital of Nanjing Medical University.
CRediT authorship contribution statement
Shuai Zhang: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. Lei Sun: Visualization, Investigation, Formal analysis. Jingfang Zuo: Visualization, Resources, Investigation. Dongjie Feng: Writing – review & editing, Methodology, Conceptualization.
Declaration of Competing Interest
On the behalf of all co-authors, we declared that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Not applicable.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2023.101866.
Appendix. Supplementary materials
References
- 1.Buhrmann C., Brockmueller A., Harsha C., Kunnumakkara A.B., Kubatka P., Aggarwal B.B., et al. Evidence that tumor microenvironment initiates epithelial-to-mesenchymal transition and calebin a can suppress it in colorectal cancer cells. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.699842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erreni M., Mantovani A., Allavena P. Tumor-associated macrophages (TAM) and inflammation in colorectal cancer. Cancer Microenviron. 2011;4(2):141–154. doi: 10.1007/s12307-010-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Caro G., Bergomas F., Grizzi F., Doni A., Bianchi P., Malesci A., et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin. Cancer Res. 2014;20(8):2147–2158. doi: 10.1158/1078-0432.Ccr-13-2590. [DOI] [PubMed] [Google Scholar]
- 4.Galon J., Costes A., Sanchez-Cabo F., Kirilovsky A., Mlecnik B., Lagorce-Pagès C., et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y., Zhao Q., Peng C., Sun L., Li X.F., Kuang D.M. Neutrophils promote motility of cancer cells via a hyaluronan-mediated TLR4/PI3K activation loop. J. Pathol. 2011;225(3):438–447. doi: 10.1002/path.2947. [DOI] [PubMed] [Google Scholar]
- 6.Fridlender Z.G., Sun J., Kim S., Kapoor V., Cheng G., Ling L., et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16(3):183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizuno R., Kawada K., Itatani Y., Ogawa R., Kiyasu Y., Sakai Y. The role of tumor-associated neutrophils in colorectal cancer. Int. J. Mol. Sci. 2019;20(3) doi: 10.3390/ijms20030529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albrengues J., Shields M.A., Ng D., Park C.G., Ambrico A., Poindexter M.E., et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409) doi: 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Templeton A.J., McNamara M.G., Šeruga B., Vera-Badillo F.E., Aneja P., Ocaña A., et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J. Natl. Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 10.Li Z., Zhao R., Cui Y., Zhou Y., Wu X. The dynamic change of neutrophil to lymphocyte ratio can predict clinical outcome in stage I-III colon cancer. Sci. Rep. 2018;8(1):9453. doi: 10.1038/s41598-018-27896-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao H.L., Chen J.W., Li M., Xiao Y.B., Fu J., Zeng Y.X., et al. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients' adverse prognosis. PLoS One. 2012;7(1):e30806. doi: 10.1371/journal.pone.0030806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galdiero M.R., Bianchi P., Grizzi F., Di Caro G., Basso G., Ponzetta A., et al. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int. J. Cancer. 2016;139(2):446–456. doi: 10.1002/ijc.30076. [DOI] [PubMed] [Google Scholar]
- 13.Li L., Yu R., Cai T., Chen Z., Lan M., Zou T., et al. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int. Immunopharmacol. 2020;88 doi: 10.1016/j.intimp.2020.106939. [DOI] [PubMed] [Google Scholar]
- 14.Yang L., Dong Y., Li Y., Wang D., Liu S., Wang D., et al. IL-10 derived from M2 macrophage promotes cancer stemness via JAK1/STAT1/NF-κB/Notch1 pathway in non-small cell lung cancer. Int. J. Cancer. 2019;145(4):1099–1110. doi: 10.1002/ijc.32151. [DOI] [PubMed] [Google Scholar]
- 15.Deng X.X., Jiao Y.N., Hao H.F., Xue D., Bai C.C., Han S.Y. Taraxacum mongolicum extract inhibited malignant phenotype of triple-negative breast cancer cells in tumor-associated macrophages microenvironment through suppressing IL-10 /STAT3 / PD-L1 signaling pathways. J. Ethnopharmacol. 2021;274 doi: 10.1016/j.jep.2021.113978. [DOI] [PubMed] [Google Scholar]
- 16.Chen L., Shi Y., Zhu X., Guo W., Zhang M., Che Y., et al. IL‑10 secreted by cancer‑associated macrophages regulates proliferation and invasion in gastric cancer cells via c‑Met/STAT3 signaling. Oncol. Rep. 2019;42(2):595–604. doi: 10.3892/or.2019.7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yajuk O., Baron M., Toker S., Zelter T., Fainsod-Levi T., Granot Z. The PD-L1/PD-1 axis blocks neutrophil cytotoxicity in cancer. Cells. 2021;10(6) doi: 10.3390/cells10061510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo I.Y., Yang Y.E., Yang P.S., Tsai Y.J., Tzeng H.T., Cheng H.C., et al. Converged Rab37/IL-6 trafficking and STAT3/PD-1 transcription axes elicit an immunosuppressive lung tumor microenvironment. Theranostics. 2021;11(14):7029–7044. doi: 10.7150/thno.60040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 21.Turley S.J., Cremasco V., Astarita J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 2015;15(11):669–682. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- 22.Gershkovitz M., Caspi Y., Fainsod-Levi T., Katz B., Michaeli J., Khawaled S., et al. TRPM2 mediates neutrophil killing of disseminated tumor cells. Cancer Res. 2018;78(10):2680–2690. doi: 10.1158/0008-5472.Can-17-3614. [DOI] [PubMed] [Google Scholar]
- 23.Moroy G., Alix A., Sapi J., Hornebeck W., Bourguet E. Neutrophil elastase as a target in lung cancer. Anti-cancer agents in medicinal chemistry (2012), 12(6):565–579. 10.2174/187152012800617696. [DOI] [PubMed]
- 24.Kargl J., Busch S., Yang G., Kim K., Hanke M., Metz H., et al. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat. Commun. 2017;8:14381. doi: 10.1038/ncomms14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mollinedo F. Neutrophil degranulation, plasticity, and cancer metastasis. Trends Immunol. 2019;40(3):228–242. doi: 10.1016/j.it.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Lee J., Lee D., Lawler S., Kim Y. Role of neutrophil extracellular traps in regulation of lung cancer invasion and metastasis: structural insights from a computational model. PLoS Comput. Biol. 2021;17(2) doi: 10.1371/journal.pcbi.1008257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aloe C., Wang H., Vlahos R., Irving L., Steinfort D., Bozinovski S. Emerging and multifaceted role of neutrophils in lung cancer. Transl. Lung Cancer Res. 2021;10(6):2806–2818. doi: 10.21037/tlcr-20-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell K., Diao L., Karpinets T., Negrao M., Tran H., Parra E., et al. Neutrophil expansion defines an immunoinhibitory peripheral and intratumoral inflammatory milieu in resected non-small cell lung cancer: a descriptive analysis of a prospectively immunoprofiled cohort. J. Immunother. Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou S.L., Yin D., Hu Z.Q., Luo C.B., Zhou Z.J., Xin H.Y., et al. A positive feedback loop between cancer stem-like cells and tumor-associated neutrophils controls hepatocellular carcinoma progression. Hepatology. 2019;70(4):1214–1230. doi: 10.1002/hep.30630. [DOI] [PubMed] [Google Scholar]
- 30.Wang R., Lu M., Zhang J., Chen S., Luo X., Qin Y., et al. Increased IL-10 mRNA expression in tumor-associated macrophage correlated with late stage of lung cancer. J. Exp. Clin. Cancer Res. 2011;30(1):62. doi: 10.1186/1756-9966-30-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutchins A.P., Diez D., Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief Funct. Genom. 2013;12(6):489–498. doi: 10.1093/bfgp/elt028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang C., He L., He P., Liu Y., Wang W., He Y., et al. Increased drug resistance in breast cancer by tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling pathway. Med. Oncol. 2015;32(2):1–8. doi: 10.1007/s12032-014-0352-6. [DOI] [PubMed] [Google Scholar]
- 33.Van Schaeybroeck S., Kalimutho M., Dunne P.D., Carson R., Allen W., Jithesh P.V., et al. ADAM17-dependent c-MET-STAT3 signaling mediates resistance to MEK inhibitors in KRAS mutant colorectal cancer. Cell Rep. 2014;7(6):1940–1955. doi: 10.1016/j.celrep.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 34.Wu J.C., Wang C.T., Hung H.C., Wu W.J., Wu D.C., Chang M.C., et al. Heteronemin is a novel c-Met/STAT3 inhibitor against advanced prostate cancer cells. Prostate. 2016;76(16):1469–1483. doi: 10.1002/pros.23230. [DOI] [PubMed] [Google Scholar]
- 35.Siveen K.S., Sikka S., Surana R., Dai X., Zhang J., Kumar A.P., et al. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim. Biophys. Acta BBA Rev. Cancer. 2014;1845(2):136–154. doi: 10.1016/j.bbcan.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Mantovani A., Cassatella M.A., Costantini C., Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;11(8):519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.