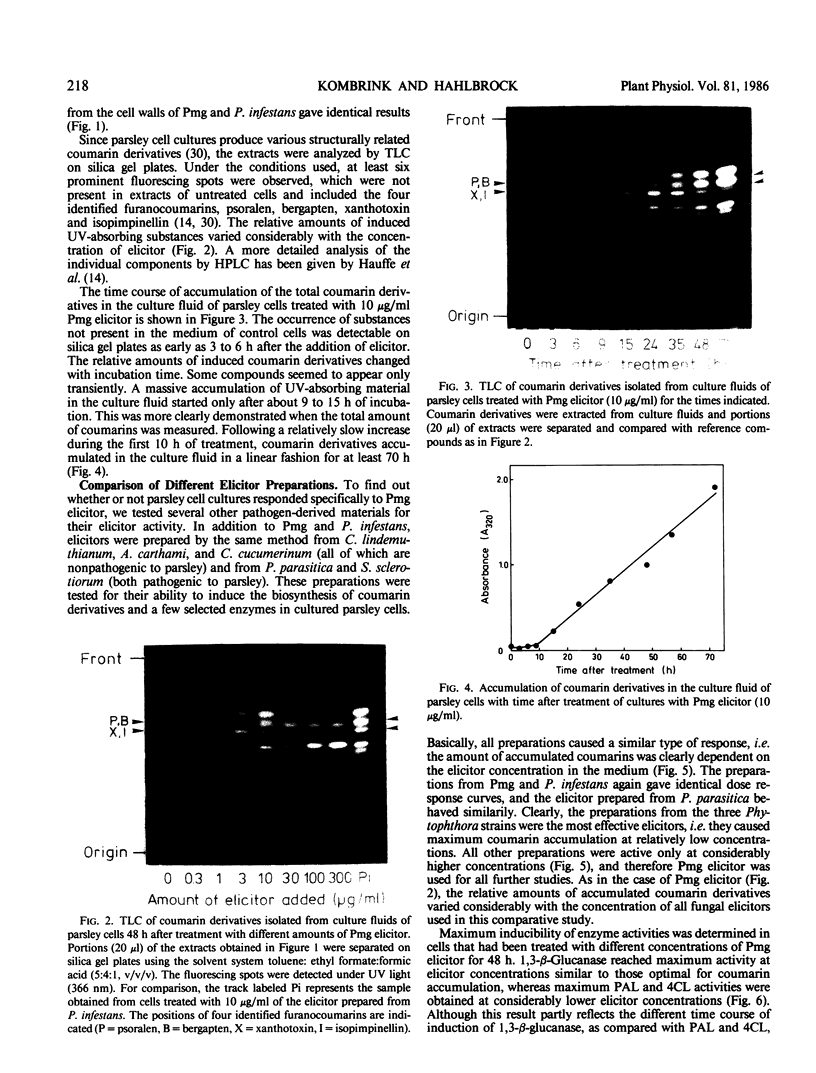
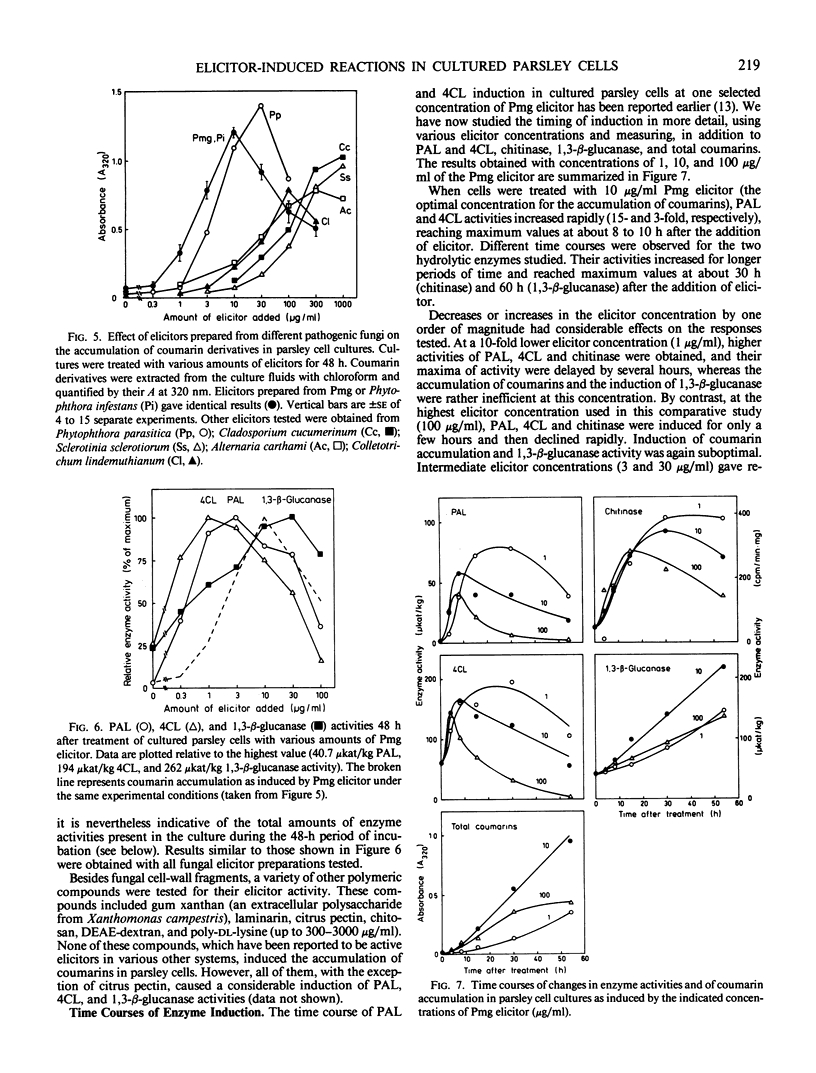
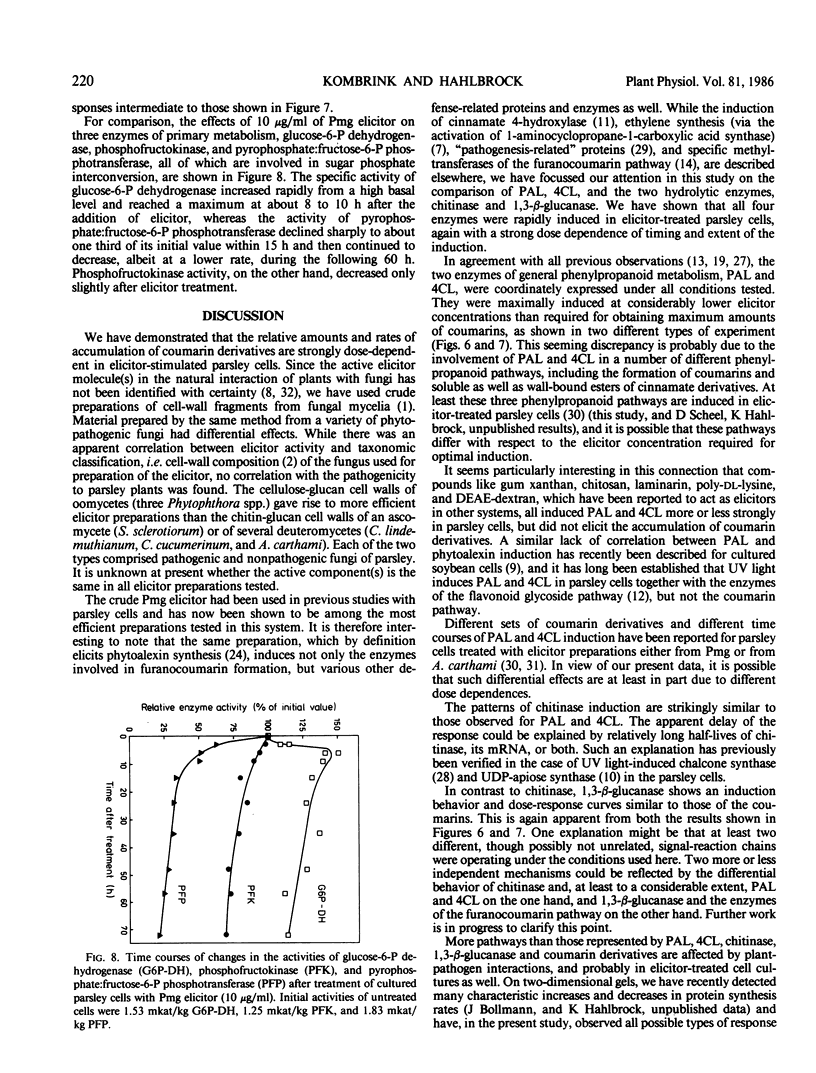
Abstract
Cultured parsley cells (Petroselinum crispum) responded to treatment with heat-released soluble cell-wall fragments (elicitors) from several different phytopathogenic fungi by forming coumarin derivatives (phytoalexins). This response was preceded in all cases by large but transient increases in the activities of two enzymes of general phenylpropanoid metabolism, phenylalanine ammonia-lyase (PAL) and 4-coumarate:CoA ligase (4CL). The activities of two hydrolytic enzymes, chitinase and 1,3-β-glucanase, also increased strongly in elicitor-treated cells, whereas the activities of three enzymes participating in primary metabolism were affected differently by the elicitor treatment. Glucose-6-phosphate dehydrogenase increased, phosphofructokinase remained almost constant, and pyrophosphate:fructose-6-phosphate phosphotransferase declined sharply in activity. Different amounts of cell-wall preparations from various phytopathogenic fungi were required for maximum elicitor activity. While three oomycetes (Phytophthora spp.) yielded the most active elicitors studied (maximum coumarin accumulation at concentrations of about 10 microgram per milliliter), cell-wall preparations from an ascomycete and three deuteromycetes gave comparable results only at 10 to 100 times higher concentrations. Optimal induction of PAL, 4CL, and chitinase with Phytophthora elicitor required only about 1 microgram per milliliter, whereas 1,3-β-glucanase induction showed a dose dependence similar to that observed for coumarins. The elicitor concentration had pronounced effects not only on the extent, but also on the timing of all induced reactions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers A. R., Ebel J., Valent B., Albersheim P. Host-Pathogen Interactions: X. Fractionation and Biological Activity of an Elicitor Isolated from the Mycelial Walls of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):760–765. doi: 10.1104/pp.57.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Börner H., Grisebach H. Enzyme induction in soybean infected by Phytophthora megasperma f.sp. glycinea. Arch Biochem Biophys. 1982 Aug;217(1):65–71. doi: 10.1016/0003-9861(82)90479-9. [DOI] [PubMed] [Google Scholar]
- Ebel J., Schmidt W. E., Loyal R. Phytoalexin synthesis in soybean cells: elicitor induction of phenylalanine ammonia-lyase and chalcone synthase mRNAs and correlation with phytoalexin accumulation. Arch Biochem Biophys. 1984 Jul;232(1):240–248. doi: 10.1016/0003-9861(84)90540-x. [DOI] [PubMed] [Google Scholar]
- Gardiner S. E., Schröder J., Matern U., Hammer D., Hahlbrock K. mRNA-dependent regulation of UDP-apiose synthase activity in irradiated plant cells. J Biol Chem. 1980 Nov 25;255(22):10752–10757. [PubMed] [Google Scholar]
- Hagmann M. L., Heller W., Grisebach H. Induction and characterization of a microsomal flavonoid 3'-hydroxylase from parsley cell cultures. Eur J Biochem. 1983 Aug 15;134(3):547–554. doi: 10.1111/j.1432-1033.1983.tb07601.x. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K., Knobloch K. H., Kreuzaler F., Potts J. R., Wellmann E. Coordinated induction and subsequent activity changes of two groups of metabolically interrelated enzymes. Light-induced synthesis of flavonoid glycosides in cell suspension cultures of Petroselinum hortense. Eur J Biochem. 1976 Jan 2;61(1):199–206. doi: 10.1111/j.1432-1033.1976.tb10012.x. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K., Lamb C. J., Purwin C., Ebel J., Fautz E., Schäfer E. Rapid Response of Suspension-cultured Parsley Cells to the Elicitor from Phytophthora megasperma var. sojae: INDUCTION OF THE ENZYMES OF GENERAL PHENYLPROPANOID METABOLISM. Plant Physiol. 1981 Apr;67(4):768–773. doi: 10.1104/pp.67.4.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen N. T. Specific elicitors of plant phytoalexin production: detenninants of race specificity in pathogens? Science. 1975 Jan 10;187(4171):74–75. doi: 10.1126/science.187.4171.74. [DOI] [PubMed] [Google Scholar]
- Keen N. T., Yoshikawa M. beta-1,3-Endoglucanase from Soybean Releases Elicitor-Active Carbohydrates from Fungus Cell Walls. Plant Physiol. 1983 Mar;71(3):460–465. doi: 10.1104/pp.71.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E., Kruger N. J., Beevers H. Kinetic properties of pyrophosphate:fructose-6-phosphate phosphotransferase from germinating castor bean endosperm. Plant Physiol. 1984 Feb;74(2):395–401. doi: 10.1104/pp.74.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D. N., Chappell J., Boudet A., Hahlbrock K. Induction of phenylalanine ammonia-lyase and 4-coumarate:CoA ligase mRNAs in cultured plant cells by UV light or fungal elicitor. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1102–1106. doi: 10.1073/pnas.81.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch F., Hadwiger L. A., Boller T. Ethylene: Symptom, Not Signal for the Induction of Chitinase and beta-1,3-Glucanase in Pea Pods by Pathogens and Elicitors. Plant Physiol. 1984 Nov;76(3):607–611. doi: 10.1104/pp.76.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragg H., Kuhn D. N., Hahlbrock K. Coordinated regulation of 4-coumarate:CoA ligase and phenylalanine ammonia-lyase mRNAs in cultured plant cells. J Biol Chem. 1981 Oct 10;256(19):10061–10065. [PubMed] [Google Scholar]
- Schröder J., Kreuzaler F., Schäfer E., Hahlbrock K. Concomitant induction of phenylalanine ammonia-lyase and flavanone synthase mRNAs in irradiated plant cells. J Biol Chem. 1979 Jan 10;254(1):57–65. [PubMed] [Google Scholar]
- Tietjen K. G., Hunkler D., Matern U. Differential response of cultured parsley cells to elicitors from two non-pathogenic strains of fungi. 1. Identification of induced products as coumarin derivatives. Eur J Biochem. 1983 Mar 15;131(2):401–407. doi: 10.1111/j.1432-1033.1983.tb07277.x. [DOI] [PubMed] [Google Scholar]
- Tietjen K. G., Matern U. Differential response of cultured parsley cells to elicitors from two non-pathogenic strains of fungi. 2. Effects on enzyme activities. Eur J Biochem. 1983 Mar 15;131(2):409–413. doi: 10.1111/j.1432-1033.1983.tb07278.x. [DOI] [PubMed] [Google Scholar]