Abstract
Background:
One strategy to reduce the burden of cardiovascular disease is the early detection and treatment of atherosclerosis. This has led to significant interest in studies of subclinical atherosclerosis, using different phenotypes, not all of which are accurate reflections of the presence of asymptomatic atherosclerotic plaques. The aim of part 2 of this series is to provide a review of the existing literature on purported measures of subclinical disease and recommendations concerning which tests may be appropriate in the prevention of incident cardiovascular disease.
Methods:
We conducted a critical review of measurements used to infer the presence of subclinical atherosclerosis in the major conduit arteries and focused on the predictive value of these tests for future cardiovascular (CV) events, independent of conventional cardiovascular risk factors, in asymptomatic people. The emphasis was on studies with >10,000 person-years of follow up, with meta-analysis of results reporting adjusted hazard ratio (HR) with 95% confidence intervals. The arterial territories were limited to carotid, coronary, aorta and lower limb arteries.
Results:
In the carotid arteries, the presence of plaque (8 studies) was independently associated with future stroke (pooled HR 1.89 [1.04,3.44]) and cardiac events (7 studies), with a pooled HR 1.77 [1.19,2.62]. Increased coronary artery calcium (5 studies) was associated with the risk of coronary heart disease events, pooled HR 1.54 [1.07,2.07] and increasing severity of calcification (by Agaston score) was associated with escalation of risk (13 studies). An ankle/brachial index of <0.9, the pooled HR for CV death from 7 studies was 2.01 [1.43,2.81]. There were insufficient studies of either, thoracic or aortic calcium, aortic diameter or femoral plaque to synthesise the data based on consistent reporting of these measures.
Conclusions:
The presence of carotid plaque, coronary artery calcium or abnormal ankle pressures appear to be valid indicators of the presence of subclinical atherosclerosis and may be considered for use in biomarker, Mendelian randomisation and similar studies.
Graphical Abstract
Background
The identification and reduction of significant atherosclerosis before it becomes symptomatic should be an important public health goal. Asymptomatic atherosclerosis is widely termed subclinical atherosclerosis and this has been defined at the histological level in part 11 . There is a large variety of tests purporting to either directly assess or be surrogate markers of subclinical atherosclerosis. These measures appear to significantly predict future atherosclerotic events such as myocardial infarction, stroke and chronic limb threatening ischemia. In the pursuit of novel intervention targets, diverse tests are being used to identify new biomarkers and biologic pathways to predict the course of subclinical atherosclerosis and future atherosclerotic events. However, insufficient attention has been given either to the validity of using these tests as markers of subclinical atherosclerosis or to their accuracy and repeatability.
The advance in biological technology has led to an ever-increasing array of candidate biomarkers and genetic loci for subclinical atherosclerosis. In this journal alone there have been 312 citations for such biomarker papers in the last 5 years (2018–22). The outcome measures, used in both these and Mendelian randomization studies vary, from skin autofluorescence to carotid plaque. Some of these outcomes do measure asymptomatic atherosclerosis but many appear to reflect other adaptive pathophysiology.
The aims of the second part of this report are to describe:
How the burden of subclinical atherosclerosis can be evaluated, preferably non-invasively, by any specific measurement to predict future atherosclerotic events and death in people without known cardiovascular disease, independent of traditional cardiovascular risk factors.
The advantages and disadvantages of the different measurement modalities with respect to population diversity, ease of measurement and repeatability.
Tests that assess vascular function or structure but do not measure subclinical atherosclerosis per se.
Methods
A series of critical reviews were undertaken of measurements used to infer the presence of subclinical atherosclerosis in the major conduit arteries. The presence of subclinical atherosclerosis was assessed from the ability of these measurements to predict future cardiovascular events, independent of traditional cardiovascular risk factors. The cardiovascular events were defined as those with target organ damage (i.e. stroke, myocardial infarction and chronic limb threatening ischemia), as well as cardiovascular death. The arterial territories investigated were limited to carotid, coronary, and femoral arteries, as well as the aorta.
The populations of particular interest were the general population, aged 18–70 years, without known cardiovascular disease and included men and women and all ethnic groups. The emphasis was on longitudinal studies with >10,000 person-years of follow up and those reporting since 2000. Studies could include comparison of people with a positive test versus those with a negative test and studies comparing different extent or severity of the specific measurement. The outcomes included were the ability to predict future cardiovascular events, independent of traditional cardiovascular risk factors, as reported by adjusted hazard ratios with 95% confidence intervals (CI). Where studies reported at several different timepoints, the study with the largest number of events and/or longest follow up was selected. Meta-analysis of results, with Forest plots was undertaken In the absence of significant heterogeneity of study design for 3 or more studies (e.g., heterogeneity in definition of outcome variable), quantitative pooled meta-analyses were conducted using inverse-variance method with a random-effects model with Hartung-Knapp (HK) adjustment and using the Paule-Mandel estimator for taû2.2,3 Heterogeneity was assessed using a chi2 test and the I2 statistic. A p value of <0.05 was considered significant. In addition to 95% confidence intervals, a prediction interval is presented. This indicates the interval within which the effect size of a new study may fall, if randomly selected from the same population.4 The Forest plots also show the prediction interval, which is the range in which the point estimate of 95% of future studies is expected to fall (assuming that the effect sizes are normally distributed).
The results are presented by arterial territory, cephalad to caudad, with recommendations for their use or non-use. The list of other tests, which do not measure the presence of asymptomatic or subclinical atherosclerosis was agreed by discussion.
Carotid artery plaque and other characteristics
Measures and imaging of carotid arteries
The presence of plaque at the carotid artery bifurcation has been extensively studied due to its role in ischemic stroke and the superficial location of the carotid arteries, thereby allowing for non-invasive imaging. Measures of carotid artery abnormalities reported include plaque presence or absence, plaque quantification (e.g. area, calcium score, stenosis severity) and morphology (ulcers or erosions, plaque density, fibrous cap thickness, plaque hemorrhage and inflammation).
Carotid plaque is most frequently assessed using ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI). Intima-media thickness has also been widely studied, although this has been disputed as a measure of subclinical atherosclerosis as intimal thickening may be a compensatory response to increased pressure and not necessarily progressive or associated with thrombo-embolic events.5–7 Each of the aforementioned imaging modalities has advantages and disadvantages (Table 1).
Table 1:
Advantages and disadvantages of different imaging modalities for assessing subclinical carotid atherosclerosis
| Modality | Advantages | Disadvantages |
|---|---|---|
| Ultrasound | Well suited to assess superficially placed carotid arteries; low cost; no radiation; | Standardization of imaging findings more difficult; resolution less than CT/ MRI |
| MRI | High resolution; no radiation exposure; | Higher cost; not as widely available as US |
| CT | High resolution; can allow multiple assessment of different arteries rapidly | Radiation exposure; Higher cost |
Previous studies assessing the association of proposed markers of subclinical carotid atherosclerosis with cardiovascular events in large asymptomatic community populations have predominantly used ultrasound. Supplemental Table 1 summarizes the findings of 18 community studies. The most common measure of subclinical atherosclerosis used was the presence or absence of carotid plaque. The definition of plaque has varied from a visualized protrusion causing some diameter reduction of the artery lumen,8 a protrusion into the artery lumen of at least 0.5mm or 50% of the surrounding intima-media thickness,9 to intimal thickening of at least 1mm10 or greater than 1.2mm11 or 1.5mm, 12,13 or focal widening of the artery compared to adjacent sites.14 A number of the studies, such as Chin-Shan Community Cardiovascular Cohort Study (CCCC),15 Monitoring of Trends and Determinants of Cardiovascular Diseases (MONICA)16 and Rotterdam14 have used plaque scoring systems to grade the severity or number of plaques present. Other studies such as Arteris Cardiovascular Outcome cohort study (ARCO)10 and Tromso17 have measured total plaque area.
Repeatability of measures of carotid plaque
The repeatability of the different measures of carotid abnormalities have been variably reported (Supplemental Table 1). The intra-observer agreement for identification of carotid plaque has been good or excellent (kappa values of 0.67,14 0.7812,13 and 0.8318), while that for inter-observer agreement has been moderate to excellent (kappa 0.5412,13 and 0.8918). Agreement on plaque area or severity score was substantial (kappa 0.6910 and 0.7015).
Association of carotid plaque measurements with atherosclerosis-related clinical events
Some8,9,12,19–21 but not all15,22–24 of the large cohort studies that tested whether presence of carotid plaque confers increased stroke risk found a positive association (Table 2). The presence of carotid plaque has also been associated with increased risk for myocardial infarction or coronary events in most9,13,25,26 but not all studies.22,23,27 A pooled meta-analysis (Figure 1), using adjusted hazard ratios from the individual studies, showed that carotid plaque presence was associated with both increased stroke risk (HR 1.89, 95% CI 1.04, 3.44, P=0.04, I2 0.0%; 8 studies, n=23,792; including 12,635 women and 11,157 men) and with increased coronary heart disease-related events (HR 1.77, 95% CI 1.19, 2.62, P=0.01, I2 0.0%; 7 studies, n=24,115; including 12,392 women and 11,723 men). An association with cardiovascular death was also detected, but this fell short of statistical significance (HR 1.72, 95% CI 0.84, 3.52, P=0.08, I2 0.0%; 3 studies, n=13,054; including 6,312 women and 6,742 men). Of note, the covariates included in these analyses varied among studies, but typically included age, sex and traditional cardiovascular risk factors. Changes in the c-statistic or net reclassification index are reported in the Supplemental Table 2.
Table 2:
Association of ultrasound measures of carotid imaging parameters with major adverse cardiovascular events examined in large community populations
| Study [ref] | Country, Year of publication | Population (% men, mean age) | Follow-up (years) | Outcome (n=) | Measurement | Adjusted HR (95% CI)* | Ethnicity and/or other comments |
|---|---|---|---|---|---|---|---|
| AGES-Reykjavik8 | Iceland, 2022 | 1836 (38%, 74.6y) | 5.2 | Stroke (258) | Plaque | 1.27 (1.01, 1.59) | NR |
| ARCO10 | Switzerland, 2021 | 2842 (62%, 50.0y) | 5.9 | CHD or Stroke (78) | Plaque area (tertiles) | 3rd tertile: 21.4 (2.8, 13.6) | NR |
| ARIC12,13 | USA 2001 | 12735 (45%, 54.1y) | 7.0 | CHD (399) | Plaque (with or without acoustic shadowing (AS)) | Plaque without AS: Female: 1.78 (1.22, 2.60) Male: 1.59 (1.22, 2.07) Plaque with AS: Female: 3.08 (1.91, 4.97) Male 1.65 (1.12, 2.42) |
73% White, 27% Black |
| ARIC11 | USA 2001 | 13123 (45%, 54.0y) | 8.0 | Stroke (226) | Plaque (with or without AS) | Lesion without AS: Women: 1.52 (0.97, 2.39), Men: 1.77 (1.21, 2.60) Lesion with AS: Women: 2.85 (1.60, 5.06), Men: 1.53 (0.89, 2.62) |
73% White, 27% Black |
| ARIC146 | USA 2010 | 13145 (43%, 54.1y) | 15.1 | CHD (1812) | Plaque | 75% White, 25% Black | |
| BLSA9 | China 2018 | 1376 (45%, 69.4y) | 5.2 | CVD (202) CHD (70) Stroke (136) |
Plaque | 2.29 (1.51, 3.48) 2.68 (1.19, 6.05) 2.17 (1.34, 3.52) |
NR |
| CCCC15 | Taiwan, 2016 | 1398 (44%, 55.9y) |
13.4 | CHD (49) Stroke (57) |
Plaque | 0.82. (0.40, 1.68) 1.30 (0.72, 2.36) |
NR |
| CCCC28 | Taiwan, 2008 | 2190 (45%, 54.1y) | 10.5 |
CHD (68) Stroke (94) |
Plaque score (0–4) | Per 1 point increase: 1.15 (1.06, 1.23) 1.11 (1.05, 1.18) |
NR |
| CHS147 | USA 2014 | 4384 (39%, 51.6y) | 10 | Stroke (482) CVD (1510) |
Plaque | Intermediate-risk plaque: 1.57 (1.15, 2.15) High-risk plaque 1.76 (1.34, 2.30) Intermediate-risk plaque: 1.45 (1.22, 1.71) High-risk plaque 1.749 (1.29, 1.72)) |
86% White, 14% Black |
| CHU Rangueil, Toulouse25 | France, 2009 | 2561 (58%, 51.6y) | 6 | CHD (94) | Plaque | 2.81 (1.84, 4.29) | NR |
| CIRCS29 | Japan 2020 | 2943 (35%, 65.1y) | 15.1 | Stroke (186) CHD (77) CVD (255) |
Plaque (homogenous vs. heterogenous) | Homogenous: 1.35 (0.91, 2.01) Heterogenous: 1.58 (1.09, 2.30) Homogenous: 1.03 (0.51, 2.07) Heterogenous: 2.11 (.20, 3.70) Homogenous: 1.25 (0.88, .77) Heterogenous: 1.71 (1.25, 2.35) |
NR |
| IMPROVE148 | Finland, France, Italy, Netherlands, Sweden, 2017 | 3703 (48%, 64.2y) | 3.0 | CVD (215) Stroke (73) CHD (125) |
Maximum IMT | Q4 vs. Q1–3 1.98 (1.47,2.67) 2.76 (1.66, 4.60) 1.58 (1.06, 2.37) |
NR |
| Kailuan22 | China 2022 | 4899 (60%, 54.2y) | 5.7 | MI or Stroke (167) Stroke (127) MI (40) |
Plaque | 1.27 (0.38, 4.28) 1.51 (0.24, 9.13) 2.16 (0.62, 7.53) |
NR |
| MDCS11 | Sweden, 2005 | 5163 (41%, 57.5y) | 7 | CHD (113) | Plaque | 1.81 (1.14, 2.87) | NR |
| MESA19 | USA 2015 | 6779 (47%, 65.2y) | 9.5 | CVD (538) CHD (388) Stroke (196) |
Plaque | 1.61 (1.17, 2.21) 1.76 (1.23, 2.52) 1.40 (1.35, 1.45) |
39% White, 28% Black, 22% Hispanic, and 12% Chinese |
| MESA20 | USA 2013 | 6562 (47%, 61.2y) | 7.8 | CVD (515) CHD (372) Stroke (139) |
Plaque | 1.45 (1.20, 1.76) 1.67 (1.33, 2.10) 1.11 (0.76, 1.62) |
39% White, 28% Black, 22% Hispanic, and 12% Chinese |
| MESA30 | USA 2017 | 4955 (43%, 61.6y) | 11.3 | CVD (487) CHD (348) Stroke (175) |
Plaque score (0–12) | Per SD increase: 1.27 (1.16, 1.40) 1.35 (1.21, 1.51) 1.15 (0.98, 1.35) |
39% White, 28% Black, 22% Hispanic, and 12% Chinese |
| MESA18 | USA 2021 | 2673 with zero baseline coronary artery calcium score (36%, 58.0y) | 16.1 | CHD (79) Stroke (80) |
Plaque |
1.66 (1.04, 2.66) 0.64 (0.39, .104) |
34% White, 30% Black, 24% Hispanic, and 12% Chinese |
| MONICA16 | Germany, 2006 | 1325 (51%, 49.7y) | 12.7 | MI (58) CV death (86) |
Plaque score (0–4) assessed at carotid and femoral arteries | Per 1 score increase: 1.20 (0.97, 1.50) 1.44 (1.18, 1.75) |
NR |
| NOMAS23 | USA 2006 | 1939 (41%, 69.0y) | 6.2 | Stroke (69) MI (102) |
Plaque | 1.6 (0.9, 2.9) 1.3 (0.8, 2.2) |
53% Hispanic, 25% Black, 22% White |
| NOMAS27 | USA 2008 | 2189 (40%, 68.0y) | 6.9 | Stroke (121) MI (118) |
Plaque (<1.9mm and >1.9mm) | <1.9mm: 0.78 (0.46, 1.35) >1.9 mm: 1.12 (0.66, 1.91) <1.9mm: 0.94 (0.52, 1.69) >1.9mm: 1.41 (0.81, 2.45) |
53% Hispanic, 25% Black, 22% White |
| Rotterdam14 | Netherlands, 2004 | 6389 (38%, 69.3y) | Not provided | MI (258) |
Plaque score (0–6) | Score=1 1.19 (0.75, 1.88) Score=2 1.28 (0.85, 1.94) Score>3 1.83 (1.27, 2.62) |
NR |
| Rotterdam24 | Netherlands, 2002 | 4217 (40%, 68.8y) | 5.2 | Stroke (160) | Plaque Plaque score (0–6) |
Presence of plaque 1.31 (0.90, 1.91) Per 1 score increase 1.13 (1.03, 1.24) |
NR |
| San Daniel Township149 | Italy 2008 | 1348 (47%, 48.0y) | 12.7 | Stroke or Vascular death (115) | Plaque | 10.4 (6.4, 17.1) | NR |
| Suita21 | Japan, 2018 | 4724 (46%, 59.7y) | 12.7 |
CVD (375) Stroke (221) |
Maximum IMT | Per SD increase 1.20 (1.10,1.30) 1.12 (0.99, 1.26) |
NR |
| Three City26 | France, 2011 | 5895 (37%, 73.9y) | 5.4 | CHD (223) |
Plaque | 1 plaque 1.5 (1.0, 2.2) >2 plaques 2.2 (1.6, 3.1) |
NR |
| Tromso17 | Norway, 2011 | 6584 (49%, 60.0y) | 10.8 | Stroke (397) | Plaque area | Per SD increase Men 1.23 (1.09, 1.38) Women 1.19 (1.04, 2.53) |
NR |
| Tromso150 | Norway, 2014 | 6257 (42%, 59.9y) | 15.4 | MI (894) |
Plaque area | Per SD increase 1.23 (1.15, 1.32) | NR |
NR ethnicity/race not reported, CHD coronary heart disease, CVD cardiovascular death, MI myocardial infarction.
Figure 1.
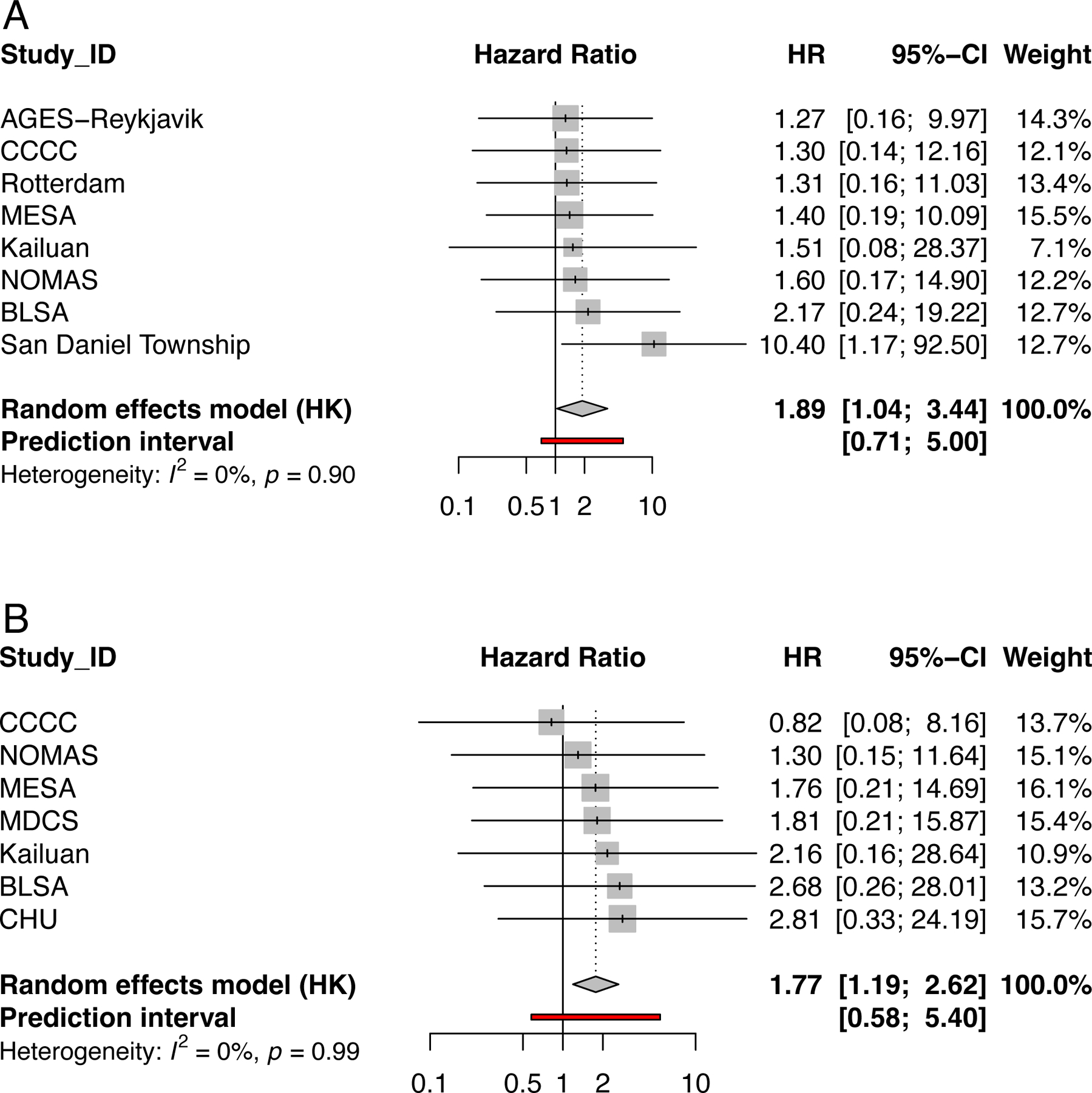
Forest plots illustrating the findings of a meta-analysis of included studies. a) Association of carotid plaque presence on ultrasound with risk of stroke in 8 studies, n=23,792; including 12,635 women and 11,157 men; b) Association of carotid plaque presence on ultrasound with risk of coronary heart related events in 7 studies, n=24,115; including 12,392 women and 11,723 men.
Among studies that examined features other than plaque presence/absence, larger plaque area was associated with increased risk of cardiovascular events.9,10 Other carotid plaque measures, including plaque score28 and heterogenous plaque29 have also been associated with cardiovascular events (Table 2). All studies included a mix of men and women with females ranging between 24%29 and 64%.18 Some of the studies performed sub-analyses to investigate sex differences in the associations found. For instance, the Atherosclerosis Risk in Communities (ARIC) study reported disparate findings in men and women.13 Carotid plaque with acoustic shadows typical of calcification were associated with significantly increased risk of stroke in women but not men, while carotid plaque without acoustic shadows were associated with significantly higher risk of stroke in men but not women.13 In contrast, plaques of both types were associated with significantly increased risk of cardiac events in both sexes.9 The Multi-Ethnic Study of Atherosclerosis (MESA) study found that presence of carotid plaque was associated with an increased risk of cardiovascular events in a mix of White, Black, Hispanic and Chinese Americans.18–20,30 Plaque was identified significantly more frequently in Caucasians than Chinese and Hispanic Americans.18 The Northern Manhattan Study (NOMAS) studied a mix of White, Black and Hispanic Americans and similarly reported plaque was significantly more frequent in Caucasians.27 However, associations of carotid plaque with risk of incident MI or stroke were not significantly increased in this population.23,27
A pooled meta-analysis was conducted using adjusted hazard ratios from the individual studies. This showed that carotid plaque presence was significantly associated with an increased risk of stroke (HR 1.89, 95% CI 1.04, 3.44, P=0.04, I2 0.0%; 8 studies, n=23,792; including 12,635 women and 11,157 men) and coronary heart disease events (HR 1.77, 95% CI 1.19, 2.62, P=0.01, I2 0.0%; 7 studies, n=24,115; including 12,392 women and 11,723 men) but not cardiovascular death (HR 1.72, 95% CI 0.84, 3.52, P=0.08, I2 0.0%; 3 studies, n=13,054; including 6,312 women and 6,742 men) [Figure 1]. Of note, the covariates included in the models varied but typically included age, sex and traditional risk factors for cardiovascular events.
Recommendations regarding the assessment of carotid plaque as a marker of subclinical atherosclerosis
Presence of carotid plaque can be reproducibly assessed and is associated with increased risk of stroke and coronary heart disease-related events, typically independent of other major cardiovascular risk factors. That said, a harmonization of how plaque is defined is recommended so that associations can be compared across studies. When ethnic/racial composition is reported, studies include a mix of White, Black, and Asian middle-aged and elderly men and women, with a relatively low number of Hispanics. It is anticipated that other forms of imaging such as MRI may be more widely used in the future, but this modality remains relatively expensive and time-consuming, limiting its use in large community populations. Further research is needed to show the consistency of the data reported here, examine ethnic and age differences in findings and determine the cost-effectiveness of examining asymptomatic populations for carotid plaque.
| Recommendation | Class | Level | Supporting References |
|---|---|---|---|
| Presence of carotid plaque, in middle aged to elderly men and women, may be considered to indicate the presence of subclinical atherosclerosis and is associated with increased risk of stroke and coronary heart disease events. There is no strong evidence to indicate whether this recommendation varies with race or ethnicity. | II | B |
Coronary artery calcium
Calcification of the coronary arteries increases with luminal narrowing and is particularly prevalent at sites of plaque rupture.1 The standard method for measuring coronary artery calcium (CAC) is scoring by the method using non-contrast cardiac gated computed tomography (CT). Using this method, the area of calcified plaque in the coronary arteries (defined as a density of >130 Hounsfield units [HU]) is multiplied by a density factor (1–4 based on peak attenuation: 1 for 130–199 HU, 2 for 200–299 HU, 3 for 300–399 HU, 4 for ≥400 HU) and then summed to produce an overall score for all coronary arteries.31 However, other methods for calcium scoring have also been developed. For example, the volumetric score was shown to be more reproducible than the Agatston score,32 but is not often used clinically. Importantly, the Agatston score is weighted upward by increased plaque density. However, plaque density is inversely associated with cardiovascular events,33,34 and many cardiovascular disease risk factors are differentially associated with CAC volume and density.35 This may explain why high-endurance athletes have increased prevalence of CAC36 and physical activity is associated with progression of CAC density37,38 as well as statin therapy being associated with increased CAC.39 Given this limitation, use of a volume score with adjustment for density consistently improves risk prediction33,40 regardless of CT scanner type.41 Additionally, use of mean density may improve risk prediction over peak density,42 but peak density may be more easily applied as it can be calculated from existing Agatston scores. Finally, the inverse association between density and events varies by level of overall CAC (i.e. significantly stronger at lower levels of CAC).42,43 The Agatston score, however, remains the clinical standard, and the remainder of this section will focus primarily on the Agatston score unless otherwise specified.
Normal and abnormal values
Given that CAC is closely associated with coronary atherosclerosis, any level of CAC may be considered abnormal. Indeed, the American College of Cardiology (ACC) / American Heart Association (AHA) Multi-Society cholesterol guidelines indicate that any level of CAC is considered an indication to consider statin therapy,44 as even low levels of CAC are associated with increased mortality risk when compared to having no CAC.45 Additionally, a CAC score of 0 is associated with very low mortality rates at 10 years,45 and with low annual risk for cardiovascular events.46 However, levels of calcium vary by age, sex and race/ethnicity. In this regard, data from the Multi-Ethnic Study of Atherosclerosis (MESA) were used to define age, sex, and race/ethnicity specific percentiles for CAC scores,47 and the percentile for an individual patient is typically provided in CAC scoring reports to provide context for the absolute score.
Reproducibility of measurements
CAC scoring is very reliable and highly reproducible in studies of multiple readers, duplicate scans, and scans using different scanner types. Imaging for CAC has been shown to be high quality, with good reproducibility and agreement between readers (κ 0.77–0.92).48 Use of calibration phantoms improves the comparability of studies between individuals by accounting for differences in body habitus and attenuation.49 Helical CT has been shown to correlate well with EBCT (electron beam computed tomography), the original standard for CAC scoring.50 Imaging results have also been shown to be highly reproducible on MDCT (multi-detector computed tomography) including newer generation MDCT scanners.51–53
Prediction of future atherosclerotic events
CAC scoring is a powerful predictor of future atherosclerotic cardiovascular events. A summary of key studies is provided in Table 3 with further details provided in Supplemental Table 3. CAC has been shown to be highly sensitive for CVD events54 and risk for cardiovascular events rises with increasing levels of CAC.55 As noted previously, the absence of CAC is a powerful prognostic indicator which is associated with a very-low 10-year mortality rate, and associated with lower mortality than even a CAC score of 1–10.45
Table 3:
Association of Coronary Artery Calcification with Atherosclerotic Events
| Study [ref] | Country Year of publication | Population (% men, mean age) | Follow-up (years) | Outcome | Measurement | Adjusted HR (95% CI) | Ethnicity and/or other comments |
|---|---|---|---|---|---|---|---|
| Arad54 | USA 1996 | 1173 (71%, 53y) | 19 months | ASCVD | Agatston | OR 25.8 (5.9–113) for CAC >100 (unadjusted) | |
| St. Francis Heart Study63 | USA 2005 | 4903 (65%, 59y) | 4.3 | ASCVD | Agatston | RR 9.6 (6.7–13.9) for CAC ≥100 | |
| PACC Project65 | USA 2005 | 2000 (82%, 43y) | 3 | CHD | Agatston | 11.82 (2.45–56.93) for any CAC | 56–72% White, 31–18% Black |
| Rotterdam Study56 | Netherlands 2005 | 1795 (42%, 71y) | 3.3 | CHD | Agatston | RR 3.1 (1.2–7.9) for CAC 101–400 | |
| Cooper Clinic58 | USA 2005 | 10746 (64%, 54y) | 3.5 | CHD | Agatston | 10.2 (3.0–35.4) for CAC 39–249 in men 4.6 (1.2–18.4) in women | >97% White |
| MESA55 | USA 2008 | 6722 (47%, 62y) | 3.8 | CHD | Agatston | 3.89 (1.72–8.79) for CAC 1–100 | 28% black 12% Chinese 22% Hispanic 39% white |
| Heinz Nixdorf Recall Study46 | Germany 2010 | 4129 (47.3%, 60y) | 5 | CHD | Agatston | RR 1.27 (1.22–1.41) | |
| Jackson Heart Study59 | USA 2016 | 2944 (35%, 60y) | CV disease | Agatston | OR 1.22 (1.12–1.32) | 100% black | |
| Framingham64 | USA 2016 | 3486 (49%, 50y) | 8 | CHD, CV disease | Agatston | 2.46 (1.75–3.48) for CHD | |
| CARDIA57 | USA 2017 | 5115 (45.4%, 40.3y) | 12.5 | CHD, CVD disease | Agatston | 5.0 (2.8–8.7) for CHD | 54.8% white |
| Western Denmark Heart Registry61 | Denmark 2020 | 23759 (44.6%, 57)y | 4.3 | CV disease | Agatston | 1.3 (1.1–1.5) for CAC 1–99 | |
| CAC Consortium68 | USA 2020 | 66636 (67%, 54y) | 12.5 | CHD and CV disease | Agatston | 5.44 (3.88–7.62) for CHD | 89% white |
| Dallas Heart Study62 | USA 2021 | 2191 (42%, 44y) | 12.2 | ASCVD | Agatston | 5.3 (3.6–7.9) for ASCVD, 2.6 (1.5–4.3) | 47% black |
| CAC Consortium60 | USA 2021 | 28025 (65%, 54y) | 11.2 | CHD and CV disease | Agatston | 1.4 (1.3–1.6) in non-statin, 1.2 (1.1–1.4) in statin users for CHD mortality | >94% white |
| MESA33 | USA 2014 | 3398 (58%, 66.4y) | 7.6 | CHD and CV disease | Volume / density | Volume: 1.81 (1.47–2.23) Density: 0.73 (0.58–0.91) for CHD |
24% Black, 12% Chinese, 20% Hispanic, 44% White individuals |
| MESA40 | USA 2017 | 3398 (58%, 66.4y) | 11.0 | CHD and CV disease | Volume / density | Volume: 1.73 (1.45–2.05), Density: 0.72 (0.60–0.86) for CHD |
24% Black, 12% Chinese, 20% Hispanic, 44% White individuals |
| CAC Consortium42 | USA 2021 | 10373 (76%, 53.4y) | 11.3 | ASCVD and CHD | Volume / density | OR 1.34 (1.17–1.54) for Agatston OR 1.37 (1.18–1.58) for score with mean density for CHD mortality |
97% White |
| MESA41 | USA 2022 | 3362 (58%, 66.5y) | 15.5 | CHD and CV disease | Volume / density | Volume: 1.92 (1.65–2.22) Density: 0.73 (0.63–0.86) for CHD |
|
| MESA43 | USA 2023 | 3316 (57.4%, 66.4y) | 16.9 | CHD | Volume / density | Volume quartile 4: 3.77 (2.48–5.74) Density quartile 4: 0.47 (0.32–0.69) |
CAC = coronary artery calcium, CHD coronary heart disease, CV cardiovascular, DHS = Dallas Heart Study, EBCT = electron beam computed tomography, MESA = Multi-Ethnic Study of Atherosclerosis.
CAC is predictive of events across a variety of populations. Specifically, CAC has been shown to be predictive in the elderly,56 young individuals,57 both men and women,58 as well as across racial/ethnic groups,55,59 and in statin users.60 Additionally, plaque burden, as represented by CAC scoring, may be a stronger predictor of cardiovascular events and death than the presence of obstructive coronary artery disease.61 CAC may even help to identify individuals more likely to benefit from aspirin for primary prevention. In an analysis of the risks and benefits associated with aspirin use, CAC >100 identified individuals more likely to derive a net benefit from aspirin.62
CAC scoring is also predictive of events independent of traditional risk factors,63,64 the Framingham risk score,64,65 and the Pooled Cohort Equations.41 CAC scoring improves risk prediction in comparison with several risk stratification tools. The CAC score has been shown to be more predictive of events than the Framingham risk score, and provided further risk stratification of individuals within Framingham predicted risk strata.63 CAC also improves risk prediction when added to traditional risk factors,55 the Framingham Risk Score,46,59,64 National Cholesterol Education Panel Adult Treatment Panel III categories,46 and the Pooled Cohort Equations.43 In the development of the Multi-Ethnic Study of Atherosclerosis (MESA) risk score, the addition of CAC improved risk prediction over risk factors alone.66
A prior study had suggested that CAC does not accurately predict coronary heart disease events in individuals at high predicted risk as the area under the curve (AUC) of the receiver operating characteristic (ROC) curve was not improved with the addition of calcium scoring.67 However, the follow-up period was relatively short (less than 4 years) and the Framingham model was used to predict risk. More recent studies have demonstrated that CAC is strongly associated with all-cause mortality and cardiovascular/CHD mortality across the spectrum of burden of cardiovascular risk factors.68 We performed a meta-analysis of studies of the association of CAC (assessed continuously or categorically) with coronary heart disease and cardiovascular disease events (Figure 2). In Figure 2A, there is a strong overall association between CAC and CHD events with a HR of 1.54 (95% CI 1.15–2.07). There is also a graded association between CAC and CHD events, with increasing risk with higher levels of CAC (Figure 2B). Lastly, a similar HR was found for CVD events but this did not meet statistical significance (HR 2.18, 95% CI 0.47–10.08).
Figure 2.
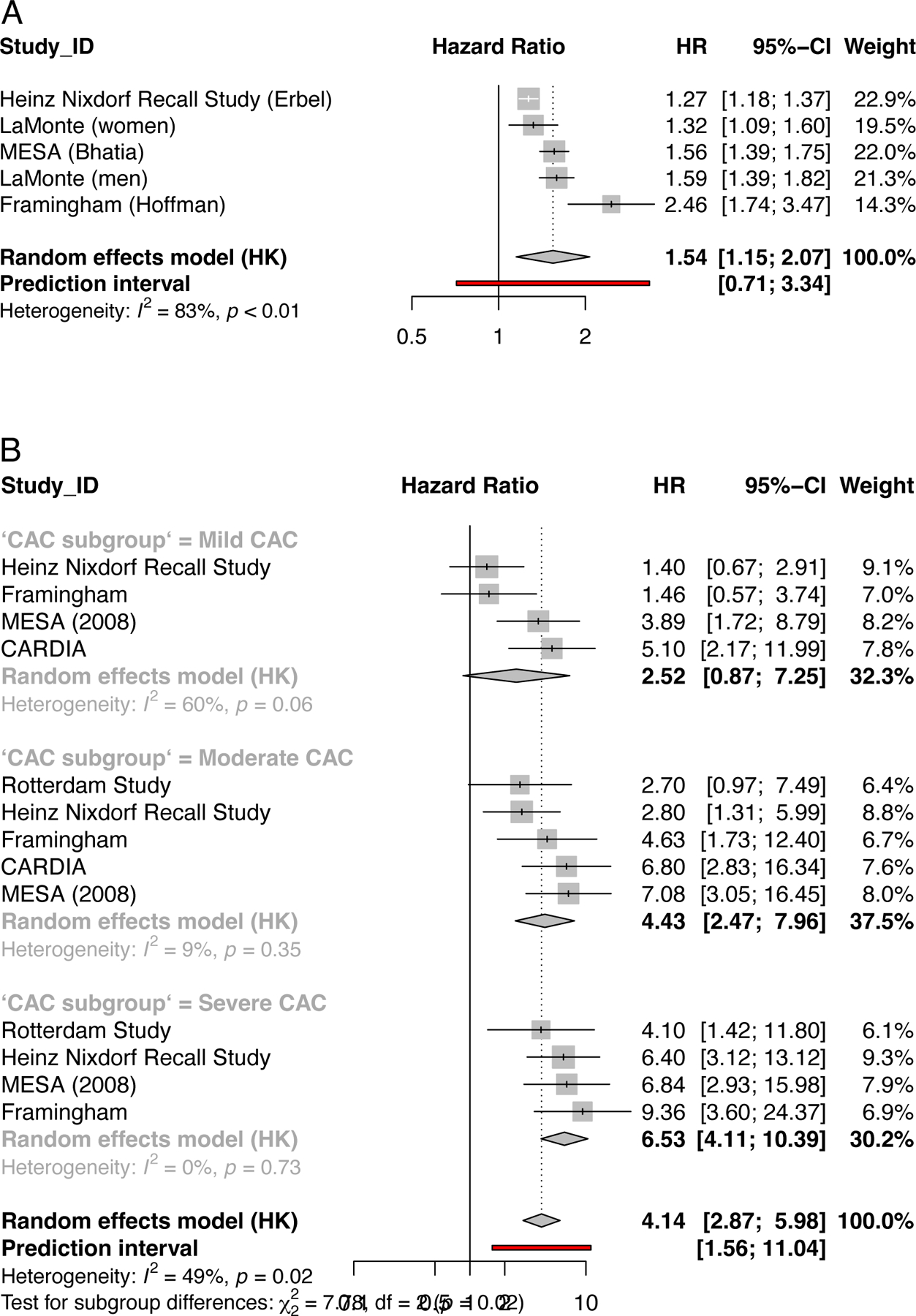
Forest plots illustrating the findings for coronary artery calcification (a) meta-analysis of 4 studies for the association of CAC severity with risk of future Coronary Heart Disease Events (n=21717). Studies included in this analysis: Erbel,46 LaMonte,58 Bhatia,41 Hoffman64. For the Heinz Nixdorf study relative risk (RR) was used. For the Heinz Nixdorf and Cooper Clinic studies, results are presented per unit of log-transformed CAC. For MESA studies, results are presented per 1-standard deviation increase in log-transformed CAC.
(b) a meta-analysis of the association of category of CAC (low moderate or severe) with risk of future events Coronary Heart Disease Events. The number of studies in each category ranged from 2 (n=10190) to 4 (n=16144). For the Heinz Nixdorf study relative risk (RR) was used.
Areas for further research
There are several areas for future research, including methods to optimize CAC scoring, particularly given the treatment of density in the Agatston score. As noted previously, the use of volume and density scoring, separately, improves risk prediction over the Agatston score, as well as over traditional risk factors.33,40 However, this has not been incorporated into typical clinical practice, likely because of the robust predictive value of the Agatston score, and the difficulty in incorporating two scores into a unified clinical assessment. As such, more research is needed in this area to optimize this method for clinical use. Notably, there may be other measures that can be captured by CAC scoring which may be considered for inclusion in scoring methods. For example, incorporation of the number of vessels with calcified plaque improves prediction when added to the Agatston score.69 Measurement of mean density may improve predictive value over the use of peak density.42
The use of coronary computed tomography angiography (CCTA) for risk stratification is an important area for further research. In the Miami Heart Study, 49% of asymptomatic individuals had prevalent coronary artery plaque by CCTA and, importantly, 16% of individuals with CAC of 0 had plaque (14.8% had non-calcified plaque), and 2.3% had high-risk plaque features, demonstrating the additional information which can be gained from CCTA versus CAC scoring.70
Recommendations and limitations
The most recent AHA/ACC Primary Prevention and Cholesterol guidelines recommend use of CAC scoring when there is uncertainty about the initiation of statin therapy in an intermediate risk individual. If no CAC is present, withholding statin therapy may be considered unless the individual has a history of diabetes, is a cigarette smoker or has a family history of premature CHD. If CAC is 1–99, statin therapy is favoured, and if CAC is ≥100 or ≥75th percentile, statin therapy is recommended.44,71 Similar recommendations are given in other major guidelines globally.72 As such, we agree with these recommendations, summarized below. However, one major limitation of prior studies and guidelines is the focus on 10-year risk; in younger individuals, a focus on long-term risk may be more appropriate, and it is not yet clear how CAC would fit into longer term risk assessment. Additionally, the radiation exposure and cost associated with CAC scoring must be considered, particularly when considering evaluation of younger individuals or serial evaluations.
| Recommendation | Class | Level | Supporting References |
|---|---|---|---|
| The presence of coronary artery calcium should be considered to indicate the presence of subclinical atherosclerosis in diverse populations of middle-aged or older men and women. | I | B |
Thoracic and abdominal aortic calcium
Aortic calcium in asymptomatic people is relatively common. Abdominal aortic calcium (AAC) is reported as frequently as in coronary arteries (55% and 56%, respectively). Thoracic aorta calcium (TAC) is reported in 39% of persons.73 Unlike the coronaries, aortic calcium is present in both the intimal and the medial vascular layers. Medial calcium is associated with heart failure and mortality, but not necessarily atherosclerotic events.74 The standard method of assessing aortic calcium is CT, usually cardiac CT, which is unable to differentiate between intimal and medial layers.75 This is one of several limitations for assessing the role of TAC and AAC. The thoracic and abdominal aorta are also much larger and heterogenous vascular beds than the coronary arteries. The abdominal aorta extends from the diaphragm to the bifurcation at the common iliac arteries, but studies use variable lengths to score AAC. The thoracic aorta includes three distinct sections: the ascending aorta, aortic arch and descending aorta segments. Calcium prevalence is low in the ascending aorta 76 and highest by proportion in the aortic arch 77; however, most studies do not capture the aortic arch given standard cardiac CT scan parameters. Some studies simply report presence or absence of calcium, some volume and density of calcium, but most use the Agatston scoring system developed for the coronary arteries as the method to report TAC and AAC data. Few studies report both TAC and AAC data in the same cohort. Given limitations at this time, this section is predominately confined to CT studies using Agatston score to assess calcium of the descending thoracic aorta and the abdominal aorta.
Normal and abnormal values
Normality is the absence of calcium. Compared to the coronary arteries, there is a higher degree of calcium in the thoracic and abdominal aorta.73 Abnormal ranges in the thoracic aorta have been considered as scores from 0 to 300 as low and greater than 300 as high.75 The abdominal aorta is one of the first vascular beds where calcium develops and typically has the highest degree of calcium with higher Agatston scores, though boundaries used to define normal and abnormal values are variable and studies have used tertiles within the cohort to define severity.78
Reproducibility of measurements
Most studies of aortic calcium assume the same reproducibility as for coronary artery calcium and do not report on the repeatability or reproducibility of the presence or scoring of TAC or AAC. For instance, a CARDIA study references the repeatability assessments for the coronary artery calcium score when assessing AAC.79 Other studies have reported that TAC scores are highly concordant and reproducible across different CAC scanner types with inter-reader variability of 3%−7.1% and interscan variability of 17–18%, as well as intrareader variability of 0.4–1.4%.80,81
Aortic calcification as a predictor of atherosclerotic events
As discussed in Part 11, the aorta is prone to subclinical calcium, but due to its larger diameter compared to other vascular beds, it is not prone to ischemic events from stenosis. However, it can be the source of both athero-emboli and thrombo-emboli that cause end-organ damage, including stroke. The aortic arch is particularly prone to atherosclerosis, 82 and is associated with stroke, subclinical cerebrovascular disease, adverse cardiovascular outcomes, and mortality.83 However, the prognostic value of TAC has been mixed (Table 4). In analyses from the Framingham Heart Study, TAC was assessed without the aortic arch was not a robust independent risk factor. In the Rotterdam Study, calcium in the aortic arch was associated with cardiovascular mortality, independent of calcium in other vascular beds.82 TAC may be more prognostically relevant if the CT field of view were expanded in more studies to include the aortic arch. TAC may also be more useful if a continuous score were consistently reported, rather than just presence versus absence or tertiles within the cohort. For instance, a TAC scores greater than 300 have been associated with CHD, stoke, and all-cause mortality, even after adjustment for conventional risk factors and CAC score; this was not seen at lower TAC scores. As such, caution is advised when interpreting and comparing studies that measure different anatomical sections and use different reporting methods.
Table 4:
Population-based studies of the association of n Thoracic Aortic Calcium and Cardiovascular Outcomes
| Study [ref] | Country, Year of Publication | Population (% men, mean age) | Follow-up (years) | Outcome (number of events) | Measurement | Adjusted HR (95% CI)* | Race/ethnicity & comments |
|---|---|---|---|---|---|---|---|
| MESA151 | USA 2015 | 5,903 (47%, 6y2) | 10.3 | CHD events (348) CHD mortality (65) |
Presence as part of extra-coronary calcium score; no aortic arch | 2.04 (1.5, 2.7) 2.66 (1.3, 5.7) |
41.4% White, 11.8% Chinese American, 25.9% African American, 20.8% Hispanic Nondiabetics |
| HNR152 | Germany 2016 | 3,630 (46%, 59y) |
9.9 | CV events (241) | Presence; no aortic arch | 1.30 (0.95, 1.8) | NR |
| San Diego73 | USA 2011 | 4,5y44 (57%, 57y) | 7.8 | CV events (40) |
Presence; with aortic arch | 3.01 (0.8, 10.9) |
NR |
| MESA84 | USA 2011 | 6,807 (47%, 62y) | 4.5 | CHD events (132) All CHD (232) |
Agatston; no aortic arch | Women: 2.42 (1.03, 5.6); Men: 1.34 (0.8, 2.2) Women: 3.04 (1.6, 5.8); Men: 1.26 (0.9, 1.8) |
38.2% White, 11.8% Chinese American, 27.9% African American, 22% Hispanic |
| FHS64 | USA 2016 | 3,486 (49%, 50y) | 8 | CHD events (59) CV events (107) |
Agatston; no aortic arch | 1.40 (1.1, 1.8) 1.18 (0.98, 1. |
NR |
| EISNER86 | USA 2009 | 2,303 (62%, 56y) | 4.4 | CHD events (16) All CHD (41) CV events (47) |
Agatston; no aortic arch | 1.20 (0.99, 1.4) 1.20 (1.04, 1.3) 1.1 (1.02, 1.3) |
NR |
| MESA153 | USA 2017 | 3,415 (37%, 56y) | 11.3 | CHD events (74) CV events (137) |
Agatston; no aortic arch | 0.94 (0.8, 1.1) 1.04 (0.9, 1.2) |
33% White, 11.7% Chinese American, 31.4% African American, 24% Hispanic No baseline CAC |
| CAC Consortium75 | USA 2021 | 30,630 (64%, 55y) | 11.2 | CV events (345) |
Agatston tertiles; no aortic arch | 4.23 (3.11, 5.76)^ | NR |
| NLST154 | USA 2019 | 5,718 (62%, 62y) | 2.7 | CV events (663) |
Agatston categories (highest level shown); with aortic arch | Men: 2.47 (1.75, 3.49) Women: 1.85 (1.05, 3.26) |
91% White, 4.3% Black, 4.4% Other All participants had ≥ 30 pack-years and were active smokers Nested case-control study |
| San Diego73 | USA 2011 | 4,544 (57%, 57y) | 7.8 | CVD (40) |
Volume; entire aorta | 1.45 (0.9, 2.3) | NR |
| Rotterdam82 | Netherlands 2015 | 2,408 (48%, 70y) |
8 | CVD (84) |
Agatston quartiles; only the aortic arch | 12.73 (1.6, 99.5) | NR |
TAC indicates thoracic aortic calcification; NR, not reported (race/ethnicity); MESA, Multi-Ethnic Study of Atherosclerosis; USA, United States of America; CHD, coronary heart disease; CV, cardiovascular disease; CVE, cardiovascular disease events; HNR, Heinz Nixdorf Recall Study; FHS, Framingham Heart Study; EISNER, Early Identification of Subclinical Atherosclerosis by Non-invasive Imaging Research; CAC, coronary artery calcification; NSLT, National Lung Screening Trial; CT, computed tomography.
All adjusted hazard ratios presented were adjusted for traditional cardiovascular risk factors.
the hazard ratio was also adjusted for CAC score.
Available data for TAC by the Agatston score are shown for CHD and CV disease events in Figures 3a and 3b, respectively. Currently limited data synthesis is possible, with only 3 studies of the descending thoracic aorta (which do not include the aortic arch). While individual studies report that TAC is significantly associated with adverse outcomes, the pooled adjusted hazard ratios do not reach statistical significance. In sex-stratified analyses, using data from MESA, TAC was associated with cardiac events in women, even after adjustment for CAC and risk factors, but not in men.84 Similarly, the Reykjavik study showed a predominance of AAC in women.85 Few studies assess whether TAC or AAC data add incremental value to risk models for cardiovascular events (Supplemental Table 4). Some studies report that TAC does not improve risk prediction beyond CAC, 86,87 while others demonstrate TAC significantly improve models beyond CAC, especially for those of intermediate ASCVD risk75. Other studies suggest that TAC is more appropriately used as part of combined calcium score using data from multiple vascular beds.75,88 There are even fewer studies of AAC and the reporting of AAC is beset by similar measurement heterogeneities (Table 5 and Supplemental Table 5). Although well-designed studies indicate that AAC independently predicts risk for cardiovascular events and mortality 79,89, there are too few studies with comparable reporting of measures and outcomes to enable proper data synthesis. As such, TAC and AAC are not recommended for screening purposes.
Figure 3.
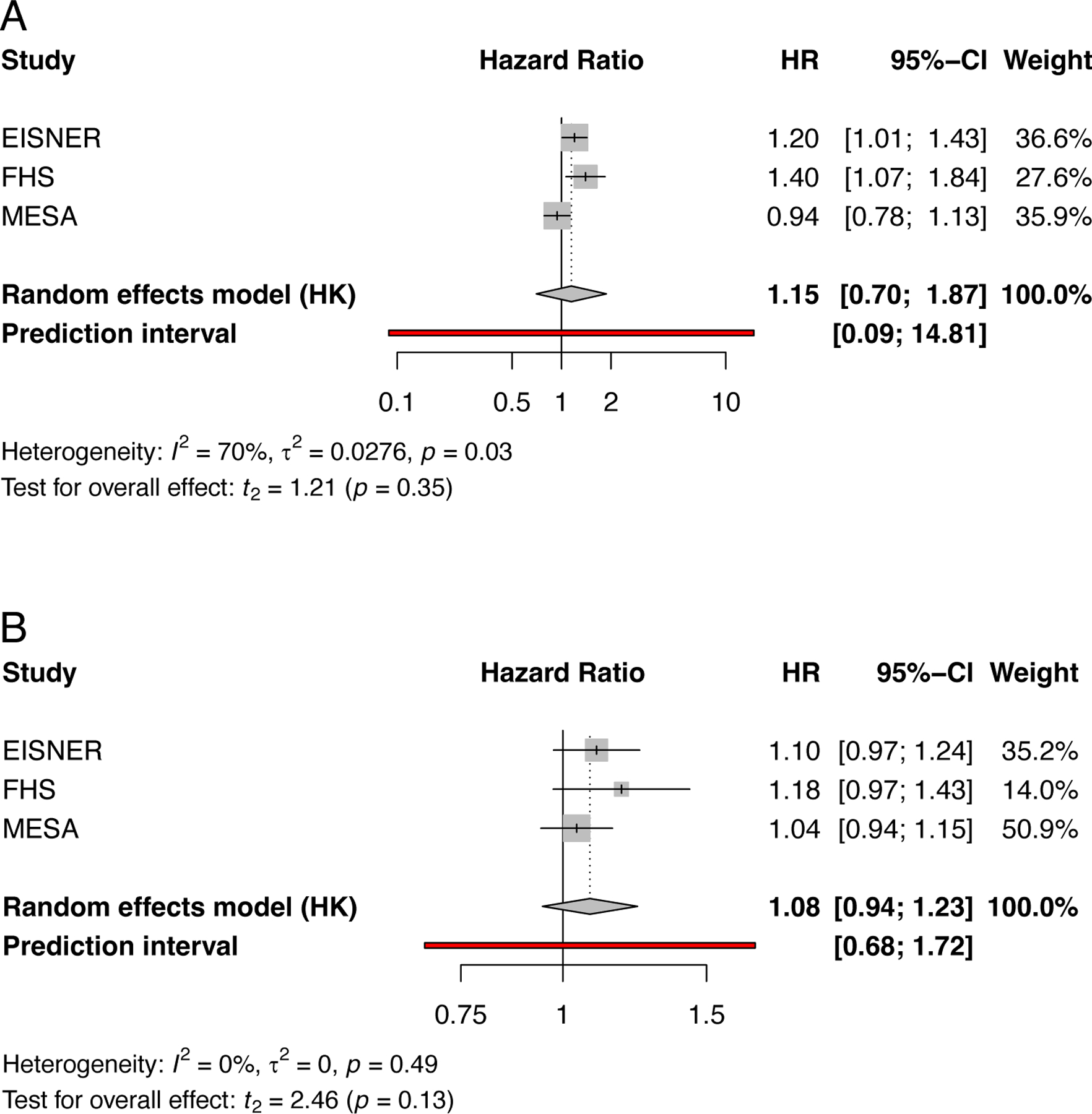
Forest plots illustrating the findings of a meta-analysis of included studies. a) Association of Thoracic Aortic Calcification by Agaston score with risk of coronary heart disease events in 3 studies, n=9204; including 12,635 women and 11,157 men; b) with cardiovascular events
Table 5:
Population-based studies on the association of Abdominal Aortic Calcification and Cardiovascular and Mortality Outcomes
|
Study [ref] |
Country Year of Publication |
Population (% men, mean age) |
Follow-up (years) | Outcomes (events) |
Measurement | Adjusted HR (95% CI)* |
Ethnicity and/or other comments |
|---|---|---|---|---|---|---|---|
| FHS64 | USA 2016 | 3,486 (49%, 50y) | 8 | CHD events (59) CV events (107) |
Agatston (L1-L4) |
1.95 (1.3, 3.0) 1.50 (1.1, 2.05) |
NR |
| MESA89 | USA 2014 | 1,974 (50%, 65y) | 5.5 | CHD events (50) CV events (83) CVD (30) |
Agatston percentiles (L2-L5) |
4.06 (1.8, 7.8) 4.00 (1.9, 5.5) 7.83 (2.2, 28.3) |
40.2% Non-Hispanic White, 25.7% Hispanic, 20.9% African-American, 13.2% Chinese |
| CARDIA 79 | USA 2021 | 3,011 (44.9%, 50y) | 8 | CHD events (55) CV events (106) |
Agatston | 1.49 (1.13, 1.98) 1.45 (1.18, 1.77) |
50% Black, 50% White |
| San Diego73 | USA 2011 | 4,544 (57%, 57y) | 7.8 | CVD (40) |
Volume (L1-L4) |
1.62 (0.9, 3.0) | NR |
| MESA 155 | USA 2016 | 997 (52%, 66y) | 9 | CHD events (77) CV events (118) |
Volume | 1.10 (0.95,1.3)^ 1.14 (1.00,1.32)^ |
47% European, 24% Hispanic, 16% African, 13% Chinese |
AAC indicates abdominal aortic calcification; NR, not reported (race/ethnicity); CHD, coronary heart disease; CV, cardiovascular; FHS, Framingham Heart Study; MESA, Multi-Ethnic Study of Atherosclerosis; CARDIA, The Coronary Artery Risk Development in Young Adults Study.
Recommendations for future use of aortic calcification measures
Consensus definitions are needed for TAC and AAC reporting. Future studies will need consistent reporting for the segment of aorta studied, method of calcium scoring, and cardiovascular outcomes. Evidence from large cohort studies including calcium of the aortic arch and proximal descending aorta is required. Studies that assess both TAC and AAC, along with repeatability of scoring, would be useful to further interpret findings.
Descending thoracic and infrarenal aortic diameters
Normal and abnormal aortic diameters
The aorta tapers in diameter from the ascending aorta to the iliac bifurcation, so it is important to specify the exact anatomical reference of the measurement. Studies of aortic root diameter have been excluded. Reference aortic diameters have been provided by the Framingham study and demonstrate that the aorta increases in diameter with age, body surface area and is greater in men than women. This is highlighted in Table 6 90. The population screening programmes for abdominal aortic aneurysm in men from Sweden and the UK also provide reference ranges for 65-year-old men. The reference ranges for Chinese and other ethnic groups may be different from the studies cited above, which included principally people of Caucasian origin. 91
Table 6:
Descending and infrarenal aortic reference diameters by age and body surface area (BSA) taken form the Framingham data showing CT diameters for the descending thoracic (DTA) and infrarenal aorta (IRA)
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Age (years) | BSA m2 | Mean DTA diameter mm | Mean IRA diameter mm | BSA m2 | Mean DTA diameter mm | Mean IRA diameter mm |
| 45–54 | <1.7 | 21.5 | 15.8 | <1.9 | 24.3 | 18.4 |
| 1.7–1.89 | 22.7 | 16.6 | 1.9–2.09 | 24.9 | 18.8 | |
| ≥1.9 | 23.6 | 17.0 | ≥2.1 | 26.0 | 19.4 | |
| >65 | <1.7 | 25.0 | 17.8 | <1.9 | 28.4 | 21.0 |
| 1.7–1.89 | 25.4 | 17.8 | 1.9–2.09 | 28.8 | 21.8 | |
| ≥1.9 | 27.1 | 18.7 | ≥2.1 | 30.3 | 22.5 | |
Increased aortic diameter is usually localised and described as an aneurysm but rarely can be generalised (arteriomegaly). The definition of an aneurysm is when the aortic dimeter is either more than 50% greater than the proximal aortic diameter or is greater than the mean±2SD population distribution.
Methods of measurement and their repeatability
Diameters have been measured by CT, MRI and ultrasonography. The advantages and disadvantages of each of these methods are like those reported for carotid arteries, although ultrasound is limited to the measurement of abdominal aortic diameters. Each method also has important intra-observer and inter-observer variability, which arise in part from the use of different equipment, anatomical landmarks for measurement, plane of measurement and where the callipers are placed on the aortic wall. Of note, many studies do not report exactly which diameter was measured e.g inner to inner or outer to outer. For instance, the repeatability of abdominal aortic diameters by either CT scan or ultrasonography has been reported to be up to ±5 mm. 92The repeatability changes little across the diameter spectrum, so that measurement errors are proportionately greater at smaller aortic diameters. The quality of aortic diameter measurement also can be quite variable. 93
Aortic diameter as a predictor of future atherosclerotic events
Five large population-based longitudinal studies were identified that evaluated the prognostic significance of aortic diameters on future cardiovascular events. However, only 2 of these provide hazard ratios which have been adjusted for conventional cardiovascular risk factors and their data are shown in Table 7. The3 remaining studies were all based on abdominal aortic aneurysm screening programmes in the general population. They show the association of aneurysmal (≥3 cm) and sub-aneurysmal (2.5–2.9 cm) infrarenal abdominal aortic diameters with cardiovascular events or death. The first study from Australia showed an association of aortic diameter with incident claudication and cardiovascular events in older men. 94 The second, a study of men and women in Norway showed a J-shaped association between aortic diameter cardiovascular death. 95 The third, from the United Kingdom showed an association in men aged 65 years at baseline with future cardiovascular events. 96
Table 7:
Population-based studies providing assessing the association of aortic diameter with future cardiovascular events and death
| Study [ref] | Country, Year of publication | Population (% men, mean age) | Follow-up (years) | Outcome (n=) | Measurement | Adjusted HR (95% CI)* | Ethnicity and/or other comments |
|---|---|---|---|---|---|---|---|
| Framingham156 |
USA 2017 |
3318 (51%, 50y) |
8.8 | Adverse CV events (177) |
CT Ascending Descending Infrarenal Above bifurcation |
Ascending: No assoc. Descending: No robust assoc. Infrarenal: 1.57 [1.06–2.32] Above bifurcation 1.53 [1.00–2.34] |
10.1161/CIRCIMAGING.117.006776 Data shown after adjusting for FRS factors model 2 Possible sex-specific effects for abdominal aortic diameters. Similar results after adjusting for CAC. Analyses for both top 10% and continuous |
| Rotterdam 157 |
Holland 2022 | 2178 (45%, 69y) |
9 | CVD (85) stroke (128) |
CT diameters adjusted for BMI Ascending Descending |
Ascending 1.33 [1.03–1.73] Descending1.38 [1.07–1.78] |
Results for women only, no association in men |
AP, anterior-posterior; CV, cardiovascular; CVD, cardiovascular death; ITI inner-to-inner aortic wall; OTO outer-to-outer aortic wall; FRS Framingham risk score.
This supersedes the earlier 2012 study of <9000 men by Duncan et al where no associations with CVD were identified. 158
Recommendations concerning the use of aortic diameters as markers of subclinical atherosclerosis.
The only evidence for aortic diameter measurements among middle aged people comes from the Framingham and Rotterdam studies, where there is weak evidence that abdominal aortic diameters are associated with either future adverse cardiovascular events or cardiovascular death respectively. This association may be related to the development of abdominal aortic aneurysm. The Framingham study suggests that there may be sex-specific effects. There are no data in either younger people or whether the observed associations apply across ethnic groups. There are conflicting data about the prognostic value of thoracic aortic diameters.
More research and evidence are required before abdominal aortic diameter can be considered a useful marker of subclinical atherosclerosis, especially in younger people and different ethnic groups. In addition, there is a need for reporting standards to improve and describe the exact measurements taken.
Femoral plaque
Definitions of normal and abnormal values
Femoral plaque is less extensively studied than carotid plaque but has been shown to be more prevalent in the asymptomatic population and thus may be a more sensitive screening tool. 97–99 Indeed, in the Progression of Early Subclinical Atherosclerosis (PESA) study, when 4184 asymptomatic 40–54 years old people were imaged with ultrasonography, plaque was most common in the iliofemoral arteries (44%), followed by the carotid arteries (31%) and aorta (25%). 97
Plaque in the lower extremities, has been variably defined in past studies. The Manheim consensus defines plaque as a focal structure encroaching into the lumen of at least 0.5mm, or 50% of the surrounding intima-media thickness (IMT), or a total thickness of >1.5mm. 100 However, other femoral studies not only report the presence of plaque but also report plaque quantification (plaque thickness, plaque volume and plaque area) and plaque morphology (ulceration, erosion, and echogenicity)101,102. In contrast, uniform femoral IMT of up to 1mm does not necessarily imply the presence of lipid and can occur as a compensatory response to hypertension 103,104.
Methods of measurement and their variability
Femoral atherosclerosis can be identified using B mode ultrasound. Femoral plaque can also be assessed using magnetic resonance imaging (MRI) and computed tomography (CT). The advantages and disadvantages of different imaging modalities is discussed in the carotid section.
A limitation of ultrasonographic assessment of femoral disease is reproducibility secondary to inter-observer variation. Of the studies included, only the MONICA study, a prospective cohort study with 1325 middle aged subjects with 13 year follow up, attempted to reduce inter-observer variation by using a single sonographer for all of the measurements.105 While many studies report a good interclass correlation coefficient, only the PESA study reported a Cohen’s Kappa Coefficient (K =0.88). 97
Measurement as a predictor of future atherosclerotic events
Three studies reported the association of femoral plaque with increased the risk of cardiovascular events. These studies utilized heterogeneous outcomes (e.g., cardiovascular events, myocardial infarction (MI) and cardiovascular death (CVD) and used variable plaque measurements (e.g., plaque presence, number of arteries affected and plaque surface irregularities).102,105–107 A fourth study, found that the number of femoral plaques identified did not significantly improve prediction of CVD risk over 10 years compared to the simple presence of plaque 108. All four studies used ultrasonography for plaque detection and found that measures of femoral plaque significantly and independently predicted future cardiovascular events or death (Table 8). 102,105–108 The presence of asymptomatic femoral plaque, therefore, may increase prediction of cardiovascular events when compared to traditional risk factors alone, but further studies are needed to substantiate this hypothesis.
Table 8:
Population studies assessing the association of femoral plaque with major adverse cardiovascular events and cardiovascular death
| Study [ref] | Country, Year of publication | Population (% men, mean age) | Follow-up (years) | Outcome (n=) |
Measurement | Adjusted HR (95% CI)* | Ethnicity and/or other comments |
|---|---|---|---|---|---|---|---|
| CHU Rangueil Toulouse25 | France 2009 | 2561 (62%, 51.6y) | 6 | Coronary Events (94) | Femoral Plaque | 2.39 [1.54–3.56] | |
| MONICA105 | Germany 2006 | 1325 (51%, 47.9y) | 13 |
MI (58) CVD (189) |
Plaque score (0–4) assessed at carotid and femoral arteries | Per 1 score increase MI: 1.20 (0.97, 1.50) CVD: 1.44 (1.18, 1.75) |
|
| Cyprus Atherosclerosis Study102 | Cyprus 2022 | 985 (45%, 58.1y) | 13 | ASCVD (154) | Number of femoral arteries with plaque | 1: 1.77 [1.03–3.05] 2: 4.25 [2.61–6.91] |
|
| CUiiDARTE108 | Uruguay 2020 | 581 (64%, 51.4y) | 10 | CVD (20) | Presence or absence of plaque | 1.114 1.95–1.22]* |
HR estimated from AUC ratio |
CHU: Centre Hopitalier Universitaire, MI myocardial infarction
MONICA: Monitoring of trends and determinants of cardiovascular disease
MI: myocardial infarction, CVD: cardiovascular death, ASCVD: atherosclerotic cardiovascular death.
approximate, derived from AUC ratios
When measuring femoral disease, two main factors appear to increase the prediction of cardiovascular events. The first is the number of arteries involved. The Cyprus Atherosclerosis Study, a prospective cohort study with 985 middle aged subjects with 13-year follow up, showed that when both femoral arteries are affected the Hazard Ratio (HR) increases from 1.77 [1.03–3.05] to 4.25 [2.61–6.91]. 102 Similar findings were shown in the MONICA study where each additional artery affected increased the HR by 1.20 (0.97, 1.50) for MI and 1.44 (1.18, 1.75) for CVD.105 The second factor is the quantity and morphology of the plaque itself. The Cyprus Atherosclerosis Study showed that femoral plaque thickness produced the highest net reclassification when added to conventional risk factors of 18.6% (3.1% correct up-reclassification and 15.5% correct down re-classification). 102 On the other hand, a smaller study in Turkey with only 215 subjects showed that plaque ulceration and surface abnormalities were a stronger predictor of major cardiovascular events (HR 23.241 [2.698–200.205]) compared to femoral and carotid atherosclerosis with accompanying coronary artery calcification (HR 2.172 [0.227–20.772]). 101 Due to the size of the study further work is needed to substantiate the increased risk with plaque surface abnormalities, especially given the significant increase in HR.
Recommendations regarding the use of specific measurement as a marker of subclinical atherosclerosis
Despite some evidence that femoral plaque is more prevalent than carotid plaque in asymptomatic, middle-aged people, it has been less studied than carotid plaque. As with atherosclerosis at other arterial sites, there is the lack of consensus on the definition of femoral plaque in the different studies. Further research in large, asymptomatic populations are needed to determine whether the presence of femoral plaque offers robust risk prediction. This is pertinent especially for younger populations, different ethnic groups, and women. Finally, although ultrasound is non-invasive and relatively inexpensive, more evidence is needed to ensure ultrasonographic measurements of femoral plaque are reproducible.
Ankle/brachial pressure index (ABI)
Definitions of normal and abnormal values
With a normal arterial tree, the systolic blood pressure at the ankle should be about 1.1 times the brachial systolic pressure. Reduction in the ankle systolic pressure indicates a blood flow restriction, due to one or more proximal stenotic lesions. Systolic ankle pressures, measured using a hand-held Doppler, are normally reported as a ratio of the brachial systolic pressure and the terms ABI, ankle/brachial index and ABPI (ankle/brachial pressure index) are both used in the literature. An ABI of ≥1.4 is considered above normal. An ABI between 1.0 and 1.39 is considered as the normal range. An ABI of ≤0.9 is considered as below normal in clinical practice, although some epidemiological studies have used values of <1.0 as being below normal.
Magnetic resonance imaging of the superficial femoral artery has shown that plaque burden increases steadily as ABI falls below 1, both in patients with intermittent claudication and in asymptomatic people. 109
An increase in ankle pressure above normal indicates that the artery walls are stiff (and potentially) calcified and may be incompressible.110 Medial, or Monckeberg’s, calcification is a histological hallmark of peripheral artery disease .1 In the presence of incompressible arteries, the toe/brachial index may be a more reliable test to detect peripheral atherosclerosis. 111
As for the coronary arteries, exercise tests are more sensitive in detecting early/borderline disease when the resting ABI is in the normal range. Only one study has reported on the association between a reduced ABI after exercise and the risk of future cardiovascular events and death (adjusted HR 2.61 [95%CI 1.67,4.06]. 112 Despite their potential advantages, neither toe/brachial index nor exercise tests are typically used in epidemiological studies.
Methods of measurement and their variability
Ankle pressures should be measured after 5 minutes rest and with the person supine (lying down). Ankle systolic pressures can be measured in either the posterior tibial or dorsalis pedis arteries, using a hand-held Doppler probe. 113 The same probe should be used for measuring brachial systolic pressures in both arms. There is significant intra-observer and inter-observer variability in measurement of ankle pressures, which may depend on training and results. These variabilities are summarised in a systematic review. 114 For intra-observer repeatability the intraclass correlation coefficients ranged from 0.42 to 0.98 and for interobserver repeatability from 0.42 to 1.0 (i.e. poor to excellent. Repeatability can be improved with training. ABI measurements are non-invasive, the equipment needed is portable and relatively inexpensive and can be performed in many different settings.
ABI as a predictor of future atherosclerotic events
In 2008 the Ankle Brachial Index Collaboration published a meta-analysis, which included additional data and information in addition to published data, with 24955 men, 23339 women and 480325 person-years of follow up. 115 The mean ages of people in the included studies ranged from 47 to 78 years (middle-aged to elderly) and people with cardiovascular disease at baseline were included in some studies. There was a reverse J-shaped relationship between ankle pressure and all-cause mortality, with lowest mortality for ABI 1.1–1.4. An ABI of <0.9 was associated with increased risk of 10-year cardiovascular death in both men and women: the risk increased with progressive decrements in ABI below 0.9. The hazard ratios (HR), compared to people with normal ABI [1.1–1.4], were 4.2 [95%CI 3.3–3.4] for men and 3.5 [2.4–5.1] for women. The Framingham Risk Score (FRS) at baseline predicted the 10-year risk of coronary heart disease in a mean (SD) of 1.1% (1.6%) to 36.1% (10.1%) in men and 7.6% (6.1%) to 14.1% (10.1%) in women in the different studies. The addition of ABI information would have reclassified the risk category of approximately 19% of men and 36% of women. There was no analysis of data by race or ethnicity.
What has been learnt since this landmark ABI collaboration publication?
There have been several confirmatory studies assessing the relationship of ABI with future atherosclerotic events in people without known cardiovascular disease at baseline. These studies were all conducted in middle-aged or elderly populations, providing data adjusted for FRS or similar risk factor combinations. The outcomes varied from cardiovascular events to cardiovascular death and the definition of cardiovascular events varied in the different studies for example, the ARTPER study. 116 included transient ischemic attack as an event but other studies only included stroke The main studies published since 2008 are shown in Table 9. Only 3 studies could be identified reporting the association of high ABI with cardiovascular events, the definition of these being heterogeneous and no meta-analysis was performed. The more recent s studies of low ABI have been combined with the published data from 4 earlier studies of cardiovascular death (CVD) in asymptomatic people (Cardiovascular Health117, Limburg118, Edinburgh Artery119 and Strong Heart120 studies, included in the 2008 meta-analysis115, to provide a Forest plot for abnormally low ABI (Figure 4a). Three studies121–123 reporting the association of future cardiovascular events (myocardial infarction and stroke) with low ABI are included in Table 9. These have been pooled with an earlier report from a 15-year follow up of the Edinburgh Artery study124 to provide the meta-analysis shown in Figure 6b. This latter report followed 1446 people (50% men) for up to 15 years, with 674 events, with adjusted HR 1.47 [1.22,1.78].124
Table 9:
Population-based studies assessing ABI as a predictor of future cardiovascular events or death published since the ABI Collaboration in 2008115
| Study [ref] | Country, Year of publication | Population (% men, mean age) | Follow-up (years) | Outcome (n=) |
Measurement | Adjusted HR (95% CI)* | Ethnicity and/or other comments |
|---|---|---|---|---|---|---|---|
| MESA159 | USA 2019 | 6669 (49%, 62y) | 14.2 | CVD (288) |
ABI<0.9 | 2.15 [1.55,3.44] |
Abstract only. Lower ABI of lower leg |
| NHANES160 | USA 2021 | 7571 (N/A, 60y) | 8 | CVD (204) |
ABI<0.9 | 3.92 [1.81–5.28] |
Abstract only |
| ARTPER116 | Spain 2018 | 2716 (43%, 62y) | 9 | CV events & death (187) | ABI<0.9 | 2.55 [1.53–4.24] |
|
| REGICOR161 | Spain 2017 | 5679 (54%, 55.4y) | 6.2 | CVD (59) |
ABI>1.4 | 3.1 [1.95–6.48] |
|
| REGICOR123 | Spain 2015 | 5248 (45%, 53.7y) | 5.9 | CV events (175) |
ABI<0.9 | 3.03 [1.86,4.95] |
5248 (45%) |
| ARIC121 | USA 2012 | 1594 (44%. 53.8y) | 10 | CV events (659) |
ABI<0.9 | 1.18 [1.10,1.27] |
24% black |
| Hoorn 162 |
Holland 2012 |
624 (49.5%, 64.7y) | 17.2 | CVD (85) | ABI<0.9 | 2.57 [1.50–4.40] |
Similar HR with/without type 2 diabetes |
| MESA 122 |
USA 2011 |
6647 (49%, 62.1y) | 5.3 | CV events (317) |
ABI>1.4 ABI<1 |
1.95 [1.0–3.43] 1.77 [1.31–2.40] |
White 38.8% Chinese 12.7% African-American 25.6% Hispanic 23.1% Consistent findings across Hispanics, Chinese, Whites, Blacks. Adds predictive value to FRS |
CVD cardiovascular death, CV cardiovascular
Figure 4.
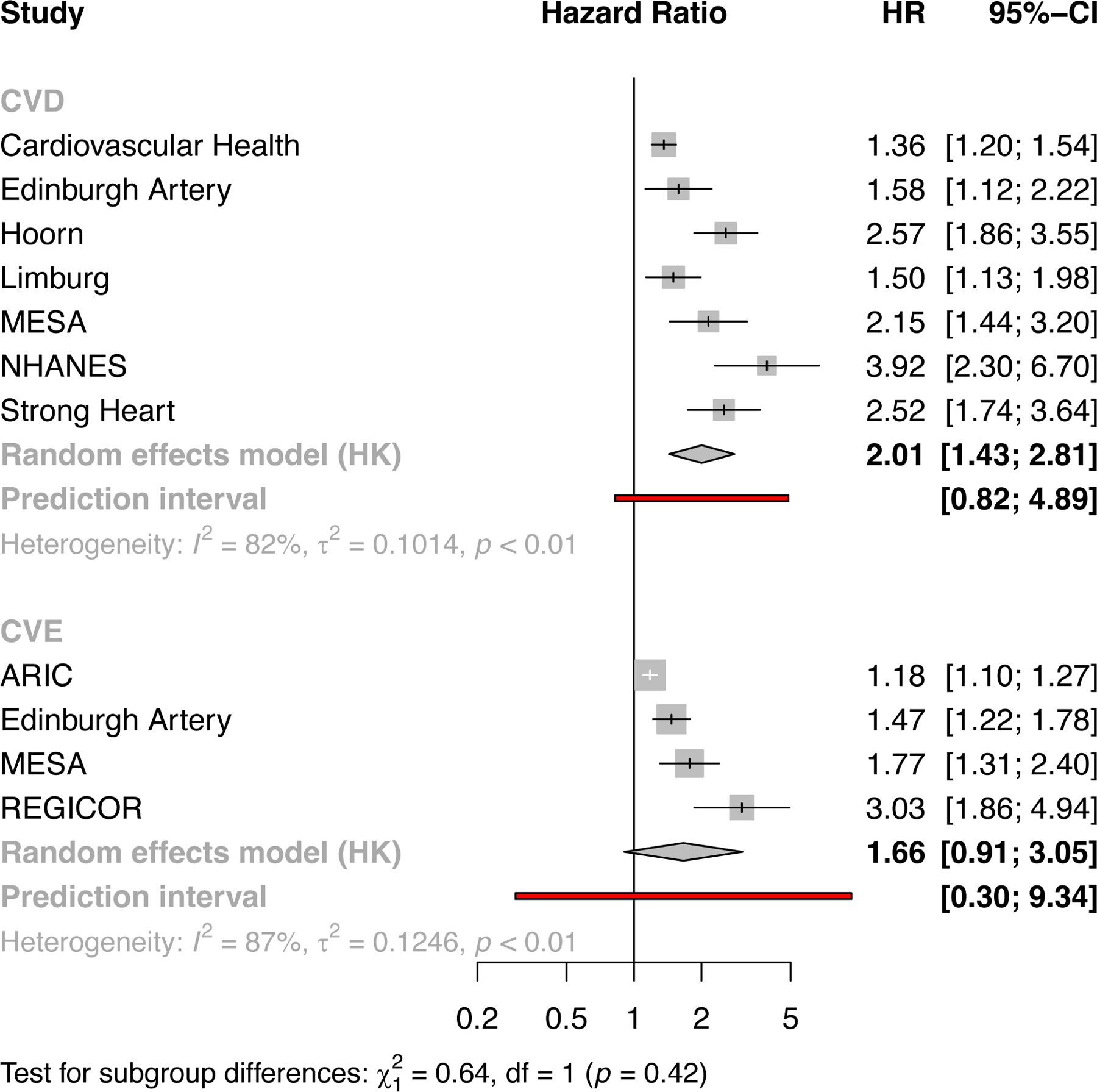
Forest plots illustrating the findings of a meta-analysis of included studies with risk of future events for people with abnormally low ABI, (a) shows studies using the outcome of cardiovascular death with 7 studies n=23890 and (b) for the outcome of cardiovascular events only with 4 studies n=14395. CVD cardiovascular death, CVE cardiovascular events. The Cardiovascular Health, Edinburgh Artery, Limburg and Strong Heart studies were include in the earlier ABI collaboration115.
The data for the association of low ABI with both cardiovascular death and cardiovascular events (myocardial infarction and stroke) showed significant heterogeneity. Some of the reasons for this include the varying baseline age of participants (mean age from 35 to 70 years) and the length of follow up (from 5 to 17 years). Figure 4 A and B show the predictive value of low ABI for future s cardiovascular death and atherosclerotic events respectively (even after adjustment for conventional risk factors). For cardiovascular death (7 studies) and cardiovascular events (4 studies) the pooled HRs were 2.01 [1.43,2.81], I2=82% 1.66 [0.91,3.05] I2=87%. and respectively.
Recommendations regarding the use of ABI as a marker of subclinical atherosclerosis
There is good evidence to indicate that low ABI is a valid measure of the presence of asymptomatic peripheral atherosclerosis and adds to the predictive value, independent of conventional cardiovascular risk factors, in predicting future cardiovascular death in middle aged to elderly men and women. The data for cardiovascular events are supportive of these findings, though limited by fewer studies with homogeneous definitions of cardiovascular events. This good evidence applies across White and Hispanic populations, with more limited support for the validity of using ABI as a marker of subclinical atherosclerosis in Black, Chinese and other ethnic groups (principally from ARIC, MESA and Strong Heart studies). There is no evidence concerning the utility of ABI as a marker of subclinical atherosclerosis in younger adult populations, although the assessment of ABI after exercise might be a useful measure in future studies. The evidence for abnormally high ABI, with stiff or incompressible arteries, is a marker of subclinical atherosclerosis is in direct and the association with cardiovascular risk may be mediated by diabetes, renal failure and other factors.
| Recommendation | Class | Level | Supporting References |
|---|---|---|---|
| Abnormally low (<0.9) ankle/brachial index in asymptomatic middle aged to elderly white and Hispanic men and women should be considered to indicate the presence of subclinical atherosclerosis. | I | B |
Measures of vascular function or anatomy but do not indicate the presence of subclinical atherosclerosis
A large variety of different tests are reported in the literature as either predicting the risk of future cardiovascular events and/or death or the presence of subclinical atherosclerosis. Since atherosclerosis is defined by the presence of lipid containing lesions, or plaque, many of these tests cannot be used to infer the presence of atherosclerosis. An example of this is given above, for abnormally high ankle/brachial pressure indices, where the association with future cardiovascular events may be mediated by mechanisms other than atherosclerosis.
One of the measures, which has been commonly reported as a measure of subclinical atherosclerosis is carotid intimal thickness. Only, when lipid pools are present (pathological intimal thickening) can this be termed subclinical atherosclerosis. Intimal thickening is almost universally present at, especially at arterial bifurcations, as an adaptive response to the altered hemodynamic environment with growth, increasing blood pressure and aging. Therefore, intimal thickening in any conduit artery cannot be termed subclinical atherosclerosis. Moreover, for the carotid arteries, comparative studies have shown that carotid plaque is a highly superior predictor of future atherosclerotic events.125 Similarly tests of microvascular or endothelial dysfunction do not directly assess subclinical atherosclerosis. Endothelial dysfunction often is measured as flow-mediated dilation in the brachial arteries. Two large population studies have failed to reveal a clinically significant independent association between impaired flow-mediated dilation of the brachial artery and future incident cardiovascular events or mortality.126,127 Arterial stiffness is measured in many different ways, including either carotid -femoral or brachial-ankle pulse wave velocity, local carotid or femoral stiffness, augmentation index, wave reflections, wave form analysis and other measures, which hinders data synthesis. Increasing arterial stiffness also reflects increasing blood pressure and medial arterial calcification. Moreover, recent data indicates that pulse wave velocity may be an important predictor of future heart failure.128 Therefore, as for abnormally high ABI, aortic stiffness may not reflect the presence of subclinical atherosclerosis and any association with cardiovascular events or death may result from other mechanisms. A variety of other tests such as retinal vessel calibre and skin autofluorescence also cannot be used to infer the presence of subclinical atherosclerosis. However, all the tests listed here are likely to provide information about other mechanisms contributing to the burden of cardiovascular disease in the community.
From the present to the future
Many of the approaches for measuring subclinical atherosclerosis in asymptomatic people have been applied with a primary goal of assessing myocardial infarction risk but today much of the focus has shifted to the identification of biomarkers or genetic loci that are associated with increased risk of future atherosclerotic events. It is logical that direct assessment of coronary atherosclerosis would be the most informative means of predicting myocardial infarction. However, direct imaging of coronary atherosclerosis (including number and degree of stenoses as well as overall plaque burden) has required invasive angiography. Notably, invasive angiography is expensive and carries procedural risks (including rare deaths). Accordingly, except for coronary calcium scoring, subclinical atherosclerosis (and myocardial infarction risk) has been evaluated in other arterial beds. Carotid and peripheral artery atherosclerosis also contribute to the overall burden of cardiovascular disease, causing end-organ damage, such as stroke and chronic limb threatening ischemia. These forms of atherosclerosis and can be diagnosed and assessed using duplex ultrasonography and other completely non-invasive techniques Since atherosclerosis is seldom confined to a single arterial bed, some of these non-invasive tests might also be used as correlates of the presence and extent of coronary atherosclerosis.
The part I section provides morphologic characteristics of plaques that constitute asymptomatic atherosclerosis and outlines the importance their identification in order to start primary prevention strategies when plaques are in their earliest stages. While the lesions of coronary arteries are discussed first, the unique characteristics of carotid and lower limb plaque also are described. Although in the last few decades we have made tremendous progress in the treatment of acute coronary syndromes, especially acute myocardial infarction, the incidence of sudden coronary death rem113ains high. Therefore, the goal of this review is to provide clinicians and researchers with a better understanding of what constitutes subclinical atherosclerosis and how it can be measured, with the goal of identifying people who are at high risk for atherosclerosis progression to symptomatic disease. These same measurement methods should be used in the biomarker and Mendelian randomisation studies to provide new insight into the mechanisms of disease and supplement current methods of risk assessment.
Each of the methods identified as valid measures of subclinical atherosclerosis in this review could also be used to assess progression of atherosclerosis and predict escalation of risk of symptomatic atherosclerosis. In the Reykjavik REFINE study129, statin use was the only factor independently associated with carotid plaque regression. In the Multi-Ethnic Study of Atherosclerosis, CAC progression has also been shown to be predictive of events. CAC progression is predictive of increased risk for coronary heart disease events among those with and without baseline CAC.130 CAC progression is also predictive of all-cause mortality risk.131 In a study of individuals with baseline CAC scoring and repeat exams at 5 years, there was a very low risk of coronary events at 10-years in those with CAC of 0 at both exams (1.4%). In contrast, there was a higher risk among those with baseline CAC of 1–399 (3.4%), particularly if there was progression in CAC at 5-years to ≥400.132 Given that individuals with baseline CAC >0 are already considered higher risk, the greatest utility for repeating a CAC score in 5-years appears to be in those with a baseline CAC of 0. The ABI collaboration115 and subsequent studies have shown how decreasing ABI increases the risk of cardiovascular death.
There are several studies that report on interventions (ranging from vitamin supplements, smoking cessation to statins) to reduce the burden of subclinical atherosclerosis. Sadly, most of the high quality studies (randomised controlled trials) have used carotid intimal medial thickness to track the progression of disease.133–135 is hugely disappointing, since ultrasonography of the carotid arteries could have measured carotid plaque, which is a valid measure of subclinical atherosclerosis and its progression (as shown herein). A systematic review of chelation therapy has concluded that there is no clear evidence that it reduces cardiovascular disease events136. Some of the included studies used CAC as an exploratory outcome and there is some evidence that lower CAC density scores were associated with worse outcomes. As such, reducing the extent of CAC could be seen as counter-productive but this remains a matter of debate. Future studies would be advised to use the methods identified in this review, namely measurement of carotid plaque, coronary artery calcium or low ankle pressures. While CAC provides direct association with coronary heart disease, it incurs some radiation burden and usually a visit to a hospital or specialist clinic. The presence of carotid artery plaque also shows a direct association with coronary heart disease, as well as stroke, but despite being non-invasive its measurement is observer dependent. Low ankle brachial index provides an alternative non-invasive technique, which uses highly portable equipment to identify subclinical atherosclerosis in the arteries of the lower limb. It is strongly association is with future cardiovascular mortality but data to support association with specific events such as myocardial infarction or stroke are more limited.
Nearly all studies identified in this series of critical reviews are of middle aged or older people and associations in younger people have not been demonstrated. Importantly, at younger ages subclinical atherosclerosis is likely to be more prevalent in men than women.137 Therefore, much work remains to be done to improve the diagnosis of subclinical atherosclerosis. This can best be done by randomized trials to assess whether early use of the safe, effective and cheap therapeutic strategies to reduce atherosclerotic burden are effective in reducing both the clinical and the health economic burden of cardiovascular disease in the longer term. Such trials will not be realised rapidly and technological advance is rapid and some of the advances which might benefit collecting this evidence are discussed below.
1. Novel imaging modalities to identify the most risky early lesions
Advances in MRI imaging have enabled the detection of lipid pools, the sine qua non of atherosclerosis in coronary and peripheral arteries.138 At the same time, new portable magnets are being developed, which might enable future use in epidemiologic studies.139 Infrared spectroscopy has been used to identify different plaque types and lipid pools in post mortem aorta.140 Near infrared spectroscopy combined with intravascular ultrasound has been used to show the presence of lipid-rich lesions in coronary arteries but this is an invasive technique.141 Technological advances to enable its application to the superficial arteries currently evaluated using duplex ultrasonography, the carotid and femoral arteries non-invasively appears to have been slow. However, there is a recent report that infrared spectroscopy and mathematical simulation can be used to image disease in the carotid artery in vivo .142 This might suggest that the application of Artificial Intelligence would render this an interesting future technology to detect subclinical atherosclerosis.
2. New approaches to identifying the most risky atherosclerotic plaques
MRI imaging also can identify plaque characteristics other than lipid pools, such as thin fibrous caps and is a potential method of identifying plaque types at greatest risk of rupture and causing ischemic events. Alternative technological advances in transcriptomics underscore the burgeoning biological data which may identify novel ways of identifying risky plaque types. There is preliminary work to indicate that it may be possible to identify plaques at highest risk of generating thrombosis.143 However, it is not clear how such information might be translated into molecular imaging, to identify plaques likely to cause clinical events.
3. Artificial intelligence to identify to both quantify plaque and identify the most risky plaque characteristics
The application of image segmentation, machine learning, deep learning and artificial intelligence for defining carotid artery lesions is work in progress that could transform the characterisation of carotid plaque. This has been applied to ultrasonography, as well as CT and MRI imaging.144 The application of these same techniques to femoral plaque would be of value, since femoral plaques are more common than carotid plaques in asymptomatic people. Similarly, the application of the same processes can allow the identification of microcalcification as an early marker of subclinical atherosclerosis, which currently is missed on CT imaging.145 The integration of such data microcalcifications with multi-omics data provides a potential route to new drug discovery.
4. Large population-based studies and randomised trials
There is no strong evidence to support early therapeutic intervention for subclinical atherosclerosis. The question of whether early therapeutic intervention (in young to middle-aged people) needs to be addressed in randomized controlled trials, which follow participants over many years for future cardiovascular events. Such trials are likely to be very costly and ideally should take place in areas with strong integrated health records, or in countries where standardized records can be integrated using advanced learning and computing techniques. For example, in Sweden there are nationwide registries which report how often prescriptions for specific drugs have been collected as well as other comprehensive registries of hospital admission and diagnoses and procedures (using standardised coding) and availability of death registration with cause. Systems and registries such as these may be necessary to conduct trials of early therapeutic interventions at reasonable cost. Other countries with insurance-based health economies also may be able to support such trials if there is a single insurer for most of the population. Any such trials should include the tools necessary to support the recruitment of diverse ethnic and socioeconomic groups.
For the here and now
The search for new biomarkers or genetic loci for the prediction of the progression of subclinical atherosclerosis to clinical cardiovascular events and new mechanistic insight should continue. Future studies should be aware that there are only a limited number of measurements which can be used to infer the presence of subclinical atherosclerosis, namely presence of carotid plaque, coronary artery calcification and low ankle/brachial pressure index. Unfortunately, in the past many studies have used surrogate outcome measures that are suboptimal or inadequate. Future studies also should use the Equator network reporting guidelines for biomarker and diagnostic accuracy (STARD and TRIPOD): these latter guidelines stress the need for separate discovery and validation cohorts, features often missing particularly in earlier biomarker studies.
That said, there are several associated areas that merit further research and improvement. First, studies of subclinical atherosclerosis typically focus on middle-aged to elderly populations, 40–70 years. The value of identifying subclinical atherosclerosis may be even greater in younger people but the tests currently available are probably not sufficiently sensitive to identify the earliest atherosclerotic lesions, which underscores the need for the development of new methods for the detection of subclinical atherosclerosis. Second, whilst some studies report that their findings are robust across all ethnic groups, further work in this area would provide a more robust examination of this important characteristic. Third, standardization of outcome reporting would facilitate future evidence synthesis. Here one of the key areas for improvement is in the measurement of arterial calcium, where establishment of gold standards would be welcome. Also, variation in the definition of clinical outcomes, such as coronary heart disease and cardiovascular death, hampers evidence harmonization. Finally, the critical reviews herein are based on summary published data. Studies based on individual patient data would provide additional insights, particularly in the 35–45 years age range, by sex and ethnic groups.
Highlights.
Many of the commonly used physiological or imaging assessments of subclinical atherosclerosis do not predict future atherosclerotic events.
Carotid plaque burden, coronary artery calcium and low ankle/brachial pressure index were the only three tests which robustly predicted future atherosclerotic events in people of middle age or older.
Accordingly, carotid plaque, coronary artery calcium and low ankle/brachial pressure index can be considered as valid indicators of subclinical atherosclerosis in biomarker and Mendelian randomisation studies of middle-aged or older people.
There is an absence of evidence about useful measurements to predict future atherosclerotic events in younger populations.
Abbreviations
- AAC
Abdominal Aortic Calcium
- ABI
Ankle Brachial Index
- ASCVD
Atherosclerotic Cardiovascular Disease
- CAC
Coronary Artery Calcium
- CHD
Coronary Heart Disease
- CT
Computed Tomography
- CV
Cardiovascular
- CVD
Cardiovascular Death
- HU
Hounsfield Units
- MI
Myocardial Infarction
- MRI
Magnetic Resonance Imaging
- TAC
Thoracic Aortic Calcium
References
Uncategorized References
- 1.Kawai K, Finn AV, Virmani R. . Subclinical atherosclerosis part 1. What is it? Csn it be defined at the histological level? Arterioscler Thromb Vasc Biol 2923. [DOI] [PubMed] [Google Scholar]
- 2.Team RStudio. RStudio: Integrated Development Environment for R RStudio, PBC, Boston, MA. 2020. [Google Scholar]
- 3.Harrer M, Cuijpers P, Furukawa T, DD E. Doing Meta-Analysis in R. A Hands on Guide https://bookdown.org/MathiasHarrer/Doing_Meta_Analysis_in_R/. 2019.
- 4.Spineli LM, Pandis N. Prediction interval in random-effects meta-analysis. Am J Orthod Dentofacial Orthop 2020;157:586–588. doi: 10.1016/j.ajodo.2019.12.011 [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1046–e1081. doi: 10.1161/CIR.0000000000000624 [DOI] [PubMed] [Google Scholar]
- 6.Urbina EM, Williams RV, Alpert BS, Collins RT, Daniels SR, Hayman L, Jacobson M, Mahoney L, Mietus-Snyder M, Rocchini A, et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension 2009;54:919–950. doi: 10.1161/HYPERTENSIONAHA.109.192639 [DOI] [PubMed] [Google Scholar]
- 7.Yan Y, Li S, Liu Y, Guo Y, Fernandez C, Bazzano L, He J, Chen W. Associations Between Life-Course Lipid Trajectories and Subclinical Atherosclerosis in Midlife. JAMA Netw Open 2022;5:e2234862. doi: 10.1001/jamanetworkopen.2022.34862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sigurdsson S, Aspelund T, Kjartansson O, Gudmundsson E, Jonsson PV, van Buchem MA, Gudnason V, Launer LJ. Cerebrovascular Risk-Factors of Prevalent and Incident Brain Infarcts in the General Population: The AGES-Reykjavik Study. Stroke 2022;53:1199–1206. doi: 10.1161/STROKEAHA.121.034130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Fang X, Hua Y, Tang Z, Guan S, Wu X, Liu H, Liu B, Wang C, Zhang Z, et al. Carotid Artery Plaques, Carotid Intima-Media Thickness, and Risk of Cardiovascular Events and All-Cause Death in Older Adults: A 5-Year Prospective, Community-Based Study. Angiology 2018;69:120–129. doi: 10.1177/0003319717716842 [DOI] [PubMed] [Google Scholar]
- 10.Romanens M, Adams A, Sudano I, Bojara W, Balint S, Warmuth W, Szucs TD. Prediction of cardiovascular events with traditional risk equations and total plaque area of carotid atherosclerosis: The Arteris Cardiovascular Outcome (ARCO) cohort study. Prev Med 2021;147:106525. doi: 10.1016/j.ypmed.2021.106525 [DOI] [PubMed] [Google Scholar]
- 11.Rosvall M, Janzon L, Berglund G, Engstrom G, Hedblad B. Incident coronary events and case fatality in relation to common carotid intima-media thickness. J Intern Med 2005;257:430–437. doi: 10.1111/j.1365-2796.2005.01485.x [DOI] [PubMed] [Google Scholar]
- 12.Hunt KJ, Evans GW, Folsom AR, Sharrett AR, Chambless LE, Tegeler CH, Heiss G. Acoustic shadowing on B-mode ultrasound of the carotid artery predicts ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke 2001;32:1120–1126. doi: 10.1161/01.str.32.5.1120 [DOI] [PubMed] [Google Scholar]
- 13.Hunt KJ, Sharrett AR, Chambless LE, Folsom AR, Evans GW, Heiss G. Acoustic shadowing on B-mode ultrasound of the carotid artery predicts CHD. Ultrasound Med Biol 2001;27:357–365. doi: 10.1016/s0301-5629(00)00353-7 [DOI] [PubMed] [Google Scholar]
- 14.van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DA, Witteman JC. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation 2004;109:1089–1094. doi: 10.1161/01.Cir.0000120708.59903.1b [DOI] [PubMed] [Google Scholar]
- 15.Chen PC, Jeng JS, Hsu HC, Su TC, Chien KL, Lee YT. Carotid Atherosclerosis Progression and Risk of Cardiovascular Events in a Community in Taiwan. Sci Rep 2016;6:25733. doi: 10.1038/srep25733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamina C, Meisinger C, Heid IM, Lowel H, Rantner B, Koenig W, Kronenberg F, Kora Study G. Association of ankle-brachial index and plaques in the carotid and femoral arteries with cardiovascular events and total mortality in a population-based study with 13 years of follow-up. Eur Heart J 2006;27:2580–2587. doi: 10.1093/eurheartj/ehl228 [DOI] [PubMed] [Google Scholar]
- 17.Mathiesen EB, Johnsen SH, Wilsgaard T, Bonaa KH, Lochen ML, Njolstad I. Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: a 10-year follow-up of 6584 men and women: the Tromso Study. Stroke 2011;42:972–978. doi: 10.1161/STROKEAHA.110.589754 [DOI] [PubMed] [Google Scholar]
- 18.Mehta A, Rigdon J, Tattersall MC, German CA, Barringer TA 3rd, Joshi PH, Sperling LS, Budoff MJ, Bertoni A, Michos ED, et al. Association of Carotid Artery Plaque With Cardiovascular Events and Incident Coronary Artery Calcium in Individuals With Absent Coronary Calcification: The MESA. Circ Cardiovasc Imaging 2021;14:e011701. doi: 10.1161/circimaging.120.011701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gepner AD, Young R, Delaney JA, Tattersall MC, Blaha MJ, Post WS, Gottesman RF, Kronmal R, Budoff MJ, Burke GL, et al. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging 2015;8. doi: 10.1161/circimaging.114.002262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polak JF, Szklo M, Kronmal RA, Burke GL, Shea S, Zavodni AE, O’Leary DH. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc 2013;2:e000087. doi: 10.1161/jaha.113.000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokubo Y, Watanabe M, Higashiyama A, Nakao YM, Nakamura F, Miyamoto Y. Impact of Intima-Media Thickness Progression in the Common Carotid Arteries on the Risk of Incident Cardiovascular Disease in the Suita Study. J Am Heart Assoc 2018;7. doi: 10.1161/JAHA.117.007720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Wang Y, Chen S, Zhao J, Su Q, Fan Y, Wu S, Li J, Hong J. Evaluation of Carotid Artery Atherosclerosis and Arterial Stiffness in Cardiovascular Disease Risk: An Ongoing Prospective Study From the Kailuan Cohort. Front Cardiovasc Med 2022;9:812652. doi: 10.3389/fcvm.2022.812652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prabhakaran S, Rundek T, Ramas R, Elkind MS, Paik MC, Boden-Albala B, Sacco RL. Carotid plaque surface irregularity predicts ischemic stroke: the northern Manhattan study. Stroke 2006;37:2696–2701. doi: 10.3389/fcvm.2022.812652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollander M, Bots ML, Del Sol AI, Koudstaal PJ, Witteman JC, Grobbee DE, Hofman A, Breteler MM. Carotid plaques increase the risk of stroke and subtypes of cerebral infarction in asymptomatic elderly: the Rotterdam study. Circulation 2002;105:2872–2877. doi: 10.1161/01.cir.0000018650.58984.75 [DOI] [PubMed] [Google Scholar]
- 25.Cournot M, Taraszkiewicz D, Cambou JP, Galinier M, Boccalon H, Hanaire-Broutin H, Chamontin B, Carrié D, Ferrières J. Additional prognostic value of physical examination, exercise testing, and arterial ultrasonography for coronary risk assessment in primary prevention. Am Heart J 2009;158:845–851. doi: 10.1016/j.ahj.2009.08.017 [DOI] [PubMed] [Google Scholar]
- 26.Plichart M, Celermajer DS, Zureik M, Helmer C, Jouven X, Ritchie K, Tzourio C, Ducimetiere P, Empana JP. Carotid intima-media thickness in plaque-free site, carotid plaques and coronary heart disease risk prediction in older adults. The Three-City Study. Atherosclerosis 2011;219:917–924. doi: 10.1016/j.atherosclerosis.2011.09.024 [DOI] [PubMed] [Google Scholar]
- 27.Rundek T, Arif H, Boden-Albala B, Elkind MS, Paik MC, Sacco RL. Carotid plaque, a subclinical precursor of vascular events: the Northern Manhattan Study. Neurology 2008;70:1200–1207. doi: 10.1212/01.wnl.0000303969.63165.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chien KL, Su TC, Jeng JS, Hsu HC, Chang WT, Chen MF, Lee YT, Hu FB. Carotid artery intima-media thickness, carotid plaque and coronary heart disease and stroke in Chinese. PLoS One 2008;3:e3435. doi: 10.1371/journal.pone.0003435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimoda S, Kitamura A, Imano H, Cui R, Muraki I, Yamagishi K, Umesawa M, Sankai T, Hayama-Terada M, Kubota Y, et al. Associations of Carotid Intima-Media Thickness and Plaque Heterogeneity With the Risks of Stroke Subtypes and Coronary Artery Disease in the Japanese General Population: The Circulatory Risk in Communities Study. J Am Heart Assoc 2020;9:e017020. doi: 10.1161/JAHA.120.017020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gepner AD, Young R, Delaney JA, Budoff MJ, Polak JF, Blaha MJ, Post WS, Michos ED, Kaufman J, Stein JH. Comparison of Carotid Plaque Score and Coronary Artery Calcium Score for Predicting Cardiovascular Disease Events: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2017;6. doi: 10.1161/jaha.116.005179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t [DOI] [PubMed] [Google Scholar]
- 32.Callister TQ, Cooil B, Raya SP, Lippolis NJ, Russo DJ, Raggi P. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology 1998;208:807–814. doi: 10.1148/radiology.208.3.9722864 [DOI] [PubMed] [Google Scholar]
- 33.Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, Carr JJ, Budoff MJ, Allison MA. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA: The Journal of the American Medical Association 2014;311:271–278. doi: 10.1001/jama.2013.282535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Rosendael AR, Narula J, Lin FY, van den Hoogen IJ, Gianni U, Al Hussein Alawamlh O, Dunham PC, Pena JM, Lee SE, Andreini D, et al. Association of High-Density Calcified 1K Plaque With Risk of Acute Coronary Syndrome. JAMA Cardiol 2020;5:282–290. doi: 10.1001/jamacardio.2019.5315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas IC, Shiau B, Denenberg JO, McClelland RL, Greenland P, de Boer IH, Kestenbaum BR, Lin GM, Daniels M, Forbang NI, et al. Association of cardiovascular disease risk factors with coronary artery calcium volume versus density. Heart 2018;104:135–143. doi: 10.1136/heartjnl-2017-311536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merghani A, Maestrini V, Rosmini S, Cox AT, Dhutia H, Bastiaenan R, David S, Yeo TJ, Narain R, Malhotra A, et al. Prevalence of Subclinical Coronary Artery Disease in Masters Endurance Athletes With a Low Atherosclerotic Risk Profile. Circulation 2017;136:126–137. doi: 10.1161/CIRCULATIONAHA.116.026964 [DOI] [PubMed] [Google Scholar]
- 37.Sung K-C, Hong YS, Lee J-Y, Lee S-J, Chang Y, Ryu S, Zhao D, Cho J, Guallar E, Lima JAC. Physical activity and the progression of coronary artery calcification. Heart 2021;107:1710–1716. doi: 10.1136/heartjnl-2021-319346 [DOI] [PubMed] [Google Scholar]
- 38.Bhatia HS, Lin F, Thomas IC, Denenberg J, Kandula NR, Budoff MJ, Criqui MH, Kanaya AM. Coronary artery calcium incidence and changes using direct plaque measurements: The MASALA study. Atherosclerosis 2022;353:41–46. doi: 10.1016/j.atherosclerosis.2022.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Rosendael AR, Van Den Hoogen IJ, Gianni U, Ma X, Tantawy SW, Bax AM, Lu Y, Andreini D, Al-Mallah MH, Budoff MJ, et al. Association of Statin Treatment With Progression of Coronary Atherosclerotic Plaque Composition. JAMA Cardiology 2021;6:1257–1266. doi: 10.1001/jamacardio.2021.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Criqui MH, Knox JB, Denenberg JO, Forbang NI, McClelland RL, Novotny TE, Sandfort V, Waalen J, Blaha MJ, Allison MA. Coronary Artery Calcium Volume and Density: Potential Interactions and Overall Predictive Value: The Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging 2017;10:845–854. doi: 10.1016/j.jcmg.2017.04.018 [DOI] [PubMed] [Google Scholar]
- 41.Bhatia HS, Thomas IC, Denenberg J, Allison M, McClelland RL, Budoff M, McVeigh ER, Criqui MH. Coronary artery calcium and cardiovascular disease prediction by scanner type: the multi-ethnic study of atherosclerosis. Clinical Radiology 2022;77:e636–e642. doi: 10.1016/j.crad.2022.04.013 [DOI] [PubMed] [Google Scholar]
- 42.Dzaye O, Razavi AC, Dardari ZA, Berman DS, Budoff MJ, Miedema MD, Obisesan OH, Boakye E, Nasir K, Rozanski A, et al. Mean Versus Peak Coronary Calcium Density on Non-Contrast CT: Calcium Scoring and ASCVD Risk Prediction. JACC Cardiovasc Imaging 2021;15:489–500. doi: 10.1016/j.jcmg.2021.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhatia HS, McClelland RL, Denenberg J, Budoff MJ, Allison MA, Criqui MH. Coronary Artery Calcium Density and Cardiovascular Events by Volume Level: The MESA. Circulation: Cardiovascular Imaging 2023;16:e014788. doi: doi: 10.1161/CIRCIMAGING.122.014788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e285–e350. doi: 10.1016/j.jacc.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 45.Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, Callister T, Raggi P, Blumenthal RS, Nasir K. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 46.Erbel R, Möhlenkamp S, Moebus S, Schmermund A, Lehmann N, Stang A, Dragano N, Grönemeyer D, Seibel R, Kälsch H, et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol 2010;56:1397–1406. doi: 10.1016/j.jacc.2010.06.030 [DOI] [PubMed] [Google Scholar]
- 47.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2006;113:30–37. doi: 10.1161/circulationaha.105.580696 [DOI] [PubMed] [Google Scholar]
- 48.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR Jr., Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology 2005;234:35–43. doi: 10.1148/radiol.2341040439 [DOI] [PubMed] [Google Scholar]
- 49.Nelson JC, Kronmal RA, Carr JJ, McNitt-Gray MF, Wong ND, Loria CM, Goldin JG, Williams OD, Detrano R. Measuring coronary calcium on CT images adjusted for attenuation differences. Radiology 2005;235:403–414. doi: 10.1148/radiol.2352040515 [DOI] [PubMed] [Google Scholar]
- 50.Carr JJ, Crouse JR 3rd, Goff DC Jr., D’Agostino RB Jr., Peterson NP, Burke GL. Evaluation of subsecond gated helical CT for quantification of coronary artery calcium and comparison with electron beam CT. AJR Am J Roentgenol 2000;174:915–921. doi: 10.2214/ajr.174.4.1740915 [DOI] [PubMed] [Google Scholar]
- 51.Detrano RC, Anderson M, Nelson J, Wong ND, Carr JJ, McNitt-Gray M, Bild DE. Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility--MESA study. Radiology 2005;236:477–484. doi: 10.1148/radiol.2362040513 [DOI] [PubMed] [Google Scholar]
- 52.Budoff MJ, Katz R, Wong ND, Nasir K, Mao SS, Takasu J, Kronmal R, Detrano RC, Shavelle DM, Blumenthal RS, et al. Effect of Scanner Type on The Reproducibility of Extracoronary Measures of Calcification: The Multi-Ethnic Study of Atherosclerosis. Academic Radiology 2007;14:1043–1049. doi: 10.1016/j.acra.2007.05.021 [DOI] [PubMed] [Google Scholar]
- 53.Budoff MJ, McClelland RL, Chung H, Wong ND, Carr JJ, Gray MM, Blumenthal RS, Detrano RC. Reproducibility of Coronary Artery Calcified Plaque with Cardiac 64-MDCT: The Multi-Ethnic Study of Atherosclerosis. American Journal of Roentgenology 2009;192:613–617. doi: 10.2214/AJR.08.1242 [DOI] [PubMed] [Google Scholar]
- 54.Arad Y, Spadaro LA, Goodman K, Lledo-Perez A, Sherman S, Lerner G, Guerci AD. Predictive value of electron beam computed tomography of the coronary arteries. 19-month follow-up of 1173 asymptomatic subjects. Circulation 1996;93:1951–1953. doi: 10.1161/01.cir.93.11.1951 [DOI] [PubMed] [Google Scholar]
- 55.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336–1345. doi: 10.1056/NEJMoa072100 [DOI] [PubMed] [Google Scholar]
- 56.Vliegenthart R, Oudkerk M, Hofman A, Oei HH, van Dijck W, van Rooij FJ, Witteman JC. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation 2005;112:572–577. doi: 10.1161/circulationaha.104.488916 [DOI] [PubMed] [Google Scholar]
- 57.Carr JJ, Jacobs DR Jr., Terry JG, Shay CM, Sidney S, Liu K, Schreiner PJ, Lewis CE, Shikany JM, Reis JP, et al. Association of Coronary Artery Calcium in Adults Aged 32 to 46 Years With Incident Coronary Heart Disease and Death. JAMA Cardiol 2017;2:391–399. doi: 10.1001/jamacardio.2016.5493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LaMonte MJ, FitzGerald SJ, Church TS, Barlow CE, Radford NB, Levine BD, Pippin JJ, Gibbons LW, Blair SN, Nichaman MZ. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol 2005;162:421–429. doi: 10.1093/aje/kwi228 [DOI] [PubMed] [Google Scholar]
- 59.Sung JH, Yeboah J, Lee JE, Smith CL, Terry JG, Sims M, Samdarshi T, Musani S, Fox E, Ge Y, et al. Diagnostic Value of Coronary Artery Calcium Score for Cardiovascular Disease in African Americans: The Jackson Heart Study. Br J Med Med Res 2016;11. doi: 10.9734/bjmmr/2016/21449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osei AD, Mirbolouk M, Berman D, Budoff MJ, Miedema MD, Rozanski A, Rumberger JA, Shaw L, Al Rifai M, Dzaye O, et al. Prognostic value of coronary artery calcium score, area, and density among individuals on statin therapy vs. non-users: The coronary artery calcium consortium. Atherosclerosis 2021;316:79–83. doi: 10.1016/j.atherosclerosis.2020.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mortensen MB, Dzaye O, Steffensen FH, Bøtker HE, Jensen JM, Rønnow Sand NP, Kragholm KH, Sørensen HT, Leipsic J, Mæng M, et al. Impact of Plaque Burden Versus Stenosis on Ischemic Events in Patients With Coronary Atherosclerosis. J Am Coll Cardiol 2020;76:2803–2813. doi: 10.1016/j.jacc.2020.10.021 [DOI] [PubMed] [Google Scholar]
- 62.Ajufo E, Ayers CR, Vigen R, Joshi PH, Rohatgi A, de Lemos JA, Khera A. Value of Coronary Artery Calcium Scanning in Association With the Net Benefit of Aspirin in Primary Prevention of Atherosclerotic Cardiovascular Disease. JAMA Cardiology 2021;6:179–187. doi: 10.1001/jamacardio.2020.4939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol 2005;46:158–165. doi: 10.1016/j.jacc.2005.02.088 [DOI] [PubMed] [Google Scholar]
- 64.Hoffmann U, Massaro JM, D’Agostino RB Sr., Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular Event Prediction and Risk Reclassification by Coronary, Aortic, and Valvular Calcification in the Framingham Heart Study. J Am Heart Assoc 2016;5. doi: 10.1161/jaha.115.003144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol 2005;46:807–814. doi: 10.1016/j.jacc.2005.05.049 [DOI] [PubMed] [Google Scholar]
- 66.McClelland RL, Jorgensen NW, Budoff M, Blaha MJ, Post WS, Kronmal RA, Bild DE, Shea S, Liu K, Watson KE, et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J Am Coll Cardiol 2015;66:1643–1653. doi: 10.1016/j.jacc.2015.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Detrano RC, Wong ND, Doherty TM, Shavelle RM, Tang W, Ginzton LE, Budoff MJ, Narahara KA. Coronary calcium does not accurately predict near-term future coronary events in high-risk adults. Circulation 1999;99:2633–2638. doi: 10.1161/01.cir.99.20.2633 [DOI] [PubMed] [Google Scholar]
- 68.Grandhi GR, Mirbolouk M, Dardari ZA, Al-Mallah MH, Rumberger JA, Shaw LJ, Blankstein R, Miedema MD, Berman DS, Budoff MJ, et al. Interplay of Coronary Artery Calcium and Risk Factors for Predicting CVD/CHD Mortality: The CAC Consortium. JACC Cardiovasc Imaging 2020;13:1175–1186. doi: 10.1016/j.jcmg.2019.08.024 [DOI] [PubMed] [Google Scholar]
- 69.Blaha MJ, Budoff MJ, Tota-Maharaj R, Dardari ZA, Wong ND, Kronmal RA, Eng J, Post WS, Blumenthal RS, Nasir K. Improving the CAC Score by Addition of Regional Measures of Calcium Distribution: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging 2016;9:1407–1416. doi: 10.1016/j.jcmg.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nasir K, Cainzos-Achirica M, Valero-Elizondo J, Ali SS, Havistin R, Lakshman S, Blaha MJ, Blankstein R, Shapiro MD, Arias L, et al. Coronary Atherosclerosis in an Asymptomatic U.S. Population. JACC: Cardiovascular Imaging 2022;15:1604–1618. doi: doi: 10.1016/j.jcmg.2022.03.010 [DOI] [PubMed] [Google Scholar]
- 71.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140:e596–e646. doi: doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Golub IS, Termeie OG, Kristo S, Schroeder LP, Lakshmanan S, Shafter AM, Hussein L, Verghese D, Aldana-Bitar J, Manubolu VS, et al. Major Global Coronary Artery Calcium Guidelines. JACC: Cardiovascular Imaging 2023;16:98–117. doi: doi: 10.1016/j.jcmg.2022.06.018 [DOI] [PubMed] [Google Scholar]
- 73.Allison MA, Hsi S, Wassel CL, Morgan C, Ix JH, Wright CM, Criqui MH. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol 2012;32:140–146. doi: 10.1161/ATVBAHA.111.235234 [DOI] [PubMed] [Google Scholar]
- 74.Lanzer P, Hannan FM, Lanzer JD, Janzen J, Raggi P, Furniss D, Schuchardt M, Thakker R, Fok PW, Saez-Rodriguez J, et al. Medial Arterial Calcification: JACC State-of-the-Art Review. J Am Coll Cardiol 2021;78:1145–1165. doi: 10.1016/j.jacc.2021.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han D, Kuronuma K, Rozanski A, Budoff MJ, Miedema MD, Nasir K, Shaw LJ, Rumberger JA, Gransar H, Blumenthal RS, et al. Implication of thoracic aortic calcification over coronary calcium score regarding the 2018 ACC/AHA Multisociety cholesterol guideline: results from the CAC Consortium. Am J Prev Cardiol 2021;8:100232. doi: 10.1016/j.ajpc.2021.100232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas IC, McClelland RL, Michos ED, Allison MA, Forbang NI, Longstreth WT Jr., Post WS, Wong ND, Budoff MJ, Criqui MH. Density of calcium in the ascending thoracic aorta and risk of incident cardiovascular disease events. Atherosclerosis 2017;265:190–196. doi: 10.1016/j.atherosclerosis.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Craiem D, Chironi G, Casciaro ME, Graf S, Simon A. Calcifications of the thoracic aorta on extended non-contrast-enhanced cardiac CT. PLoS One 2014;9:e109584. doi: 10.1371/journal.pone.0109584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leow K, Szulc P, Schousboe JT, Kiel DP, Pinto AT, Shaikh H et al. Prognostic Value of Abdominal Aortic Calcification: A Systematic Review and Meta‐Analysis of Observational Studies. J Am Heart Assoc 2021:10(12): e017205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jurgens PT, Carr JJ, Terry JG, Rana JS, Jacobs DR Jr., Duprez DA. Association of Abdominal Aorta Calcium and Coronary Artery Calcium with Incident Cardiovascular and Coronary Heart Disease Events in Black and White Middle-Aged People: The Coronary Artery Risk Development in Young Adults Study. J Am Heart Assoc 2021;10:e023037. doi: 10.1161/JAHA.121.023037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Budoff MJ, Katz R, Wong ND, Nasir K, Mao SS, Takasu J, Kronmal R, Detrano RC, Shavelle DM, Blumenthal RS, et al. Effect of scanner type on the reproducibility of extracoronary measures of calcification: the multi-ethnic study of atherosclerosis. Acad Radiol 2007;14:1043–1049. doi: 10.1016/j.acra.2007.05.021 [DOI] [PubMed] [Google Scholar]
- 81.Budoff MJ, Takasu J, Katz R, Mao S, Shavelle DM, O’Brien KD, Blumenthal RS, Carr JJ, Kronmal R. Reproducibility of CT measurements of aortic valve calcification, mitral annulus calcification, and aortic wall calcification in the multi-ethnic study of atherosclerosis. Acad Radiol 2006;13:166–172. doi: 10.1016/j.acra.2005.09.090 [DOI] [PubMed] [Google Scholar]
- 82.Bos D, Leening MJ, Kavousi M, Hofman A, Franco OH, van der Lugt A, Vernooij MW, Ikram MA. Comparison of Atherosclerotic Calcification in Major Vessel Beds on the Risk of All-Cause and Cause-Specific Mortality: The Rotterdam Study. Circ Cardiovasc Imaging 2015;8. doi: 10.1161/CIRCIMAGING.115.003843 [DOI] [PubMed] [Google Scholar]
- 83.Tugcu A, Jin Z, Homma S, Elkind MS, Rundek T, Yoshita M, DeCarli C, Nakanishi K, Shames S, Wright CB, et al. Atherosclerotic Plaques in the Aortic Arch and Subclinical Cerebrovascular Disease. Stroke 2016;47:2813–2819. doi: 10.1161/STROKEAHA.116.015002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Budoff MJ, Nasir K, Katz R, Takasu J, Carr JJ, Wong ND, Allison M, Lima JA, Detrano R, Blumenthal RS, et al. Thoracic aortic calcification and coronary heart disease events: the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis 2011;215:196–202. doi: 10.1016/j.atherosclerosis.2010.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Danielsen R, Sigvaldason H, Thorgeirsson G, Sigfusson N. Predominance of aortic calcification as an atherosclerotic manifestation in women: the Reykjavik study. J Clin Epidemiol 1996;49:383–387. doi: 10.1016/0895-4356(95)00547-1 [DOI] [PubMed] [Google Scholar]
- 86.Wong ND, Gransar H, Shaw L, Polk D, Moon JH, Miranda-Peats R, Hayes SW, Thomson LE, Rozanski A, Friedman JD, et al. Thoracic aortic calcium versus coronary artery calcium for the prediction of coronary heart disease and cardiovascular disease events. JACC Cardiovasc Imaging 2009;2:319–326. doi: 10.1016/j.jcmg.2008.12.010 [DOI] [PubMed] [Google Scholar]
- 87.Kalsch H, Lehmann N, Berg MH, Mahabadi AA, Mergen P, Mohlenkamp S, Bauer M, Kara K, Dragano N, Hoffmann B, et al. Coronary artery calcification outperforms thoracic aortic calcification for the prediction of myocardial infarction and all-cause mortality: the Heinz Nixdorf Recall Study. Eur J Prev Cardiol 2014;21:1163–1170. doi: 10.1177/2047487313482281 [DOI] [PubMed] [Google Scholar]
- 88.Yeboah J, Carr JJ, Terry JG, Ding J, Zeb I, Liu S, Nasir K, Post W, Blumenthal RS, Budoff MJ. Computed tomography-derived cardiovascular risk markers, incident cardiovascular events, and all-cause mortality in nondiabetics: the Multi-Ethnic Study of Atherosclerosis. Eur J Prev Cardiol 2014;21:1233–1241. doi: 10.1177/2047487313492065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Criqui MH, Denenberg JO, McClelland RL, Allison MA, Ix JH, Guerci A, Cohoon KP, Srikanthan P, Watson KE, Wong ND. Abdominal aortic calcium, coronary artery calcium, and cardiovascular morbidity and mortality in the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2014;34:1574–1579. doi: 10.1161/ATVBAHA.114.303268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rogers IS, Massaro JM, Truong QA, Mahabadi AA, Kriegel MF, Fox CS, Thanassoulis G, Isselbacher EM, Hoffmann U, O’Donnell CJ. Distribution, Determinants, and Normal Reference Values of Thoracic and Abdominal Aortic Diameters by Computed Tomography (from the Framingham Heart Study). The American Journal of Cardiology 2013;111:1510–1516. doi: 10.1016/j.amjcard.2013.01.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li S, Zhuang B, Yin G, Yang X, Zhao S, Lu M. Reference values of thoracic aorta and pulmonary artery diameters by age and gender in healthy Chinese adults assessed by cardiac magnetic resonance imaging: data from national center for cardiovascular diseases of China. The International Journal of Cardiovascular Imaging 2021;37:1423–1431. doi: 10.1007/s10554-020-02116-9 [DOI] [PubMed] [Google Scholar]
- 92.Mora C, Marcus C, Barbe C, Ecarnot F, Long A. Measurement of Maximum Diameter of Native Abdominal Aortic Aneurysm by Angio-CT: Reproducibility is Better with the Semi-automated Method. European Journal of Vascular and Endovascular Surgery 2014;47:139–150. doi: 10.1016/j.ejvs.2013.10.013 [DOI] [PubMed] [Google Scholar]
- 93.Long A, Rouet L, Lindholt JS, Allaire E. Measuring the Maximum Diameter of Native Abdominal Aortic Aneurysms: Review and Critical Analysis. European Journal of Vascular and Endovascular Surgery 2012;43:515–524. doi: 10.1016/j.ejvs.2012.01.018 [DOI] [PubMed] [Google Scholar]
- 94.Lakshmanan R, Hyde Z, Jamrozik K, Hankey GJ, Norman PE. Population-based observational study of claudication in older men: the Health in Men Study. Medical Journal of Australia 2010;192:641–645. doi: 10.5694/j.1326-5377.2010.tb03663.x [DOI] [PubMed] [Google Scholar]
- 95.Forsdahl SH, Solberg S, Singh K, Jacobsen BK. Abdominal aortic aneurysms, or a relatively large diameter of non-aneurysmal aortas, increase total and cardiovascular mortality: the Tromsø study. International Journal of Epidemiology 2009;39:225–232. doi: 10.1093/ije/dyp320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sidloff DA, Saratzis A, Thompson J, Katsogridakis E, Bown MJ. Editor’s Choice - Infra-Renal Aortic Diameter and Cardiovascular Risk: Making Better Use of Abdominal Aortic Aneurysm Screening Outcomes. Eur J Vasc Endovasc Surg 2021;62:38–45. doi: 10.1016/j.ejvs.2021.03.013 [DOI] [PubMed] [Google Scholar]
- 97.Fernandez-Friera L, Penalvo JL, Fernandez-Ortiz A, Ibanez B, Lopez-Melgar B, Laclaustra M, Oliva B, Mocoroa A, Mendiguren J, Martinez de Vega V, et al. Prevalence, Vascular Distribution, and Multiterritorial Extent of Subclinical Atherosclerosis in a Middle-Aged Cohort: The PESA (Progression of Early Subclinical Atherosclerosis) Study. Circulation 2015;131:2104–2113. doi: 10.1161/CIRCULATIONAHA.114.014310 [DOI] [PubMed] [Google Scholar]
- 98.Laclaustra M, Casasnovas JA, Fernandez-Ortiz A, Fuster V, Leon-Latre M, Jimenez-Borreguero LJ, Pocovi M, Hurtado-Roca Y, Ordovas JM, Jarauta E, et al. Femoral and Carotid Subclinical Atherosclerosis Association With Risk Factors and Coronary Calcium: The AWHS Study. J Am Coll Cardiol 2016;67:1263–1274. doi: 10.1016/j.jacc.2015.12.056 [DOI] [PubMed] [Google Scholar]
- 99.Bermudez-Lopez M, Martinez-Alonso M, Castro-Boque E, Betriu A, Cambray S, Farras C, Barbe F, Pamplona R, Lecube A, Mauricio D, et al. Subclinical atheromatosis localization and burden in a low-to-moderate cardiovascular risk population: the ILERVAS study. Rev Esp Cardiol (Engl Ed) 2021;74:1042–1053. doi: 10.1016/j.rec.2020.09.015 [DOI] [PubMed] [Google Scholar]
- 100.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Fatar M, et al. Mannheim carotid intima-media thickness consensus (2004–2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis 2007;23:75–80. doi: 10.1159/000097034 [DOI] [PubMed] [Google Scholar]
- 101.Kocyigit D, Gurses KM, Taydas O, Poker A, Ozer N, Hazirolan T, Tokgozoglu L. Role of femoral artery ultrasound imaging in cardiovascular event risk prediction in a primary prevention cohort at a medium-term follow-up. J Cardiol 2020;75:537–543. doi: 10.1016/j.jjcc.2019.09.012 [DOI] [PubMed] [Google Scholar]
- 102.Nicolaides AN, Panayiotou AG, Griffin M, Tyllis T, Bond D, Georgiou N, Kyriacou E, Avraamides C, Martin RM. Arterial Ultrasound Testing to Predict Atherosclerotic Cardiovascular Events. J Am Coll Cardiol 2022;79:1969–1982. doi: 10.1016/j.jacc.2022.03.352 [DOI] [PubMed] [Google Scholar]
- 103.Belcaro G, Nicolaides AN, Ramaswami G, Cesarone MR, De Sanctis M, Incandela L, Ferrari P, Geroulakos G, Barsotti A, Griffin M, et al. Carotid and femoral ultrasound morphology screening and cardiovascular events in low risk subjects: a 10-year follow-up study (the CAFES-CAVE study(1)). Atherosclerosis 2001;156:379–387. doi: 10.1016/s0021-9150(00)00665-1 [DOI] [PubMed] [Google Scholar]
- 104.Sun P, Dwyer KM, Merz CNB, Sun W, Johnson CA, Shircore AM, Dwyer JH. Blood Pressure, LDL Cholesterol, and Intima-Media Thickness. Arteriosclerosis, Thrombosis, and Vascular Biology 2000;20:2005–2010. doi: doi: 10.1161/01.ATV.20.8.2005 [DOI] [PubMed] [Google Scholar]
- 105.Lamina C, Meisinger C, Heid IM, Löwel H, Rantner B, Koenig W, Kronenberg F. Association of ankle-brachial index and plaques in the carotid and femoral arteries with cardiovascular events and total mortality in a population-based study with 13 years of follow-up. Eur Heart J 2006;27:2580–2587. doi: 10.1093/eurheartj/ehl228 [DOI] [PubMed] [Google Scholar]
- 106.Lamina C, Meisinger C, Heid IM, Löwel H, Rantner B, Koenig W, Kronenberg F, Group ftKS. Association of ankle-brachial index and plaques in the carotid and femoral arteries with cardiovascular events and total mortality in a population-based study with 13 years of follow-up. European Heart Journal 2006;27:2580–2587. doi: 10.1093/eurheartj/ehl228 [DOI] [PubMed] [Google Scholar]
- 107.Cournot M, Taraszkiewicz D, Cambou J-P, Galinier M, Boccalon H, Hanaire-Broutin H, Chamontin B, Carrié D, Ferrières J. Additional prognostic value of physical examination, exercise testing, and arterial ultrasonography for coronary risk assessment in primary prevention. American Heart Journal 2009;158:845–851. doi: 10.1016/j.ahj.2009.08.017 [DOI] [PubMed] [Google Scholar]
- 108.Marin M, Bia D, Zocalo Y. Carotid and Femoral Atherosclerotic Plaques in Asymptomatic and Non-Treated Subjects: Cardiovascular Risk Factors, 10-Years Risk Scores, and Lipid Ratios Capability to Detect Plaque Presence, Burden, Fibro-Lipid Composition and Geometry. J Cardiovasc Dev Dis 2020;7. doi: 10.3390/jcdd7010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McDermott MM, Liu K, Carr J, Criqui MH, Tian L, Li D, Ferrucci L, Guralnik JM, Kramer CM, Yuan C, et al. Superficial Femoral Artery Plaque, the Ankle-Brachial Index, and Leg Symptoms in Peripheral Arterial Disease. Circulation: Cardiovascular Imaging 2011;4:246–252. doi: doi: 10.1161/CIRCIMAGING.110.962183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kröger K, Stang A, Kondratieva J, Moebus S, Beck E, Schmermund A, Möhlenkamp S, Dragano N, Siegrist J, Jöckel K-H, et al. Prevalence of Peripheral Arterial Disease – Results of the Heinz Nixdorf Recall Study. European Journal of Epidemiology 2006;21:279–285. doi: 10.1007/s10654-006-0015-9 [DOI] [PubMed] [Google Scholar]
- 111.Herraiz-Adillo Á, Cavero-Redondo I, Álvarez-Bueno C, Pozuelo-Carrascosa DP, Solera-Martínez M. The accuracy of toe brachial index and ankle brachial index in the diagnosis of lower limb peripheral arterial disease: A systematic review and meta-analysis. Atherosclerosis 2020;315:81–92. doi: 10.1016/j.atherosclerosis.2020.09.026 [DOI] [PubMed] [Google Scholar]
- 112.Liefde IId Verhagen HJ, Stolker RJ Domburg RTv, Poldermans D. The value of treadmill exercise test parameters together in patients with known or suspected peripheral arterial disease. European Journal of Preventive Cardiology 2012;19:192–198. doi: 10.1177/1741826711399986 [DOI] [PubMed] [Google Scholar]
- 113.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FGR, Hiatt WR, Jönsson B, Lacroix P, et al. Measurement and Interpretation of the Ankle-Brachial Index. Circulation 2012;126:2890–2909. doi: doi: 10.1161/CIR.0b013e318276fbcb [DOI] [PubMed] [Google Scholar]
- 114.Casey S, Lanting S, Oldmeadow C, Chuter V. The reliability of the ankle brachial index: a systematic review. Journal of Foot and Ankle Research 2019;12:39. doi: 10.1186/s13047-019-0350-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA: The Journal of the American Medical Association 2008;300:197–208. doi: 10.1001/jama.300.2.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Forés R, Alzamora MT, Pera G, Baena-Díez JM, Mundet-Tuduri X, Torán P. Contribution of the ankle-brachial index to improve the prediction of coronary risk: The ARTPER cohort. PLOS ONE 2018;13:e0191283. doi: 10.1371/journal.pone.0191283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Newman AB SL, Manolio TA, Cushman M, Mittelmark M, Polak JF, Powe NR, Siscovick D. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. [published correction appears in 545]. Arterioscler Thromb Vasc Biol 1888;19. [DOI] [PubMed] [Google Scholar]
- 118.Hooi JD KA, Stoffers HE, Rinkens PE, Knottnerus JA, van Ree JW. Asymptomatic periph-eral arterial occlusive disease predicted cardiovascu-lar morbidity and mortality in a 7-year follow-up study. J Clin Epidemiol 2004;57:294–300. [DOI] [PubMed] [Google Scholar]
- 119.Leng GC FF, Lee AJ, Dunbar J, Housley E, Ruckley CV. Use of ankle brachial pressure index to predict cardiovascular events and death: a cohort study BMJ : British Medical Journal 1996;313:1440–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Resnick HELR, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, Howard BV. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation 2004;109:733–739. [DOI] [PubMed] [Google Scholar]
- 121.Murphy TP, Dhangana R, Pencina MJ, D’Agostino RB Sr. Ankle-brachial index and cardiovascular risk prediction: an analysis of 11,594 individuals with 10-year follow-up. Atherosclerosis 2012;220:160–167. doi: 10.1016/j.atherosclerosis.2011.10.037 [DOI] [PubMed] [Google Scholar]
- 122.Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, Ix JH, Burke GL, Liu K, Shea S. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2010;56:1506–1512. doi: 10.1016/j.jacc.2010.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Velescu A, Cara A/, Prenenal J, Ramos R, Marti R, Garau M, Degano ir., Marrugat J Adding low ankle brachial index to classical risk factors improves the prediction of major cardiovascular events. The REGICOR study. Atherosclerosis 2015;241:357–363. [DOI] [PubMed] [Google Scholar]
- 124.Wild SH BC, Smith FB, Lee AJ, Fowkes. Diabetes Care 2006;29:637–642. doi: 10.2337/diacare.29.03.06.dc05-1637 [DOI] [PubMed] [Google Scholar]
- 125.Johnsen HS, Mathiesen E Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Curr Cardiol Rep 2009;``:21–27. doi: 10.1007/s11886-009-0004-1. [DOI] [PubMed]
- 126.Shimbo D, Grahame-Clarke. D, Miyake Y,Y, rodriguez C, Sciacca R, di Tullio M, et al. The association between endothelial dysfunction and cardiovascular outcomes in a population-based multi-ethnic cohort. Atherosclerosis 2007;192:197–203. doi: 10.1016/j.atherosclerosis.2006.05.005 [DOI] [PubMed] [Google Scholar]
- 127.Schnabel RB, Magnussen C, Schulz A, Ojeda FM, Schmitt VH, Arnold N, et al. Non-invasive peripheral vascular function, incident cardiovascular disease, and mortality in the general populationSchnabel. Cardiovasc Res 2022;118:904–912. doi: 10.1093/cvr/cvab087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zheng H, Wu S, Liu X, Qiu G, Chen S, Wu Y, Li J, Yin C, Zhang Q Association Between Arterial Stiffness and New-Onset Heart Failure: The Kailuan Study. Arterioscler Thromb Vasc Biol 2023;43. doi: 10.1161/ATVBAHA.122.317715. [DOI] [PubMed] [Google Scholar]
- 129.Sturlaugsdottir R, Aspelund T, Bjornsdottir G, Sigurdsson S, Thorsson B, Eiriksdottir G, Gudnason V. Predictors of carotid plaque progression over a 4-year follow-up in the Reykjavik REFINE-study. Atherosclerosis 2018;269:57–62. doi: 10.1016/j.atherosclerosis.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 130.Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, Detrano RC, Bild DE, Guerci AD, Liu K, et al. Progression of Coronary Calcium and Incident Coronary Heart Disease Events. Journal of the American College of Cardiology 2013;61:1231–1239. doi: doi: 10.1016/j.jacc.2012.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, DeMoss D, Nuguri V, Nabavi V, Ratakonda R, et al. Progression of Coronary Artery Calcium Predicts All-Cause Mortality. JACC: Cardiovascular Imaging 2010;3:1229–1236. doi: doi: 10.1016/j.jcmg.2010.08.018 [DOI] [PubMed] [Google Scholar]
- 132.Lehmann N, Erbel R, Mahabadi AA, Rauwolf M, Möhlenkamp S, Moebus S, Kälsch H, Budde T, Schmermund A, Stang A, et al. Value of Progression of Coronary Artery Calcification for Risk Prediction of Coronary and Cardiovascular Events. Circulation 2018;137:665–679. doi: doi: 10.1161/CIRCULATIONAHA.116.027034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hodis HN, Mack WJ, Dustin L, Mahrer PR, Azen SP, Detrano R, Selhub J, Alaupovic P, Liu CR, Liu CH, et al. High-dose B vitamin supplementation and progression of subclinical atherosclerosis: a randomized controlled trial. Stroke 2009;40:730–736. doi: 10.1161/STROKEAHA.108.526798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Johnson HM, Piper ME, Baker TB, Fiore MC, Stein JH. Effects of smoking and cessation on subclinical arterial disease: a substudy of a randomized controlled trial. PLoS One 2012;7:e35332. doi: 10.1371/journal.pone.0035332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tam LS, Li EK, Shang Q, Tomlinson B, Lee VW, Lee KK, Li M, Kuan WP, Li TK, Tseung L, et al. Effects of rosuvastatin on subclinical atherosclerosis and arterial stiffness in rheumatoid arthritis: a randomized controlled pilot trial. Scand J Rheumatol 2011;40:411–421. doi: 10.3109/03009742.2011.586649 [DOI] [PubMed] [Google Scholar]
- 136.Ibad A, Khalid R, Thompson PD. Chelation therapy in the treatment of cardiovascular diseases. J Clin Lipidol 2016;10:58–62. doi: 10.1016/j.jacl.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 137.Ibanez B, Fernandez-Ortiz A, Fernandez-Friera L, Garcia-Lunar I, Andres V, Fuster V. Progression of Early Subclinical Atherosclerosis (PESA) Study: JACC Focus Seminar 7/8. J Am Coll Cardiol 2021;78:156–179. doi: 10.1016/j.jacc.2021.05.011 [DOI] [PubMed] [Google Scholar]
- 138.Chhoudhuri RP FV, Badimon JJ, Fisher EA, Fayad ZA. MRI and characterization of atherosclerotic plaque: emerging applications and molecular imaging. Arterioscler Thromb Vasc Biol 2002;22:1065–1074. [DOI] [PubMed] [Google Scholar]
- 139.Cho A MRI for all. Science 2023;379:748–751. [DOI] [PubMed] [Google Scholar]
- 140.Moreno P, Lodder RA, K.Purushothaman R, Charash WE, . O’Connor WN, Muller JE. Detection of Lipid Pool, Thin Fibrous Cap, and Inflammatory Cells in Human Aortic Atherosclerotic Plaques by Near-Infrared Spectroscopy Circulation 2002;105:923–927. [DOI] [PubMed] [Google Scholar]
- 141.Bambagioni G DMC, Torguspm R, Demola P, Ali Z, Singh V et a. ipid-rich plaques detected by near-infrared spectroscopy predict coronary events irrespective of age: A Lipid Rich Plaque sub-study. Atherosclerosis 2021;34:17–33. [DOI] [PubMed] [Google Scholar]
- 142.Anastassopoulou J MV, Mylonas E, Kolovou P, Mamarelis I, Kotoulas C et al. Infrared Spectroscopic Study and Mathematical Simulations of Carotid Atherosclerosis. In vivo 2022;36:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mokry M BK, Slenders L, Bel-Bordes G, Brouwer KCE, Mekke JM et al. Transcriptomic-based clustering of human atherosclerotic plaques identifies subgroups with different underlying biology and clinical presentation. Nature Caridovascular Research 2022;1:1140–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saba L SS, Gupta SK, Koppula VK, John AM, Khanna NN et al. Multimodality carotid plaque tissue characterization and classification in the artificial intelligence paradigm: a narrative review for stroke application. ATM Annals of Translational Medicine 2021;9:1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rogers MAAE. Cardiovascular calcification: artificial intelligence and big data accelerate mechanistic discovery. Nature Rev Cardiol 2019;16:261–274. [DOI] [PubMed] [Google Scholar]
- 146.Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, Volcik K, Boerwinkle E, Ballantyne CM. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol 2010;55:1600–1607. doi: 10.1016/j.jacc.2009.11.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gardin JM, Bartz TM, Polak JF, O’Leary DH, Wong ND. What do carotid intima-media thickness and plaque add to the prediction of stroke and cardiovascular disease risk in older adults? The cardiovascular health study. J Am Soc Echocardiogr 2014;27:998–1005 e1002. doi: 10.1016/j.echo.2014.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Amato M, Veglia F, de Faire U, Giral P, Rauramaa R, Smit AJ, Kurl S, Ravani A, Frigerio B, Sansaro D, et al. Carotid plaque-thickness and common carotid IMT show additive value in cardiovascular risk prediction and reclassification. Atherosclerosis 2017;263:412–419. doi: 10.1016/j.atherosclerosis.2017.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Prati P, Tosetto A, Vanuzzo D, Bader G, Casaroli M, Canciani L, Castellani S, Touboul PJ. Carotid intima media thickness and plaques can predict the occurrence of ischemic cerebrovascular events. Stroke 2008;39:2470–2476. doi: 10.1161/STROKEAHA.107.511584 [DOI] [PubMed] [Google Scholar]
- 150.Hald EM, Lijfering WM, Mathiesen EB, Johnsen SH, Løchen ML, Njølstad I, Wilsgaard T, Rosendaal FR, Brækkan SK, Hansen JB. Carotid atherosclerosis predicts future myocardial infarction but not venous thromboembolism: the Tromso study. Arterioscler Thromb Vasc Biol 2014;34:226–230. doi: 10.1161/atvbaha.113.302162 [DOI] [PubMed] [Google Scholar]
- 151.Tison GH, Guo M, Blaha MJ, McClelland RL, Allison MA, Szklo M, Wong ND, Blumenthal RS, Budoff MJ, Nasir K. Multisite extracoronary calcification indicates increased risk of coronary heart disease and all-cause mortality: The Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr 2015;9:406–414. doi: 10.1016/j.jcct.2015.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mahabadi AA, Lehmann N, Mohlenkamp S, Pundt N, Dykun I, Roggenbuck U, Moebus S, Jockel KH, Erbel R, Kalsch H, et al. Noncoronary Measures Enhance the Predictive Value of Cardiac CT Above Traditional Risk Factors and CAC Score in the General Population. JACC Cardiovasc Imaging 2016;9:1177–1185. doi: 10.1016/j.jcmg.2015.12.024 [DOI] [PubMed] [Google Scholar]
- 153.Kim J, Budoff MJ, Nasir K, Wong ND, Yeboah J, Al-Mallah MH, Shea S, Dardari ZA, Blumenthal RS, Blaha MJ, et al. Thoracic aortic calcium, cardiovascular disease events, and all-cause mortality in asymptomatic individuals with zero coronary calcium: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2017;257:1–8. doi: 10.1016/j.atherosclerosis.2016.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lessmann N, de Jong PA, Celeng C, Takx RAP, Viergever MA, van Ginneken B, Isgum I. Sex Differences in Coronary Artery and Thoracic Aorta Calcification and Their Association With Cardiovascular Mortality in Heavy Smokers. JACC Cardiovasc Imaging 2019;12:1808–1817. doi: 10.1016/j.jcmg.2018.10.026 [DOI] [PubMed] [Google Scholar]
- 155.Forbang NIMRL, Remegio-Baker RA, Allison MA, Sandfort V, Michos ED et al. Associations of cardiovascular disease risk factors with abdominal aortic calcium volume and density: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2016;255:54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Qazi S, Massaro JM, Chuang ML, D’Agostino RB, Hoffmann U, O’Donnell CJ. Increased Aortic Diameters on Multidetector Computed Tomographic Scan Are Independent Predictors of Incident Adverse Cardiovascular Events. Circulation: Cardiovascular Imaging 2017;10:e006776. doi: doi: 10.1161/CIRCIMAGING.117.006776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rueda-Ochoa OL, Bons LR, Zhu F, Rohde S, Ghoul KE, Budde RPJ, Ikram MK, Deckers JW, Vernooij MW, Franco OH, et al. Thoracic Aortic Diameter and Cardiovascular Events and Mortality among Women and Men. Radiology 2022;304:208–215. doi: 10.1148/radiol.210861 [DOI] [PubMed] [Google Scholar]
- 158.Duncan JL, Harrild KA, Iversen L, Lee AJ, Godden DJ. Long term outcomes in men screened for abdominal aortic aneurysm: prospective cohort study. BMJ : British Medical Journal 2012;344:e2958. doi: 10.1136/bmj.e2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Unkart J, Allison M, Araneta MR, Ix J, McDermott M, Criqui M Alternative Ankle Brachial Index Calculation and CVD Events and Mortality: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2019;139:P305. doi: 10.1161/circ.139.suppl_1.P305 [DOI] [Google Scholar]
- 160.Penson P, Henney NC, Banach M. Association between ankle-brachial index and cardiovascular mortality in a cohort from the United States of America. European Heart Journal 2021;42. doi: 10.1093/eurheartj/ehab724.2476 [DOI] [Google Scholar]
- 161.Velescu A, Clara A, Martí R, Ramos R, Perez-Fernandez S, Marcos L, Grau M, Degano IR, Marrugat J, Elosua R. Abnormally High Ankle–Brachial Index is Associated with All-cause and Cardiovascular Mortality: The REGICOR Study. European Journal of Vascular and Endovascular Surgery 2017;54:370–377. doi: 10.1016/j.ejvs.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 162.NMJ H Associations Between the Ankle-Brachial Index and Cardiovascular and All-Cause Mortality Are Similar in Individuals Without and With Type 2 Diabetes. Diabetes Care 2012;35:1731–1735. doi: 10.2337/dc12-0178 [DOI] [PMC free article] [PubMed] [Google Scholar]


