Abstract
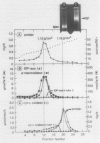
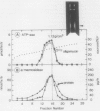
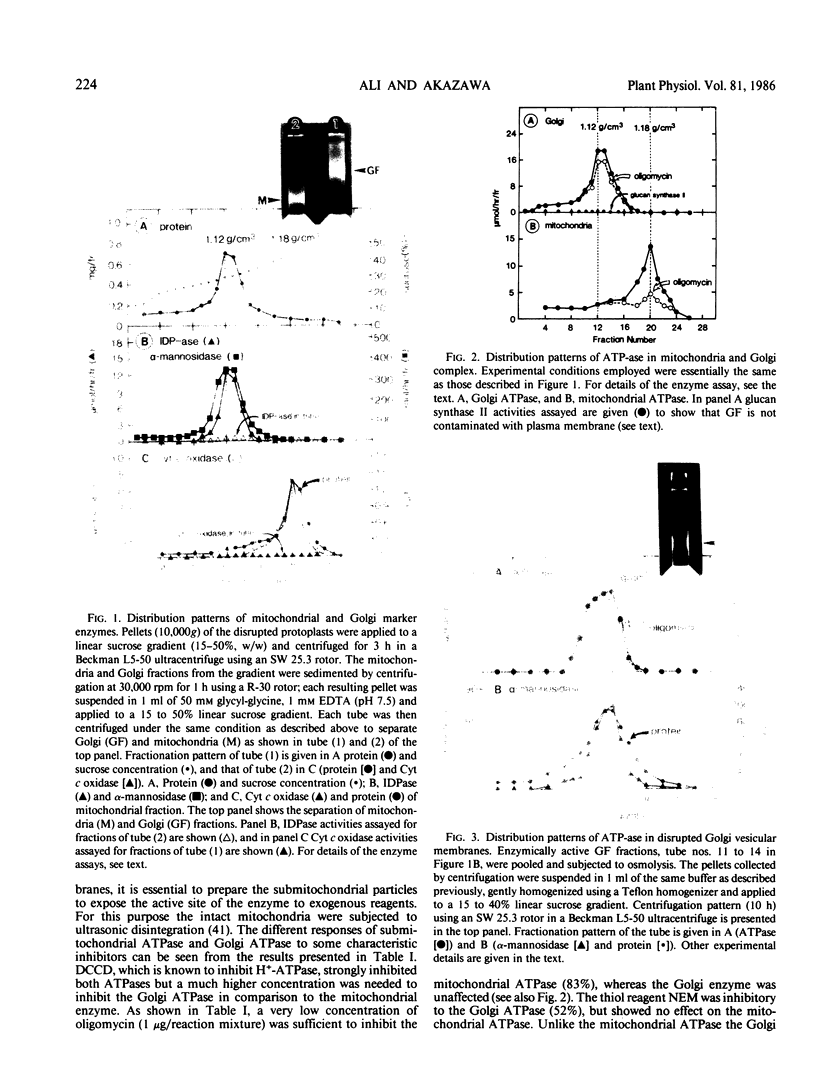
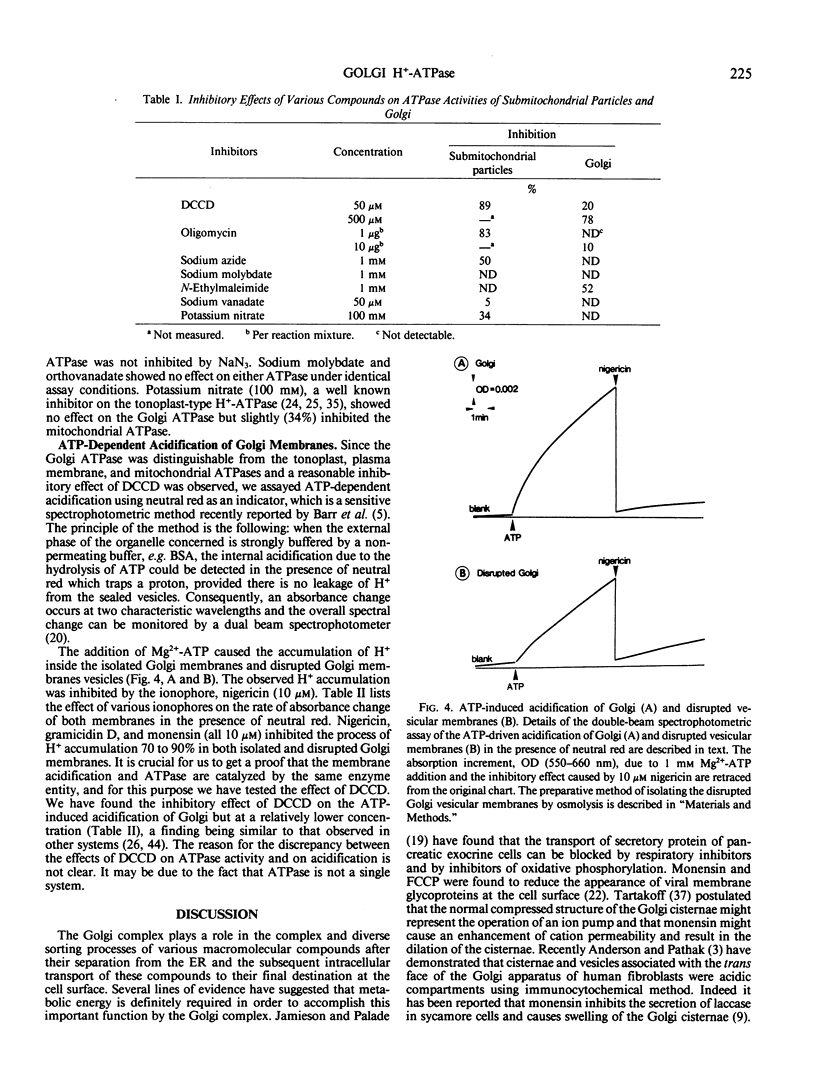
The Golgi complex and the disrupted vesicular membranes were prepared from suspension-cultured cells of sycamore (Acer pseudoplatanus L.) using protoplasts as the starting material and employing linear sucrose density gradient centrifugation followed by osmolysis (Ali et al. [1985] Plant Cell Physiol 26: 1119-1133). The isolated Golgi fraction was found to be enriched with marker enzyme activities and depleted of the activity of a typical mitochondrial marker enzyme, cytochrome c oxidase. Golgi complex, and vesicular membranes derived thereof were found to contain the specific ATPase (specific activity of about 0.5 to 0.7 micromoles per minute per milligram protein). Inhibitor studies suggested that the ATPase of Golgi was different from plasma membrane, tonoplast and mitochondrial ATPases as it was not inhibited by sodium vanadate, potassium nitrate, oligomycin and sodium azide. The sensitivity to N-ethylmaleimide further distinguished the Golgi ATPase from F0 to F1 ATPase of mitochondria. The internal acidification was measured by monitoring the difference in absorbance at 550 nanometers minus 600 nanometers using neutral red as a probe. The maximum rate detected with Golgi and disrupted membrane system was 0.49 and 0.61 optical density unit per minute per milligram protein, at pH 7.5, respectively, indicating that the proton pump activity was tightly associated with the Golgi membranes. In both cases, the acidification was inhibited 70 to 90% by various ionophores, indicating that the proton pump was electrogenic in nature. Both the Golgi ATPase activity and ATP-dependent acidification were profoundly inhibited by N,N′-dicyclohexylcarbodiimide, which also indicate that the two activities are catalyzed by the same enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Pathak R. K. Vesicles and cisternae in the trans Golgi apparatus of human fibroblasts are acidic compartments. Cell. 1985 Mar;40(3):635–643. doi: 10.1016/0092-8674(85)90212-0. [DOI] [PubMed] [Google Scholar]
- Apps D. K., Pryde J. G., Sutton R., Phillips J. H. Inhibition of adenosine triphosphatase, 5-hydroxytryptamine transport and proton-translocation activities of resealed chromaffin-granule 'ghosts'. Biochem J. 1980 Aug 15;190(2):273–282. doi: 10.1042/bj1900273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr R., Safranski K., Sun I. L., Crane F. L., Morré D. J. An electrogenic proton pump associated with the Golgi apparatus of mouse liver driven by NADH and ATP. J Biol Chem. 1984 Nov 25;259(22):14064–14067. [PubMed] [Google Scholar]
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binari L. L., Racusen R. H. Membrane-associated ATPases in isolated secretory vesicles. Plant Physiol. 1983 Mar;71(3):594–597. doi: 10.1104/pp.71.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson A., McNaughton E., Taiz L. Evidence for a KCl-Stimulated, Mg-ATPase on the Golgi of Corn Coleoptiles. Plant Physiol. 1984 Oct;76(2):498–507. doi: 10.1104/pp.76.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson A., Taiz L. Evidence for an ATP-Dependent Proton Pump on the Golgi of Corn Coleoptiles. Plant Physiol. 1985 Jun;78(2):232–240. doi: 10.1104/pp.78.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman J., Croen K., Kelly S., Al-Awqati Q. Golgi membranes contain an electrogenic H+ pump in parallel to a chloride conductance. J Cell Biol. 1983 Oct;97(4):1303–1308. doi: 10.1083/jcb.97.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. IV. Metabolic requirements. J Cell Biol. 1968 Dec;39(3):589–603. doi: 10.1083/jcb.39.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge W., Ausländer W., McGeer A. J., Runge T. The buffering capacity of the internal phase of thylakoids and the magnitude of the pH changes inside under flashing light. Biochim Biophys Acta. 1979 Apr 11;546(1):121–141. doi: 10.1016/0005-2728(79)90175-0. [DOI] [PubMed] [Google Scholar]
- Kakinuma Y., Ohsumi Y., Anraku Y. Properties of H+-translocating adenosine triphosphatase in vacuolar membranes of SAccharomyces cerevisiae. J Biol Chem. 1981 Nov 10;256(21):10859–10863. [PubMed] [Google Scholar]
- Käriäinen L., Hashimoto K., Saraste J., Virtanen I., Penttinen K. Monensin and FCCP inhibit the intracellular transport of alphavirus membrane glycoproteins. J Cell Biol. 1980 Dec;87(3 Pt 1):783–791. doi: 10.1083/jcb.87.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S., Taiz L. Partial purification of a tonoplast ATPase from corn coleoptiles. Plant Physiol. 1985 Jun;78(2):327–333. doi: 10.1104/pp.78.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S., Taiz L. Proton transport in isolated vacuoles from corn coleoptiles. Plant Physiol. 1985 May;78(1):104–109. doi: 10.1104/pp.78.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama Y., Takano T., Ohkuma S. Proton translocating ATPase in lysosomal membrane ghosts. Evidence that alkaline Mg2+-ATPase acts as a proton pump. J Biochem. 1984 Apr;95(4):995–1007. doi: 10.1093/oxfordjournals.jbchem.a134726. [DOI] [PubMed] [Google Scholar]
- O'Neal S. G., Rhoads D. B., Racker E. Vanadate inhibition of sarcoplasmic reticulum Ca2+-ATPase and other ATPases. Biochem Biophys Res Commun. 1979 Aug 13;89(3):845–850. doi: 10.1016/0006-291x(79)91855-2. [DOI] [PubMed] [Google Scholar]
- Patel L., Kaback H. R. The role of the carbodiimide-reactive component of the adenosine-5'-triphosphatase complex in the proton permeability of Escherichia coli membrane vesicles. Biochemistry. 1976 Jun 29;15(13):2741–2746. doi: 10.1021/bi00658a005. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D. L. ATP-dependent acidification of intact and disrupted lysosomes. Evidence for an ATP-driven proton pump. J Biol Chem. 1981 Apr 25;256(8):3858–3864. [PubMed] [Google Scholar]
- Serrano R. Plasma membrane ATPase of fungi and plants as a novel type of proton pump. Curr Top Cell Regul. 1984;23:87–126. doi: 10.1016/b978-0-12-152823-2.50007-6. [DOI] [PubMed] [Google Scholar]
- Serrano R. Purification of the proton pumping ATPase from plant plasma membranes. Biochem Biophys Res Commun. 1984 Jun 15;121(2):735–740. doi: 10.1016/0006-291x(84)90243-2. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Tognoli L. Partial purification and characterization of an anion-activated ATPase from radish microsomes. Eur J Biochem. 1985 Feb 1;146(3):581–588. doi: 10.1111/j.1432-1033.1985.tb08691.x. [DOI] [PubMed] [Google Scholar]
- Toll L., Howard B. D. Evidence that an ATPase and a protonmotive force function in the transport of acetylcholine into storage vesicles. J Biol Chem. 1980 Mar 10;255(5):1787–1789. [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assesmbly of the mitochondrial membrane system. VI. Mitochondrial synthesis of subunit proteins of the rutamycin-sensitive adenosine triphosphatase. J Biol Chem. 1972 Jan 25;247(2):594–603. [PubMed] [Google Scholar]
- Vara F., Serrano R. Partial purification and properties of the proton-translocating ATPase of plant plasma membranes. J Biol Chem. 1982 Nov 10;257(21):12826–12830. [PubMed] [Google Scholar]
- Virk S. S., Kirk C. J., Shears S. B. Ca2+ transport and Ca2+-dependent ATP hydrolysis by Golgi vesicles from lactating rat mammary glands. Biochem J. 1985 Mar 15;226(3):741–748. doi: 10.1042/bj2260741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X. S., Stone D. K., Racker E. Activation and partial purification of the ATPase of clathrin-coated vesicles and reconstitution of the proton pump. J Biol Chem. 1984 Oct 10;259(19):11676–11678. [PubMed] [Google Scholar]
- Xie X. S., Stone D. K., Racker E. Determinants of clathrin-coated vesicle acidification. J Biol Chem. 1983 Dec 25;258(24):14834–14838. [PubMed] [Google Scholar]
- Zhang F., Schneider D. L. The bioenergetics of Golgi apparatus function: evidence for an ATP-dependent proton pump. Biochem Biophys Res Commun. 1983 Jul 29;114(2):620–625. doi: 10.1016/0006-291x(83)90825-2. [DOI] [PubMed] [Google Scholar]