Most gram-negative bacteria in search of nutrients and space in which to multiply release toxins that selectively kill competing microorganisms while causing no harm to the producing cell. The highly specific protection function is conferred on producing cells by immunity proteins produced from the same plasmid or chromosomal region as the toxin (56, 67). Studies of these toxins should not only unravel the biological principles underlying microbial warfare but could also give useful information for designing antibiotics.
Colicins are toxins, encoded by plasmids, that are produced by and active against Escherichia coli and closely related bacteria. They are produced in large amounts and are generally released into the extracellular medium (101, 102). It is now possible to purify these 30- to 70-kDa colicins in large amounts, allowing many biochemical, biophysical, and structural investigations. The three-dimensional (3-D) structure of several of these toxins has been determined in recent years (40, 92, 123).
Each colicin shows the same type of organization, coherent with their mechanism of action. Their structure comprises three distinct domains: (i) a domain involved in the recognition of a specific receptor, (ii) a domain involved in translocation, and (iii) a domain responsible for the lethal activity. By using hybrid colicins, it has been shown that each determinant for translocation is contained in the N-terminal domain of colicins (8).
To be taken up by sensitive cells, colicins must cross a formidable physical barrier: the outer membrane with lipopolysaccharide (LPS) molecules on the outer face of its bilayer and its small pores (10 to 20 Å in diameter). The outer membrane is a permeability barrier, and the pores are the sites of nutrient uptake by two different mechanisms. The passive diffusion pathway is used by low-Mr (<700) hydrophilic molecules that diffuse across the membrane through porins (90). Bulky nutrients, such as siderophores and vitamin B12, that exceed the diffusion limit of the outer membrane porins are imported across the outer membrane by energy-dependent ligand-gated receptors. The TonB protein mediates energy transfer from the inner membrane to the outer membrane receptor (21, 97). However, there are exceptions to this rule; for example, maltodextrins are imported through the maltoporin (111) and a small substrate (ferric dicitrate) uses a TonB-dependent pathway through FecA (100).
COLICIN IMPORT
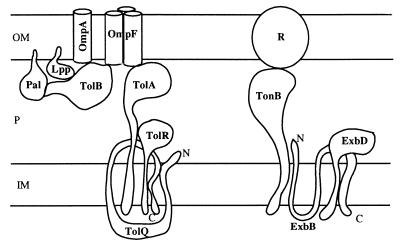
The two import pathways of colicins.
Both the passive diffusion pathway and the energy-dependent pathway have been parasitized by colicins. Genetic studies have shown that, in addition to porins, a set of proteins called Tol (for tolerant) are required for colicin import (27). Group A colicins (A, E1 to E9, K, L, N, bacteriocin 28b, and cloacin DF13) and filamentous phages (f1, fd, and M13) use the Tol system (27, 88), whereas group B colicins (B, D, Ia, Ib, M, V, 5, and 10) and phages T1 and Φ80 use the TonB system (28).
Some components of the Tol system and the TonB system are homologous (Fig. 1). Therefore, the two systems probably originated from a common ancestor (22) and their physiological functions seem to be essential because they are conserved in many gram-negative bacteria (Haemophilus influenzae, H. ducreyi, Brucella abortus, Pseudomonas putida, P. aeruginosa, etc.) (14, 26, 29, 39, 57, 96, 107, 119).
FIG. 1.
Tol and Ton systems. OM, outer membrane; IM, inner membrane; P, periplasm; R, TonB-dependent receptor; C, C terminus; N, N terminus.
Receptors of group A and B colicins.
To enter cells, colicin parasitizes multiprotein systems used by sensitive cells for important biological functions. These proteins include porins, vitamin B12, siderophore and nucleoside receptors, and multiprotein systems that cooperate with these proteins.
The receptors used by various colicins are presented in Table 1. The protein most frequently used is BtuB, the vitamin B12 receptor, which defines the so-called E type of colicins (E2 to E9) (56, 106). High-affinity receptors for iron siderophores are also used by many colicins. The nucleoside Tsx porin and the major porin OmpF are also used as receptors (Table 1). Depending on the colicin class, the colicin receptors function in cooperation with either the TonB system or the Tol system.
TABLE 1.
Characteristics of colicins
| Colicin(s) | Colicin group | Activity | Receptor | Translocation system | Reference |
|---|---|---|---|---|---|
| E2E7E8E9 | A | DNase | BtuB | OmpF TolABQR | 56 |
| E3E6 | A | RNase | BtuB | OmpF TolABQR | 56 |
| DF13 | A | RNase | IutA | TolAQR | 118 |
| E1 | A | Pore formation | BtuB | TolCAQ | 56 |
| A | A | Pore formation | BtuB | OmpF, TolABQR | 8 |
| N | A | Pore formation | OmpF | OmpF, TolAQ | 16 |
| K | A | Pore formation | Tsx | OmpFA, TolABQR | 94 |
| Col5 | B | Pore formation | Tsx | TolC, TonB, ExbBD | 94 |
| Col10 | B | Pore formation | Tsx | TolC, TonB, ExbBD | 93 |
| Ia, Ib | B | Pore formation | Cir | TonB, ExbBD | 123 |
| B | B | Pore formation | FepA | TonB, ExbBD | 113 |
| D | B | Inhibition of protein synthesis | FepA | TonB, ExbBD | 113 |
| M | B | Inhibition of synthesis of murein and LPS | FhuA | TonB, ExbBD | 123 |
TonB system.
The tonB gene is located at 28 min on the E. coli chromosomal map (3). The genes encoding the two other proteins of the TonB system, ExbB and ExbD, form an operon at 65 min on the map, and insertions in ExbB have a polar effect on the expression of ExbD (2, 38). However, the genetic organization of the three genes is different in other bacterial species. tonB expression is repressed in the presence of iron through the Fur protein and hyperexpressed under anaerobic conditions (97, 124).
Most of the polypeptide chain of TonB (239 residues) is located in the periplasm, and it is anchored in the inner membrane via an N-terminal transmembrane segment (97, 99, 108). Its rigid structure is due to X-Pro amino acid repeated sequences (17% of the residues in TonB are proline) between positions 70 and 102 (68).
ExbB (244 residues) is an integral membrane protein of the inner membrane with three transmembrane segments (62) and a large cytoplasmic domain. ExbD (141 residues) is an inner membrane protein anchored in the cytoplasmic membrane via an N-terminal transmembrane segment and a periplasmic domain of 101 residues.
Various convergent results lead to the conclusion that TonB, ExbB, and ExbD form a complex in the inner membrane. In exbB and exbD mutants, not only is TonB function altered but TonB is also unstable (2, 44, 114). The stability of ExbD is also decreased in the absence of ExbB (64). Moreover, the transmembrane anchor of TonB specifies the dependence with respect to ExbB and ExbD (64). After chemical cross-linking with formaldehyde, a 59-kDa complex was detected in the wild type but not in exbB mutants (115). When the TonB anchor was replaced with the first transmembrane segment of TetA, the TonB-ExbB complex was absent (58). More recently, a point mutation in the transmembrane segment of TonB was shown to be partially suppressed by a mutation in the first transmembrane segment of ExbB, thus suggesting that the TonB anchor may interact with the first transmembrane segment of ExbB (69). These genetic and biochemical data were recently confirmed by in vitro demonstration of the interaction between ExbD and TonB on histidine-tagged ExbB (23). Moreover, a point mutant ExbD (D25N) competes with wild-type ExbD, suggesting that the C-terminal region may be involved in the interaction with ExbB and/or TonB (23). To conclude, the three proteins ExbB, ExbD, and TonB interact via their transmembrane segments (Fig. 1). However, one cannot rule out interactions between other domains of the proteins. The complex of these three proteins is required for iron siderophore and vitamin B12 transport across the outer membrane. Each of the TonB-dependent transport systems comprises (i) a specific high-affinity outer membrane receptor, (ii) a periplasmic ligand-binding protein, and (iii) an inner membrane protein complex for import into the cytoplasm. In the import process, TonB is involved in transport across the outer membrane and the TonB-ExbB-ExbD system mediates the energy of the electrochemical potential from the inner membrane to the outer membrane (21, 98). The facts that transport across the outer membrane depends upon the proton motive force and that transport across the inner membrane is ATP dependent suggest that the two transport mechanisms are likely to be independent.
The mechanisms allowing TonB-mediated transport across the outer membrane are being studied. Various data on the interaction between TonB and TonB-dependent receptors are now available.
The use of electron spin resonance spectroscopy (ESR) in vivo (61) has recently led to a breakthrough. The time-resolved operation of the E. coli ferric enterobactin receptor FepA was observed with ESR by monitoring the mobility of covalently bound nitroxide spin labels. A ligand-binding surface loop of FepA, which normally closes its transmembrane channel, exhibited energy-dependent structural changes during iron and colicin transport. These changes were not merely associated with ligand binding but occurred during ligand uptake through the outer membrane. These results demonstrate that gated-porin channels open and close during membrane transport in vivo.
In each of the TonB-dependent receptors, there is a sequence designated the TonB box in the N-terminal region (113). Mutations in the TonB box of BtuB, FhuA, and Cir can be partially suppressed by a Q160L or Q160K mutation in the periplasmic domain of TonB (5, 51, 112). Moreover, the presence of a synthetic peptide with the TonB box sequence of FhuE (Glu-Thr-Val-Ile-Val) protects bacteria from the action of colicins B and Ia and from phages φ80 and T5, which require FepA, Cir, and FhuA, respectively (120). This suggests that the TonB protein may interact directly with the receptors via their TonB box. The interaction between TonB and TonB-dependent receptors was confirmed by chemical cross-linking experiments that provided evidence for a TonB-FepA complex (115). A complex between FhuA and TonB has also been evidenced by chemical cross-linking in vivo and without cross-linking by copurification of TonB with His-tagged FhuA (85). In vivo, overexpressed TonB is stabilized by overexpressed FhuA (50). It is not known whether the putative interaction between TonB and the receptors is transitory or permanent. The interaction between TonB and FepA has been detected by chemical cross-linking in the absence of ligand (115). However, the TonB-FhuA interaction is greatly increased in the presence of ferrichrome (85). It was then shown that this is also the case for the TonB-FepA interaction in the presence of enterocholin (98a). Recently, it has been reported that following E. coli membrane fractionation, some of the TonB is localized in the outer membrane fraction, due to its interaction with the receptors (74).
The regions of interaction in the receptors and in TonB have not been clearly identified. Indeed, the TonB box may only allow the receptor to assume a certain conformation that allows it to interact with TonB via other, unidentified sites (69). Moreover, through site-directed mutagenesis and spin labeling, it has been shown that the residues of the TonB box in the TonB-dependent receptors probably belong to the first transmembrane β strand of the protein and thus are located within the outer membrane bilayer (65a). The suppressor mutations in TonB described above concern Gln-160. Again, however, this does not mean that the residue is directly involved in the interaction. The region surrounding this residue is not sufficient for the interaction with the receptors because a TonB mutant truncated at residue 191 is unable to interact with FepA (69). In membrane fractionation experiments, the last 65 amino residues of TonB were found to be required for outer membrane localization (74). Thus, the C-terminal region of TonB is probably required for its interaction with receptors in the outer membrane.
TonB-dependent receptors are gated channels.
Deletion of the fifth (out of 14) external loop of FepA transforms the ligand-specific receptor into a nonspecific channel (109). The mutant no longer binds enterobactin, although this siderophore, like others, is still transported. Moreover, the transport no longer depends upon TonB. The FepA protein may thus form a channel closed by a loop, and the function of TonB may be to open the channel when the ligand binds. This has been recently confirmed (61). Similarly, deletion of the 8th external loop of FhuA (out of 16) transformed this ligand-specific TonB-dependent channel into a nonspecific pore independent of TonB (65).
However, the rate of siderophore import by wild-type FepA and FhuA is much higher than that of these deletion mutants. TonB may thus not only open the receptor channel but may also help dissociate the siderophore-receptor interaction to allow import. TonB may behave like a regulating protein acting on the conformation of another protein. The opening caused by ligand transport must be very short-lived, since it does not alter the permeability of the outer membrane. This question can now been addressed by using spin labels.
Tol-Pal system.
The genes tolQ, tolR, tolA, tolB, and pal are clustered at 17 min on the E. coli chromosome. They are organized in two operons: orf1, tolA, tolQ, and tolR under the control of promoter P1 and tolB, pal, and orf2 under the control of PB (122). The expression of tolQ, tolR, and tolA is regulated by RcsC, an inner membrane protein, probably through the action of an unidentified factor (24). RcsC is the sensor of the two-component system RcsC-RcsB involved in the regulation of capsule synthesis (47).
Orf1 is a gene product of the same operon including tolA, tolQ, and tolR. However, an orf1 chromosomal tranposon insertion mutant does not have a Tol phenotype (117). TolQ is an integral membrane protein with three transmembrane segments (63, 121). Its insertion in the cytoplasmic membrane is independent of the Sec system but requires the membrane potential (78). TolR has an N-terminal transmembrane anchor and a periplasmic domain of about 100 residues (63, 87). The 24 C-terminal residues may form an amphipatic helix able to interact with the inner membrane (73). TolR appears to form dimers, and its central periplasmic domain seems to be involved in its dimerization (61a). Each of the three domains of TolR is required for its activity.
TolA, like TolR, is anchored by an N-terminal transmembrane segment in the inner membrane and has a periplasmic region, which is itself subdivided into two domains (75, 76). Central domain II is separated from N-terminal domain I by five glycines and from C-terminal domain III by three glycines. It contains 10 repeats of the sequence Lys1-2–Ala2-4 (Glu-Asp) and has mainly an α-helix structure (76).
TolB is a periplasmic protein of 408 residues resulting from the cleavage of its 21-residue signal sequence (53, 75). However, a small amount is always found in outer membrane fractions (53). In its central region, there are three homologous segments that are successively repeated. Similar regions are found in various other proteins: the oligogalacturonate lyase from Erwinia chrysanthemi and E. carotovora (104), the apical gut membrane polyprotein (GA1) from Haemonchus contorssus, a homologous protein from Caenorhabditis elegans (59), and the dipeptidyl-peptidases from various species. Crystals of a TolB derivative tagged with six histidines have been obtained. Frozen crystals diffract to 1.9 Å resolution, and the 3-D structure is being determined (1).
Pal (152 residues) is a lipoprotein associated with the peptidoglycan (71, 83). It features a Ser residue at position +2 with respect to the N-terminal modified cysteine and, accordingly, is transported to the outer membrane, probably by the general transport system of lipoproteins, consisting of LolA and LolB (81). The C-terminal region of the protein is homologous to that of the OmpA family of proteins that have in common a strong, noncovalent interaction with the peptidoglycan (32). An amphipatic α-helix between residues 94 and 114 may be the region interacting with the peptidoglycan (5a, 19a, 66, 70b).
Orf2 is a periplasmic protein. An orf2 insertion mutant does not have a Tol phenotype (63). However, it seems that the porin content of the outer membrane is affected and that this protein may interact with TolB-Pal (70c). Like that of Orf1, the function of Orf2 is unknown.
The first evidence suggesting that the Tol proteins form a complex was from membrane fractionation experiments in which the Tol proteins were found to be localized mainly in a fraction supposed to account for the contact sites between the inner and outer membranes. When cells were treated with colicin A, the quantity of Tol proteins in this fraction was doubled (48). Studies with TonB-TolA hybrids also suggest interactions between proteins of the Tol-Pal system (64). As the interactions with TolQR and ExbBD depend on the transmembrane anchors of TolA and TonB, respectively, it seemed probable that these two proteins interact with TolQR and ExbBD via their membrane anchors. This view was reinforced by a genetic analysis in which mutations in the TolR transmembrane anchor were found to partially suppress a point mutation (A177V) in the third transmembrane segment of TolQ (73). Another suppressor mutation was found at the C-terminal end of TolR (residue 139), suggesting that this region may be in contact with the inner membrane directly or indirectly. The interactions between TolA and TolQ and between TolA and TolR have been biochemically demonstrated by using chemical cross-linking with formaldehyde in intact cells. With antibodies directed against TolA, both TolA-TolQ and TolA-TolR complexes were evidenced (33). More recently, the same technique, using TolR antibodies and various TolR constructs, has shown that the membrane anchor of TolR is involved in complex formation (61a). Since the TolA N-terminal (anchor) domain interacts with TolQ and TolR, it is likely that the three proteins (TolA, TolQ, and TolR) interact via their transmembrane regions (33). Moreover, recent genetic experiments allowed the isolation of numerous mutations in the TolA anchor that alter the interactions with TolQ and TolR, thus confirming intramembrane helix-helix interaction (46a). Suppressor mutations that were found in the first transmembrane segment of TolQ allowed the identification of the helix faces of TolA and TolQ that interact. The situation is thus very similar to that of TonB-ExbB (69) (Fig. 1).
Function of the Tol-PaL system.
The TolQRA and TolB proteins are required for the entry of most group A colicins, and the TolQRA proteins are required for uptake of filamentous phage DNA. However, in contrast to the TonB system, the function of the Tol-PaL proteins in the cell is not clear. Electron microscopy reveals that tol and pal mutants form blebs in their outer membrane. This phenotype was not observed in tolC and rfaD mutant cells. The blebs or vesicles contain outer membrane trimeric porins correctly exposed at the surface (13). Accordingly, it appears that the Tol-PaL system is required to maintain the integrity of the outer membrane. Indeed, tol and pal mutants have a pleiotropic phenotype: they are leaky for periplasmic proteins that are released to the extracellular medium and hypersensitive to drugs and detergents (71, 88). These mutants are also mucoid when grown at low temperature (20 to 30°C), due to the activation of the cps gene by RcsCB regulators that are believed to detect outer membrane defects (24).
Domain II of TolA and TolB can interact in the presence of sodium dodecyl sulfate with trimeric porins of E. coli but not with OmpA (35, 105). The Tol-Pal system also interacts with Lpp and OmpA via TolB and Pal, and the production of TolB and Pal is increased in lpp ompA mutants (25). As TolB and Pal can be cross-linked (17), it has been suggested that this set of proteins (TolB, Pal, Lpp, and OmpA) forms a complex with a structural function, ensuring the anchoring of the outer membrane to the peptidoglycan (25).
At least three different functions have been proposed for the Tol-Pal system. The first is a function in the biogenesis of porins. The Tol-Pal proteins may be involved at various steps during export across the periplasm and assembly in the outer membrane. However, a problem with this hypothesis is that porins are correctly assembled in tol and pal mutants even though less OmpF and LamB is found in the outer membrane of these mutants (72). The effect of the Tol and Pal proteins on the porin content of the outer membrane may be indirect. The tol and pal mutations may alter other components (LPS or phospholipids), which may, in turn, affect outer membrane integrity.
Alternatively, the Tol-Pal system may have a function in porin activity. The opening of nonspecific porins may be regulated by the periplasmic polyamines and membrane-derived oligosaccharides (30, 31). The Tol-Pal system may be involved in this mechanism, although this possibility is not supported by any data. Finally, a purely structural function in the scaffolding of interactions between the outer membrane and peptidoglycan is the most likely function.
Comparison of the TonB and Tol-Pal systems.
The proteins TolQ and TolR are homologous to ExbB and ExbD, with 26.3 and 25% identity and 79.1 and 70% similarity, respectively (38). They are also functionally homologous (22). exbB tolQ and exbD tolR double mutants are entirely tolerant to colicins, and their TonB-dependent transport function for vitamin B12 and siderophores is abolished. The complexes formed by the proteins of the two systems are also very similar (see below), at least in the inner membrane. In contrast, TolA and TonB have no sequence homology other than in their transmembrane anchor. They both have elongated conformations, probably spanning the periplasm, but with different structures: TolA has an α-helical structure, and TonB has X-Pro repeats. Moreover, the Tol-Pal complex contains two additional components compared to the TonB system: the TolB and Pal proteins. In some species, these two systems are similar in gene organization: tolQ tolR tolA and exbB exbD tonB (for example, in Pseudomonas putida).
COLICIN TRANSPORT
Transport of group A colicins across the outer membrane.
Each of the group A colicins (but not colicin E1) requires a porin, sometimes in addition to its specific receptor, for the translocation step. Colicin N uses OmpF both as a receptor and for translocation. Colicin A uses the BtuB receptor and OmpF for translocation. Colicins E2 to E9 require at least one porins OmpF, OmpC, or PhoE, for translocation (84). In contrast, colicin E1 requires TolC for its translocation (7, 88).
An interaction between colicin A and OmpF has been repeatedly demonstrated; however, the region of colicin A involved in this interaction has not been mapped precisely (16, 41), although its OmpF dependence requires the N-terminal domain (1). The affinity constant of colicin N for OmpF was determined by microcalorimetry experiments (43). The association constant is 5 × 105 M−1, with a stoichiometry of three colicin N molecules per OmpF trimer. Surprisingly, OmpC and PhoE had colicin N association constants in the same range but very different thermodynamics. This indicates that colicin N-OmpF binding involves significant structural rearrangement, which may be important for translocation (43). This is consistent with reports showing that unfolding is a prerequisite for colicin translocation (11, 36).
Four colicin N- and A-resistant OmpF mutants have been identified that contain only single amino acid substitutions (45). The G119D mutation is located in the narrowest part of the OmpF pore, and although it increases the temperature sensitivity of OmpF trimers (45), the overall protein structure is unchanged, except for partial occlusion of the pore (60). Although the mutation may affect colicin binding and/or translocation, it is possible that both colicins A and N are translocated through the OmpF pore and that their translocation is prevented by steric blockage of the pore in this mutant. It might be that the other group A colicins which are dependent on porins for their translocation are also translocated through the pore of these porins (70). However, the space available in the OmpF pore when the L3 loop is in the conformation determined by X-ray crystallography is 11 by 7Å (60), and polypeptides containing secondary structural elements would thus be unable to pass through the pore with L3 in place. As disulfide bonds which fix the conformation of the C-terminal domain of colicin A have little effect on translocation (36), it seems implausible that this domain is translocated through the lumen of the OmpF pore.
OmpF cysteine mutant proteins which fix L3 in the conformation determined by X-ray analysis were constructed recently. The sensitivity to colicins A and N of E. coli cells expressing these mutant proteins indicated that gross movement of L3 is not required for colicin N or A activity. It was thus concluded that neither of these colicins crosses the outer membrane of E. coli through the lumen of the OmpF pore (4). However, unstructured translocation (N-terminal) domains of colicins may use this pathway to penetrate the periplasm because the isolated domain, at least with colicins N (103) and A (70a), appears to be unstructured.
Transport of group B colicins across the outer membrane.
The binding sites of colicin M and of phages T1, T5, and φ80 on the FhuA receptor are on the eighth external loop of FhuA, which appears to gate the pore (65). Similarly, colicins B and D can bind to the fifth loop of FepA, which also gates this receptor’s pore (89). As for group A colicins, the subsequent steps are unknown. However, in contrast to these colicins, the energy source for translocation is not so obscure: TonB receptors can use the inner membrane proton motive force mediated by the TonB system.
The movement of a spin label bound to a cysteine in the center of the fifth external loop of FepA can be monitored (79). It was thus possible to monitor the movement of this loop in vivo upon passage of a TonB-dependent ligand or upon the addition of colicin B. Siderophore transport is accompanied by movement of the loop toward a position where the spin label is more mobile, and then it returns to its original position. In contrast, upon colicin B transport, there is nonreversible movement of the loop towards a more constrained position (61), indicating that colicin B probably transits across the FepA pore.
Interaction of group A colicins with the Tol system.
Many studies suggest that colicins interact with the Tol protein upon translocation. The first evidence for a direct interaction between one of the Tol proteins and group A colicins was obtained by Western blotting experiments (9). It was reported that TolA domain III exported to the periplasm prevented group A colicin uptake, presumably by competition, which suggested an interaction between this domain and colicins (77). Tol proteins have also been shown to be preferentially localized in a membrane fraction, operationally defined as contact sites between the inner and outer membranes, and the amount of Tol proteins in this fraction doubled when cells were treated with colicin A. This suggested that the colicin recruits Tol proteins upon its translocation (49). Finally, a mutant with a mutation in the C-terminal domain of colicin A, unable to form a pore, remained in interaction with the translocation system and prevented wild-type colicins A and N from killing the cells (37).
Colicins A and E1 bind TolA domain III blotted onto a nitrocellulose membrane. The N-terminal domain of colicin A alone could bind TolA, and this was confirmed by surface plasmon resonance experiments. The Kd with the N-terminal domains of colicins A and E1 were 0.2 and 0.6 μM, respectively, with immobilized TolA domain III. This technique indicated that it is also the N-terminal domain of colicin E1 that binds TolA (34). The colicin N-TolA domain III interaction was studied by isothermic titration microcalorimetry. The dissociation constants for TolA domain III were 18 μM with whole colicin N, 8 μM with the colicin N pore-forming domain alone, and 1.4 μM with the colicin N translocation domain alone (103). TolA domain III interacts with protein g3p of filamentous phage f1, which binds to the F pilus of target cells (52). Furthermore, a hybrid consisting of the first two-thirds of g3p fused to the RNase domain of colicin E3 was active on BtuB mutant cells by using the pilus as a receptor and the tol gene cluster as a transporter (54). The N-terminal domain (D1) of g3p interacts with TolA domain III just like the N-terminal domain of group A colicins and with similar affinity, although there is no significant homology beyond the fact that both contain glycine-rich regions. The disruption of the interaction between the N-terminal domain (D1) and the central domain (D2) of g3p upon binding to the F pilus allows D1 to interact with TolA domain III, as inferred from nuclear magnetic resonance and crystallographic studies (52, 80). It may be that partial unfolding of colicin A or N upon receptor binding favors the interaction of the ColA or ColN N-terminal domain with TolA domain III (as suggested by the Kds).
Despite the similar Kds for TolA domain III, there are probably differences between the translocation steps of different group A colicins. Colicin E1 uses TolC and not OmpF in the outer membrane, and colicin E1 requires neither TolB nor TolR. Colicin N and cloacin DF13 do not require TolB. These differences must reflect differences in their translocation mechanisms. The activities of colicins A and E1 are not affected in the same way in TolA deletion mutants (110), and, significantly, colicins with nuclease activities and pore-forming colicins must reach different sites in the cell.
The N-terminal domain of group A colicins contains all of the information needed for the translocation step which follows receptor binding (8). TolA domain III binds to the group A colicin N-terminal domains (9, 34), but the exact site of interaction and its physical nature are unknown. It has been suggested that there is a conserved sequence in some group A colicins, which may be the TolA box (46, 88), but it is now clear that this is the TolB binding site (18, 19). Thus, the nature of the TolA binding sites is unknown, although amino acids 51 to 98 in colicin A have been implicated (16a, 19). Residues essential for TolA binding, for example, Y62 in colicin N, have been characterized by mutagenesis, isothermal titration calorimetry, and tryptophan fluorescence studies. A region of at least 20 residues that is involved in colicin N binding to TolA domain III was thereby identified (103).
The colicin A N-terminal domain can also form a trimeric complex with TolA and TolB (19). The region involved in the interaction between colicin A and TolA is only conserved in colicin K (19). This suggests that during translocation, group A colicins use the same Tol proteins in different ways, perhaps interacting with different domains.
Group B colicins and proteins of the TonB system.
There have been few studies of the translocation mechanism of group B colicins. The recent determination of the structure of colicin Ia will probably give a strong impetus to the examination of this topic. The few data available are summarized below. The N-terminal region of each of the group B colicins contains a sequence homologous to the TonB box found in TonB-dependent receptors. Mutations in the TonB box of colicins or in that of receptors make them inactive. Since the receptor-binding step and pore formation are not altered, the translocation step must be blocked. The translocation of colicins carrying mutations in the TonB box can be restored in TonB mutants (82). These TonB mutants are the same as those that suppress mutations in the TonB box of FhuA and BtuB receptors (51, 112). Similar results were obtained with colicin M (95). Nevertheless, it seemed that in this case the TonB box mutations in colicin M were suppressed in an allele-specific way by TonB mutations. This suggests that colicins may interact with TonB via the TonB box. However, there is no direct evidence for such interactions. The translocation mechanism of group B colicins is undoubtedly very similar to that of siderophores and vitamin B12. Nevertheless, in ESR experiments, the dynamics of the external FepA loop is different, which probably reflects colicin B remaining bound at the translocation site when its pore has formed (61).
With colicins 5 and 10, which are TonB dependent, TolC and Tsx are required, although neither is a TonB-dependent receptor (20, 73). These two colicins presumably use a different mechanism. It is unclear in each case how the colicins gain access to TonB and how this interaction can then favor the entry of the colicin C-terminal domain. In the case of colicins that use a TonB-dependent receptor, it is likely that the colicin chain is translocated across the receptor channel opened in a TonB-dependent way. This would explain the requirement for a functional receptor not mutated in its TonB box, and part of the translocation mechanism of TonB-dependent colicins may involve competition between the corresponding TonB boxes in the receptor and the colicin for the putative interaction with TonB.
Unfolding of colicins.
Pore-forming colicins kill cells by depolarizing their cytoplasmic membrane and by inducing a phosphate efflux which leads to depletion of cytoplasmic ATP (48). The opening of the pore also induces an efflux of cytoplasmic K+, an efflux which can be continuously recorded. In this way, the progression of the toxin from its receptor (in the outer membrane) to its target, the inner membrane, can be monitored “on line.” The kinetics of K+ efflux caused by colicin A have been extensively studied. The K+ efflux reflects the opening of the colicin A channel. It is preceded by a lag time which corresponds to the binding of colicin A to its receptor and its translocation through the cell envelope. Under particular conditions, the time needed for the translocation step determines the lag time observed. Therefore, the lag time measured is a good approximation of the translocation time (15). This allowed the demonstration that the colicin A translocation step is accelerated if the colicin is urea denatured before being added to the cells (11), a result suggesting that colicin A is unfolded during its translocation. Two different results confirm this view. First, externally added trypsin cleaves the colicin polypeptide chain and thereby induces the closure of the colicin A pore while having access neither to the periplasmic space nor to the inner membrane (11). Second, a disulfide bond-engineered colicin A mutant that is able to be translocated but unable to open its pore prevents wild-type colicin A from binding to its receptor and from being translocated (37). These results both indicate that colicin A maintains an extended conformation across the cell envelope and is still in contact with its receptor and its translocation machinery when its pore has been formed in the inner membrane. The unfolding of colicin A is initiated very early in the translocation process by its binding to the receptor (36).
Translocation of the C-terminal domain of colicins.
The transport of the C-terminal domain of colicins across the outer membrane is still poorly understood. It is indeed difficult to understand how the N-terminal domain and the C-terminal domain can be translocated while the central domain remains bound to the receptor at the cell surface.
Few data have been reported concerning translocation across or insertion into the inner membrane. The C-terminal domain of colicin A fused to the presequence of cytochrome c1, when produced inside the bacterial cell, is exported to the periplasm and can form voltage-sensitive channels (42). These channels were formed in tol mutant cells, which strongly suggests that these proteins are not required for the insertion of the C-terminal domain of entire pore-forming colicins into the inner membrane.
In the case of the C-terminal domain of colicins with nuclease activity, the question is even more intriguing because at least part of the colicin must cross the inner membrane to exert its action in the cytoplasmic target. Cells treated with colicin E2 can be rescued by addition of trypsin (12, 91). This is similar to the situation with colicin A (11) and suggests that the colicin polypeptide is still accessible from the external medium and thus not cleaved when the C-terminal domain of colicin E2 reaches the cytoplasm. It is not known whether Tol proteins are involved in this crossing of the inner membrane and whether the N-terminal domain is involved. As mentioned above, a hybrid consisting of the 372 N-terminal residues of g3p (from phage f1) fused to the C-terminal RNase domain of colicin E3 is bactericidal. However, this activity was TolB dependent, as well as TolQRA dependent, whereas the f1 phage does not require TolB (54). This suggests that the C-terminal domain of colicin E3 may require TolB to cross the inner membrane.
In the case of colicin Ia, a long stretch of the C-terminal polypeptide chain can cross the inner membrane in a voltage-sensitive way (116), and it has been recently shown that foreign polypeptide regions inserted into this domain can also be translocated (55). Possibly, the C-terminal domain or part of it, at least of colicins with nuclease activity, has the ability to be translocated across the inner membrane in the same way.
STRUCTURES OF COLICINS A, E1, N, AND IA
The pore-forming domains of colicins A and E1, all of colicin Ia, and colicin N with its N-terminal 90 residues truncated have been crystallized, and their 3-D structures have been determined (40, 67a, 92, 123). In each case, the toxins are pore-forming colicins and the four C-terminal domains have the same type of structure. These domains consist of a bundle of eight amphipathic helices burying too hydrophobic helices. The structures of the four proteins confirm the organization of colicins into distinct structural and functional domains.
The crystal structure of colicin Ia has been determined to 3 Å resolution (123). This 626-amino-acid protein is about 210 Å long and consists of three functional domains separated by a pair of helices 160 Å long. A central domain at the bend of the hairpin-like structure mediates binding to the outer membrane receptor. The N-terminal domain mediates translocation across the outer membrane via the TonB pathway. The TonB box recognition motif of colicin Ia is on one side of three 80-Å-long helices arranged as a helical sheet. The third (C-terminal) domain, made up of 10 α-helices, is the pore-forming domain (see below). The two exceptionally long (160 Å) α-helices may enable colicin Ia to span the periplasmic space between the outer and inner membranes (123). This type of structure, which will most likely be found in every colicin, gives strong clues as to how these toxins exploit the target cell machinery to get across the periplasmic space.
A MODEL OF COLICIN TRANSLOCATION
It has been shown that: (i) colicin A unfolds upon binding to its receptor (36); (ii) the N-terminal domains of colicins A, E1, E3, and N interact with Tol proteins (9, 18, 19); (iii) after insertion of the pore-forming domain, the colicin A polypeptide chain spans the whole cell envelope (11); (iv) partial occlusion of the lumen of the OmpF pore prevents colicin A and N translocation (60); and (v) movement of the L3 loops of OmpF is not required for translocation (4). There are about 1,000 translocation sites for colicin A on sensitive cells (6, 36). With all of the information now available, especially concerning colicin A, previous models of colicin translocation (6, 70) can be improved (Fig. 2).
FIG. 2.
Proposed model of colicin A translocation. T, domain involved in translocation (N-terminal region); RB, receptor binding (central) domain; C, pore-forming domain (C-terminal region); OM, outer membrane; IM, inner membrane; P, periplasm; PG, peptidoglycan.
In the first step, colicin A is substantially unfolded after interaction with its receptor (BtuB), allowing the N-terminal (T) domain to access the OmpF pore. As soon as it is exposed to the inner face of the outer membrane, the T domain interacts with TolB, attached to Pal, and eventually displaces TolB from Pal. Pal may then interact with peptidoglycan because the same region of Pal is involved in the interaction with TolB and peptidoglycan (16a). TolB is likely to be widely spread over the inner face of the outer membrane, because it can interact with trimeric porins (105), with Lpp, and with OmpA (25). Downstream from the T domain is the TolA box (19), which allows TolA to bind. This cascade of interactions probably allows unidirectional translocation and unfolding of the polypeptide chain. It is likely that the central domain of the colicin remains in contact with the receptor. We do not know how the C-terminal pore-forming domain is translocated through the outer membrane. However, the simple interaction of this domain with phospholipids can induce its slow conformational change into a “molten-globule” conformation (the tertiary structure is destroyed, whereas most of the secondary structure is preserved) (86, 125). One possibility is that there is further conformational change and movement to the opposite side of the membrane. In the last step, the C-terminal domain may insert into the inner membrane and form a channel, provided that there is a trans-negative membrane potential of greater than 80 mV (15).
Little is known about the translocation step of nuclease-type colicins. Immunity proteins specific for these enzymatic colicins bind the toxin in the producing cell, and the two molecules are released into the medium as a heterodimeric complex. As these toxins begin their passage into a bacterium, the immunity protein must be lost, but how and where this occurs is not known. The translocation across the outer membrane and the periplasmic space might be similar to that of pore-forming colicins because a hybrid colicin having the N-terminal and central domains of colicin A and the C-terminal domain of colicin E3 has been shown to be active (10). Nothing is known about how these colicins with nuclease activity traverse the inner membrane. Do the Tol proteins play a role? Are other proteins needed?
Many aspects of the colicin translocation process remain obscure and speculative. However, the structural studies of the Tol proteins and the proteins of the TonB system that are now in progress and better knowledge of the dynamics of the interactions between proteins of the Tol and TonB systems and between colicins and Tol and TonB proteins should lead to a more detailed understanding of the translocation mechanism at the molecular level.
ACKNOWLEDGMENTS
We are grateful to M. Gavioli for technical assistance and to B. Videau for preparing the manuscript.
This research was supported by the Life Science Department and the program “Physique et Chimie du Vivant” of the CNRS; by the Program Environnement Santé 96, Convention d’Aide EN96C3; by ACC-SV no. 6 of the Ministry of Research; and by EC Biotech contract BIO-4CT97-2313.
REFERENCES
- 1.Abergel C, Rigal A, Chenivesse S, Lazdunski C, Claverie J M, Bouveret E, Bénédetti H. Crystallization and preliminary crystallographic study of a component of the Escherichia coli Tol system: TolB. Acta Cristallogr. 1997;D54:101–104. doi: 10.1107/s0907444997008020. [DOI] [PubMed] [Google Scholar]
- 2.Ahmer B M M, Thomas M G, Larsen R A, Postle K. Characterization of the exbBD operon of Escherichia coli and the role of ExbB and ExbD in TonB function and stability. J Bacteriol. 1995;177:4742–4747. doi: 10.1128/jb.177.16.4742-4747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann B J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990;54:30–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bainbridge G, Armstrong G A, Whelan K F, Dover L G, Lakey J H. Colicins and Other Bacteriocins Workshop. Norwich, England: University of East Anglia; 1998. Inhibition of OmpF loop 3 movement reduces colicin N toxicity in vivo, abstr. W1; p. 35. [Google Scholar]
- 5.Bell P E, Nau C D, Brown J T, Konisky J, Kadner R J. Genetic suppression demonstrates interaction of TonB protein with outer membrane transport proteins in Escherichia coli. J Bacteriol. 1990;172:3826–3829. doi: 10.1128/jb.172.7.3826-3829.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Bénédetti, H. Unpublished data.
- 6.Bénédetti H, Géli V. Colicin transport, channel formation and inhibition. In: Konings W N, Kaback H R, Lolkema J S, editors. Handbook of biological physics. Vol. 2. New York, N.Y: Elsevier; 1996. pp. 665–691. [Google Scholar]
- 7.Bénédetti H, Frenette M, Baty D, Lloubès R, Géli V, Lazdunski C. Comparison of the uptake systems for the entry of various BtuB group colicins into Escherichia coli. J Gen Microbiol. 1989;135:3413–3420. doi: 10.1099/00221287-135-12-3413. [DOI] [PubMed] [Google Scholar]
- 8.Bénédetti H, Frenette M, Baty D, Knibiehler M, Pattus F, Lazdunski C. Individual domains of colicins confer specificity in colicin uptake, in pore-properties and in immunity requirements. J Mol Biol. 1991;217:429–439. doi: 10.1016/0022-2836(91)90747-t. [DOI] [PubMed] [Google Scholar]
- 9.Bénédetti H, Lazdunski C, Lloubès R. Protein import into Escherichia coli: colicins A and E1 interact with a component of their translocation system. EMBO J. 1991;10:1989–1995. doi: 10.1002/j.1460-2075.1991.tb07728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bénédetti H, Letellier L, Lloubès R, Géli V, Baty D, Lazdunski C. Study of the import mechanisms of colicins through protein engineering and K+ efflux kinetics. NATO Adv Study Inst Ser. 1992;H65:215–223. [Google Scholar]
- 11.Bénédetti H, Lloubès R, Lazdunski C, Letellier L. Colicin A unfolds during its translocation in Escherichia coli cells and spans the whole cell envelope when its pore has formed. EMBO J. 1992;11:441–447. doi: 10.1002/j.1460-2075.1992.tb05073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beppu T, Kawabata K, Arima K. Specific inhibition of cell division by colicin E2 without degradation of deoxyribonucleic acid in a new colicin sensitivity mutant of Escherichia coli. J Bacteriol. 1972;110:485–493. doi: 10.1128/jb.110.2.485-493.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernadac, A., M. Gavioli, J. C. Lazzaroni, S. Raina, and R. Lloubès.Escherichia coli tol-pal mutants form outer membrane vesicles. J. Bacteriol., in press. [DOI] [PMC free article] [PubMed]
- 14.Bitter W, Tommassen J, Weisbeek P J. Identification and characterization of the exbB, exbD and tonB genes. Mol Microbiol. 1993;24:169–179. doi: 10.1111/j.1365-2958.1993.tb01103.x. [DOI] [PubMed] [Google Scholar]
- 15.Bourdineaud J P, Boulanger P, Lazdunski C, Letellier L. In vivo properties of colicin A: channel activity is voltage dependent but translocation may be voltage independent. Proc Natl Acad Sci USA. 1990;87:1037–1041. doi: 10.1073/pnas.87.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bourdineaud J P, Fierobe H, Lazdunski C, Pagès J M. Involvement of OmpF during reception and translocation steps of colicin N entry. Mol Microbiol. 1990;4:1737–1743. doi: 10.1111/j.1365-2958.1990.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 16a.Bouveret, E. Unpublished data.
- 17.Bouveret E, Derouiche R, Rigal A, Lloubès R, Lazdunski C, Bénédetti H. Peptidoglycan-associated lipoprotein-TolB interaction. J Biol Chem. 1995;270:11071–11077. doi: 10.1074/jbc.270.19.11071. [DOI] [PubMed] [Google Scholar]
- 18.Bouveret E, Rigal A, Lazdunski C, Bénédetti H. The N-terminal domain of colicin E3 interacts with TolB, which is involved in the colicin translocation step. Mol Microbiol. 1997;23:909–920. doi: 10.1046/j.1365-2958.1997.2751640.x. [DOI] [PubMed] [Google Scholar]
- 19.Bouveret E, Rigal A, Lazdunski C, Bénédetti H. Distinct regions of the colicin A translocation domain are involved in the interaction with TolA and TolB proteins upon import into Escherichia coli. Mol Microbiol. 1998;27:143–157. doi: 10.1046/j.1365-2958.1998.00667.x. [DOI] [PubMed] [Google Scholar]
- 19a.Bouveret, E., A. Rigal, C. Lazdunski, and H. Bénédetti. Unpublished data.
- 20.Bradley D E, Howard S P. A new colicin that adsorbs to outer-membrane protein Tsx is dependent on the tonB instead of the tolQ membrane transport system. J Gen Microbiol. 1992;135:1857–1863. doi: 10.1099/00221287-138-12-2721. [DOI] [PubMed] [Google Scholar]
- 21.Braun V. Energy-coupled transport and signal transduction through the Gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol Rev. 1995;16:295–307. doi: 10.1111/j.1574-6976.1995.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 22.Braun V, Herrmann C. Evolutionary relationship of uptake systems for biopolymers in Escherichia coli: cross-complementation between the TonB-ExbB-ExbD and the TolA-TolQ-TolR proteins. Mol Microbiol. 1993;8:261–268. doi: 10.1111/j.1365-2958.1993.tb01570.x. [DOI] [PubMed] [Google Scholar]
- 23.Braun V, Gaisser S, Herrmann C, Kampfenkel K, Killmann H, Traub I. Energy-coupled transport across the outer membrane of Escherichia coli: ExbB binds ExbD and TonB in vitro, and leucine 132 in the periplasmic region and aspartate 25 in the transmembrane region are important for ExbD activity. J Bacteriol. 1996;178:2836–2845. doi: 10.1128/jb.178.10.2836-2845.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clavel T, Lazzaroni J C, Vianney A, Portalier R. Expression of the tolQRA genes of Escherichia coli K-12 is controlled by the RcsC sensor protein involved in capsule synthesis. Mol Microbiol. 1996;19:19–25. doi: 10.1046/j.1365-2958.1996.343880.x. [DOI] [PubMed] [Google Scholar]
- 25.Clavel, T., P. Germon, A. Vianney, R. Portalier, and J. C. Lazzaroni. The TolB and Pal proteins of Escherichia coli K-12 form a complex with Lpp and OmpA to link the outer membrane and the peptidoglycan. Mol. Microbiol., in press. [DOI] [PubMed]
- 26.Cornelis P, Sierra J C, Lim A, Jr, Malur A, Tungpradabkul S, Tazka H, Leitao A, Martins C V, Di Perna C, Brys L, De Baetselier P, Hamers R. Development of new cloning vectors for the production of immunogenic outer membrane fusion proteins in Escherichia coli. Nat Biotechnol. 1996;14:203–208. doi: 10.1038/nbt0296-203. [DOI] [PubMed] [Google Scholar]
- 27.Davies J K, Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group B. J Bacteriol. 1975;123:96–101. doi: 10.1128/jb.123.1.96-101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies J K, Reeves P. Genetics of resistance to colicins in Escherichia coli K-12. Cross-resistance among colicins of group A. J Bacteriol. 1975;123:102–117. doi: 10.1128/jb.123.1.102-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deich R A, Metcalf B J, Finn C W, Farley J E, Green B A. Cloning of genes encoding a 15,000-dalton peptidoglycan-associated outer membrane lipoprotein and an antigenically related 15,000-dalton protein from Haemophilus influenzae. J Bacteriol. 1988;170:489–498. doi: 10.1128/jb.170.2.489-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dela Vega A L, Delcour A H. Cadaverine induces closing of E. coli porins. EMBO J. 1995;14:6058–6065. doi: 10.1002/j.1460-2075.1995.tb00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delcour A H, Kung C, Adler J, Martinac B. Membrane-derived oligosaccharides (MDOs) promote closing of an E. coli porin channel. FEBS Lett. 1992;304:216–220. doi: 10.1016/0014-5793(92)80622-n. [DOI] [PubMed] [Google Scholar]
- 32.De Mot R, Vanderleyden J. The C-terminal sequence conservation between OmpA-related outer membrane proteins and MotB suggests a common function in both Gram-positive and Gram-negative bacteria, possibly in the interaction of these domains with peptidoglycan. Mol Microbiol. 1994;12:333–334. doi: 10.1111/j.1365-2958.1994.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 33.Derouiche R, Bénédetti H, Lazzaroni J C, Lazdunski C, Lloubès R. Protein complex within Escherichia coli inner membrane—TolA N-terminal domain interacts with TolQ and TolR proteins. J Biol Chem. 1995;270:11078–11084. doi: 10.1074/jbc.270.19.11078. [DOI] [PubMed] [Google Scholar]
- 34.Derouiche R, ZederLutz G, Bénédetti H, Gavioli M, Rigal A, Lazdunski C, Lloubès R. Binding of colicins A and E1 to purified TolA domains. Microbiology. 1997;143:3185–3192. doi: 10.1099/00221287-143-10-3185. [DOI] [PubMed] [Google Scholar]
- 35.Derouiche R, Gavioli M, Bénédetti H, Prilipov A, Lazdunski C, Lloubès R. TolA central domain interacts with Escherichia coli porins. EMBO J. 1997;15:6408–6415. [PMC free article] [PubMed] [Google Scholar]
- 36.Duché D, Baty D, Chartier M, Letellier L. Unfolding of colicin A during its translocation through the Escherichia coli envelope as demonstrated by disulfide bond engineering. J Biol Chem. 1994;269:24820–24825. [PubMed] [Google Scholar]
- 37.Duché D, Letellier L, Géli V, Bénédetti H, Baty D. Quantification of group A colicin import sites. J Bacteriol. 1995;177:4935–4939. doi: 10.1128/jb.177.17.4935-4939.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eick-Helmerich K, Braun V. Import of biopolymers into Escherichia coli: nucleotide sequences of the exbB and exbD genes are homologous to those of the tolQ and tolR genes, respectively. J Bacteriol. 1989;171:5117–5127. doi: 10.1128/jb.171.9.5117-5126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elkins C, Totton P A, Olsen B, Thomas C E. Role of the Haemophilus ducreyii Ton system in internalization of heme from hemoglobin. Infect Immun. 1998;66:151–160. doi: 10.1128/iai.66.1.151-160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elkins P A, Bunker A, Cramer W A, Stauffacher C V. A mechanism for toxin insertion into membranes is suggested by the crystal structure of the channel-forming domain of colicin E1. Structure. 1997;5:443–458. doi: 10.1016/s0969-2126(97)00200-1. [DOI] [PubMed] [Google Scholar]
- 41.El Kouhen R, Hoenger A, Engel A, Pagès J M. In vitro approaches to investigation of the early steps of colicin-OmpF interaction. Eur J Biochem. 1994;224:723–728. doi: 10.1111/j.1432-1033.1994.0723a.x. [DOI] [PubMed] [Google Scholar]
- 42.Espesset D, Corda Y, Cunningham K, Bénédetti H, Lloubès R, Lazdunski C, Géli V. The colicin A pore-forming domain fused to mitochondrial intermembrane space sorting signals can be functionally inserted into the Escherichia coli plasma membrane by a mechanism that bypasses the Tol proteins. Mol Microbiol. 1994;13:1121–1131. doi: 10.1111/j.1365-2958.1994.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 43.Evans L J A, Cooper A, Lakey J H. Direct measurement of the association of a protein with a family of membrane receptors. J Mol Biol. 1996;255:559–563. doi: 10.1006/jmbi.1996.0047. [DOI] [PubMed] [Google Scholar]
- 44.Fisher E, Günter K, Braun V. Involvement of ExbB and TonB in transport across the outer membrane of Escherichia coli: phenotypic complementation of exb mutants by overexpressed tonB and physical stabilization of TonB by ExbB. J Bacteriol. 1989;171:5127–5134. doi: 10.1128/jb.171.9.5127-5134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fourel D, Mizushima S, Bernadac A, Pagès J M. Specific regions of Escherichia coli OmpF involved in antigenic and colicin receptor sites and in stable trimerization. J Bacteriol. 1992;175:2754–2757. doi: 10.1128/jb.175.9.2754-2757.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garinot-Schneider C, Penfold C N, Moore G R, Kleanthous C, James R. Identification of residues in the putative TolA box which are essential for the toxicity of the endonuclease toxin colicin E9. Microbiology. 1997;143:2931–2938. doi: 10.1099/00221287-143-9-2931. [DOI] [PubMed] [Google Scholar]
- 46a.Germon, P., T. Clavel, A. Vianney, R. Portalier, and J. C. Lazzaroni. Unpublished data.
- 47.Gottesman S, Stout V. Regulation of capsular polysaccharide synthesis in Escherichia coli K12. Mol Microbiol. 1991;5:1599–1606. doi: 10.1111/j.1365-2958.1991.tb01906.x. [DOI] [PubMed] [Google Scholar]
- 48.Guihard G, Bénédetti H, Besnard M, Letellier L. Phosphate efflux through the channels formed by colicins and phage T5 in Escherichia coli cells is responsible for the fall in cytoplasmic ATP. J Biol Chem. 1993;268:17775–17780. [PubMed] [Google Scholar]
- 49.Guihard G, Boulanger P, Bénédetti H, Lloubès R, Besnard M, Letellier L. Colicin A and the Tol proteins involved in its translocation are preferentially located in the contact sites between the inner and outer membranes of Escherichia coli cells. J Biol Chem. 1993;269:5874–5880. [PubMed] [Google Scholar]
- 50.Günter K, Braun V. In vivo evidence for FhuA outer membrane receptor interaction with the TonB inner membrane protein of Escherichia coli. FEBS Lett. 1990;274:85–88. doi: 10.1016/0014-5793(90)81335-l. [DOI] [PubMed] [Google Scholar]
- 51.Heller K, Kadner R J, Günther K. Suppression of the btuB451 mutation by mutations of the tonB gene suggests a direct interaction between TonB and Ton-dependent receptor proteins in the outer membrane of Escherichia coli. Gene. 1988;64:147–153. doi: 10.1016/0378-1119(88)90488-x. [DOI] [PubMed] [Google Scholar]
- 52.Holliger P, Riechmann L. A conserved infection pathway for filamentous bacteriophages is suggested by the structure of the membrane penetration domain of the minor coat protein g3p from phage fd. Structure. 1997;5:265–275. doi: 10.1016/s0969-2126(97)00184-6. [DOI] [PubMed] [Google Scholar]
- 53.Isnard M, Rigal A, Lazzaroni J C, Lazdunski C, Lloubès R. Maturation and localization of the TolB protein required for colicin import J. Bacteriol. 1994;176:6392–6396. doi: 10.1128/jb.176.20.6392-6396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jakes K S, Davis N G, Zinder N D. A hybrid toxin from bacteriophage f1 attachment protein and colicin E3 has altered cell receptor specificity. J Bacteriol. 1988;170:4231–4238. doi: 10.1128/jb.170.9.4231-4238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jakes K S, Kienker P K, Slatin S L, Finkelstein A. Translocation of inserted foreign epitopes by a channel-forming protein. Proc Natl Acad Sci USA. 1998;95:4321–4326. doi: 10.1073/pnas.95.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.James R, Kleanthous C, Moore G R. The biology of E colicins: paradigms and paradoxes. Microbiology. 1996;142:1569–1580. doi: 10.1099/13500872-142-7-1569. [DOI] [PubMed] [Google Scholar]
- 57.Jarosik G P, Hansen E J. Cloning and sequencing of the Haemophilus influenzae exbB and exbD genes. Gene. 1995;152:89–92. doi: 10.1016/0378-1119(94)00675-i. [DOI] [PubMed] [Google Scholar]
- 58.Jaskula J C, Letain T E, Roof S K, Skare J T, Postle K. Role of the TonB amino terminus in energy transduction between membranes. J Bacteriol. 1994;175:2326–2338. doi: 10.1128/jb.176.8.2326-2338.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jasmer D P, Perryman L E, McGuire T C. Haemonchus contortus GA1 antigens: related, phospholipase C-sensitive, apical gut membrane proteins encoded as a polyprotein and released from the nematode during infection. Proc Natl Acad Sci USA. 1996;93:8642–8647. doi: 10.1073/pnas.93.16.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jeanteur D, Schirmer T, Fourel D, Simonet V, Rummel G, Widmer C, Rosenbusch J P, Pattus F, Pagès J M. Structural and functional alterations of a colicin-resistant mutant of OmpF porin from Escherichia coli. Proc Natl Acad Sci USA. 1994;91:10675–10679. doi: 10.1073/pnas.91.22.10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang X, Payne M A, Cao Z, Foster S B, Feix J B, Newton M C, Klebba P E. Ligand-specific opening of a gated-porin channel in the outer membrane of living bacteria. Science. 1997;276:1261–1264. doi: 10.1126/science.276.5316.1261. [DOI] [PubMed] [Google Scholar]
- 61a.Journet, L., A. Rigal, C. Lazdunski, and H. Bénédetti. Unpublished data.
- 62.Kampfenkel K, Braun V. Topology of the ExbB protein in the cytoplasmic membrane of Escherichia coli. J Biol Chem. 1992;268:6050–6057. [PubMed] [Google Scholar]
- 63.Kampfenkel K, Braun V. Membrane topologies of the TolQ and TolR proteins of Escherichia coli: inactivation of TolQ by a missense mutation in the proposed first transmembrane segment. J Bacteriol. 1993;175:4485–4491. doi: 10.1128/jb.175.14.4485-4491.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karlsson M, Hannavy K, Higgins C F. A sequence-specific function for the N-terminal signal-like sequence of the TonB protein. Mol Microbiol. 1993;8:379–388. doi: 10.1111/j.1365-2958.1993.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 65.Killmann H, Vedenov G, Jung G, Schwarz H, Braun V. Identification of receptor binding sites by competitive peptide mapping: phages T1, T5, and F80 and colicin M bind the gating loop. J Bacteriol. 1995;177:694–698. doi: 10.1128/jb.177.3.694-698.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65a.Klebba, P. Personal communication.
- 66.Koebnik R. Proposal for a peptidoglycan-associated alpha-helical motif in the C-terminal regions of some bacterial cell-surface proteins. Mol Microbiol. 1995;16:1269–1270. doi: 10.1111/j.1365-2958.1995.tb02348.x. [DOI] [PubMed] [Google Scholar]
- 67.Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- 67a.Lakey, J. Unpublished data.
- 68.Larsen R A, Wood G E, Postle K. The conserved proline-rich motif is not essential for energy transduction by Escherichia coli TonB protein. Mol Microbiol. 1993;10:943–953. doi: 10.1111/j.1365-2958.1993.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 69.Larsen R A, Thomas M G, Wood G E, Postle K. Partial suppression of an Escherichia coli TonB transmembrane domain mutation (DV17) by a missense mutation in ExbB. Mol Microbiol. 1994;13:627–640. doi: 10.1111/j.1365-2958.1994.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 70.Lazdunski C J. Colicin import and pore formation: a system for studying protein transport across membranes? Mol Microbiol. 1995;16:1059–1066. doi: 10.1111/j.1365-2958.1995.tb02331.x. [DOI] [PubMed] [Google Scholar]
- 70a.Lazdunski, C. J. Unpublished data.
- 70b.Lazzaroni, J. C. Unpublished data.
- 70c.Lazzaroni, J. C. Personal communication.
- 71.Lazzaroni J C, Portalier R. The excC gene of Escherichia coli K-12 required for cell envelope integrity encodes the peptidoglycan-associated lipoprotein (Pal) Mol Microbiol. 1992;6:735–742. doi: 10.1111/j.1365-2958.1992.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 72.Lazzaroni J C, Fognini-Lefebvre N, Portalier R. Effects of lkyB mutations on the expression of ompF, ompC and lamB porin structural genes in Escherichia coli K-12. FEMS Microbiol Lett. 1986;33:235–239. [Google Scholar]
- 73.Lazzaroni J C, Vianney A, Popot J L, Benedetti H, Samatey F, Lazdunski C, Portalier R, Geli V. Transmembrane alpha-helix interactions are required for the functional assembly of the Escherichia coli Tol complex. J Mol Biol. 1995;246:1–7. doi: 10.1006/jmbi.1994.0058. [DOI] [PubMed] [Google Scholar]
- 74.Letain T E, Postle K. TonB protein appears to transduce energy by shuttling between the cytoplasmic membrane and the outer membrane in Escherichia coli. Mol Microbiol. 1997;24:271–283. doi: 10.1046/j.1365-2958.1997.3331703.x. [DOI] [PubMed] [Google Scholar]
- 75.Levengood S K, Webster R E. Nucleotide sequences of the tolA and tolB genes and localization of their products, components of a multistep translocation system in Escherichia coli. J Bacteriol. 1989;171:6600–6609. doi: 10.1128/jb.171.12.6600-6609.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levengood S K, Beyer W F, Jr, Webster R E. TolA: a membrane protein involved in colicin uptake contains an extended helical region. Proc Natl Acad Sci USA. 1991;88:5939–5943. doi: 10.1073/pnas.88.14.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Levengood-Freyermuth S K, Click E M, Webster R E. Role of the carboxy-terminal domain of TolA in protein import and integrity of the outer membrane. J Bacteriol. 1993;175:222–228. doi: 10.1128/jb.175.1.222-228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lewin T M, Webster R E. Membrane insertion characteristics of the various transmembrane domains of the Escherichia coli TolQ protein. J Biol Chem. 1996;271:14143–14149. doi: 10.1074/jbc.271.24.14143. [DOI] [PubMed] [Google Scholar]
- 79.Liu J, Rutz J M, Klebba P E, Feix J B. A site-directed spin-labeling study of ligand-induced conformational change in the ferric enterobactin receptor, FepA. Biochemistry. 1994;33:13274–13283. doi: 10.1021/bi00249a014. [DOI] [PubMed] [Google Scholar]
- 80.Lubkowski J, Hennecke F, Plückthun A, Wlodawer A. The structural basis of phage display elucidated by the crystal structure of the N-terminal domains of g3p. Nat Struct Biol. 1998;5:140–147. doi: 10.1038/nsb0298-140. [DOI] [PubMed] [Google Scholar]
- 81.Matsuyama S, Yokota N, Tokuda H. A novel outer membrane lipoprotein, LolB (HemM), involved in the LolA (p20)-dependent localization of lipoproteins to the outer membrane of Escherichia coli. EMBO J. 1997;16:6947–6955. doi: 10.1093/emboj/16.23.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mende J, Braun V. Import-defective colicin B derivatives mutated in the TonB box. Mol Microbiol. 1990;4:1523–1533. doi: 10.1111/j.1365-2958.1990.tb02063.x. [DOI] [PubMed] [Google Scholar]
- 83.Mizuno T. A novel peptidoglycan-associated lipoprotein found in the cell envelopes of Pseudomonas aeruginosa and Escherichia coli. J Biochem. 1979;86:991–1000. doi: 10.1093/oxfordjournals.jbchem.a132631. [DOI] [PubMed] [Google Scholar]
- 84.Mock M, Pugsley A P. The BtuB group Col plasmids and homology between the colicins they encode. J Bacteriol. 1982;150:1069–1076. doi: 10.1128/jb.150.3.1069-1076.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moeck G S, Bazzaz B S F, Gras M F, Ravi T S, Ratcliffe M J H, Coulton J W. Genetic insertion and exposure of a reporter epitope in the ferrichrome-iron receptor of Escherichia coli K-12. J Bacteriol. 1994;176:4250–4259. doi: 10.1128/jb.176.14.4250-4259.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muga A, Gonzalez-Manas J M, Lakey J H, Pattus F, Surewicz W S. pH-dependent stability and membrane interaction of the pore-forming domain of colicin A. J Biol Chem. 1993;268:1553–1557. [PubMed] [Google Scholar]
- 87.Müller M M, Vianney A, Lazzaroni J C, Webster R E, Portalier R. Membrane topology of the Escherichia coli TolR protein required for cell envelope integrity. J Bacteriol. 1993;175:6059–6061. doi: 10.1128/jb.175.18.6059-6061.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nagel de Zwaig R, Luria S E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967;94:1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Newton S M C, Allen J S, Cao Z, Jiang X, Spencel C, Igo J D, Foster S B, Payne M A, Klebba P E. Double mutagenesis of a positive charge cluster in the ligand-binding site of the ferric enterobactin receptor, FepA. Proc Natl Acad Sci USA. 1997;94:4560–4565. doi: 10.1073/pnas.94.9.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nikaïdo H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nose K, Mizuno D. Degradation of ribosomes in Escherichia coli cells treated with colicin E2. J Biochem. 1968;64:1–6. doi: 10.1093/oxfordjournals.jbchem.a128853. [DOI] [PubMed] [Google Scholar]
- 92.Parker M W, Pastman J P M, Pattus F, Tucker A D, Tsenoglou D. Refined structure of the pore-forming domain of colicin A at 2.4 Å resolution. J Mol Biol. 1992;224:639–657. doi: 10.1016/0022-2836(92)90550-4. [DOI] [PubMed] [Google Scholar]
- 93.Pilsl H, Braun V. Novel colicin 10: assignment of four domains to TonB- and TolC-dependent uptake via the Tsx receptor and to pore formation. Mol Microbiol. 1995;16:57–67. doi: 10.1111/j.1365-2958.1995.tb02391.x. [DOI] [PubMed] [Google Scholar]
- 94.Pilsl H, Braun V. Strong function-related homology between the pore-forming colicins K and 5. J Bacteriol. 1995;177:6973–6977. doi: 10.1128/jb.177.23.6973-6977.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pilsl H, Glaser C, Gross P, Killman H, Olschläger T, Braun V. Domains of colicin M involved in uptake and activity. Mol Gen Genet. 1993;240:103–112. doi: 10.1007/BF00276889. [DOI] [PubMed] [Google Scholar]
- 96.Poole K, Zhao Q, Neshat S, Heinrichs D E, Dean C R. The Pseudomonas aeruginosa tonB gene encodes a novel TonB protein. Microbiology. 1996;142:1449–1458. doi: 10.1099/13500872-142-6-1449. [DOI] [PubMed] [Google Scholar]
- 97.Postle K. TonB protein and the gram-negative dilemma. Mol Microbiol. 1990;4:2019–2026. doi: 10.1111/j.1365-2958.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 98.Postle K. TonB protein and energy transduction between membranes. J Bioenerg Biomembr. 1993;25:591–601. doi: 10.1007/BF00770246. [DOI] [PubMed] [Google Scholar]
- 98a.Postle, K. Unpublished data.
- 99.Postle K, Skare J T. Escherichia coli TonB proteins exported from the cytoplasm without proteolytic cleavage of its amino terminus. J Biol Chem. 1988;263:11000–11007. [PubMed] [Google Scholar]
- 100.Pressler U, Staudenmaier H, Zimmermann L, Braun V. Genetics of the iron dicitrate transport system of Escherichia coli. J Bacteriol. 1981;170:2716–2724. doi: 10.1128/jb.170.6.2716-2724.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pugsley A P. The ins and outs of colicins. Part I. Production and translocation across membranes. Microbiol Sci. 1984;1:168–175. [PubMed] [Google Scholar]
- 102.Pugsley A P. The ins and outs of colicins. Part II. Lethal action, immunity and ecological implications. Microbiol Sci. 1984;1:203–205. [PubMed] [Google Scholar]
- 103.Raggett E M, Bainbridge G, Evans L J, Cooper A, Lakey J H. Colicins and Other Bacteriocins Workshop. Norwich, England: University of East Anglia; 1998. Biophysical characterisation of a novel colicin N-TolA binding region, abstr. 8; p. 15. [Google Scholar]
- 104.Reverchon S, Huan Y, Bourson C, Robert-Boudouy J. Nucleotide sequences of Erwinia chrysanthemi ogl and pelE genes negatively regulated by the kdgR gene product. Gene. 1989;85:125–134. doi: 10.1016/0378-1119(89)90472-1. [DOI] [PubMed] [Google Scholar]
- 105.Rigal A, Bouveret E, Lloubès R, Lazdunski C, Bénédetti H. The TolB protein interacts with the porins of Escherichia coli. J Bacteriol. 1997;179:7274–7279. doi: 10.1128/jb.179.23.7274-7279.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Riley M A. Positive selection for colicin diversity in bacteria. Mol Biol Evol. 1993;10:1048–1059. doi: 10.1093/oxfordjournals.molbev.a040054. [DOI] [PubMed] [Google Scholar]
- 107.Rodriguez-Herva J J, Ramos-Gonzalez M I, Ramos J L. The Pseudomonas putida peptidoglycan-associated outer membrane lipoprotein is involved in maintenance of the integrity of the cell envelope. J Bacteriol. 1996;178:1699–1706. doi: 10.1128/jb.178.6.1699-1706.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roof S K, Allard J D, Bertrand K P, Postle K. Analysis of Escherichia coli TonB membrane topology by use of PhoA fusions. J Bacteriol. 1991;173:5554–5557. doi: 10.1128/jb.173.17.5554-5557.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rutz J M, Liu J, Lyons J A, Gotanson J, Amstrong S K, McIntosh M A, Feix J B, Klebba P E. Formation of a gated channel by a ligand-specific transport protein in the bacterial membrane. Science. 1992;258:471–475. doi: 10.1126/science.1411544. [DOI] [PubMed] [Google Scholar]
- 110.Schendel S L, Click E M, Webster R E, Cramer W A. The TolA protein interacts with colicin E1 differently than with other group A colicins. J Bacteriol. 1997;179:3683–3690. doi: 10.1128/jb.179.11.3683-3690.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schirmer T, Keller T A, Wang Y F, Rosenbush J P. Structural basis for sugar translocation through maltoporin channels at 3.1 Å resolution. Science. 1995;267:512–514. doi: 10.1126/science.7824948. [DOI] [PubMed] [Google Scholar]
- 112.Schöffler H, Braun V. Transport across the outer membrane of Escherichia coli via the FhuA receptor is regulated by the TonB protein of the cytoplasmic membrane. Mol Gen Genet. 1989;217:378–383. doi: 10.1007/BF02464907. [DOI] [PubMed] [Google Scholar]
- 113.Schramm E, Mende J, Braun V, Kamp R M. Nucleotide sequence of the colicin B activity gene cba: consensus pentapeptide among TonB-dependent colicins and receptors. J Bacteriol. 1987;169:3350–3357. doi: 10.1128/jb.169.7.3350-3357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Skare J T, Postle K. Evidence for a TolB-dependent energy transduction complex in Escherichia coli. Mol Microbiol. 1991;5:2883–2890. doi: 10.1111/j.1365-2958.1991.tb01848.x. [DOI] [PubMed] [Google Scholar]
- 115.Skare J T, Ahmer B M M, Seachord C L, Darveau R P, Postle K. Energy transduction between membranes. TonB, a cytoplasmic membrane protein, can be chemically cross-linked in vitro to the outer membrane receptor FepA. J Biol Chem. 1993;268:16302–16308. [PubMed] [Google Scholar]
- 116.Slatin S L, Qui X Q, Jakes K S, Finkelstein A. Identification of a translocated protein segment in a voltage-dependent channel. Nature. 1994;371:158–161. doi: 10.1038/371158a0. [DOI] [PubMed] [Google Scholar]
- 117.Sun T P, Webster R E. Nucleotide sequence of a gene cluster involved in entry of E colicins and single-stranded DNA of infecting filamentous bacteriophages into Escherichia coli. J Bacteriol. 1987;169:2667–2674. doi: 10.1128/jb.169.6.2667-2674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thomas J A, Valvano M A. Role of tol genes in cloacin DF13 susceptibility of Escherichia coli K-12 stains expressing the cloacin DF13-aerobactin receptor IutA. J Bacteriol. 1993;175:548–552. doi: 10.1128/jb.175.2.548-552.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tibor A, Weynants V, Denoel P, Lichtfouse B, De Bolle X, Saman E, Limet J N, Letesson J-J. Molecular cloning, nucleotide sequence, and occurrence of a 16.5-kilodalton outer membrane protein of Brucella abortus with similary to Pal lipoproteins. Infect Immun. 1994;62:3633–3639. doi: 10.1128/iai.62.9.3633-3639.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tuckman M, Osburn M S. In vivo inhibition of TonB-dependent processes by a TonB box consensus pentapeptide. J Bacteriol. 1992;174:320–323. doi: 10.1128/jb.174.1.320-323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vianney A, Lewin T M, Beyer Jr W F, Lazzaroni J C, Portalier R, Webster R E. Membrane topology and mutational analysis of the TolQ protein of Escherichia coli required for the uptake of macromolecules and cell envelope integrity. J Bacteriol. 1994;176:822–829. doi: 10.1128/jb.176.3.822-829.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vianney A, Müller M M, Clavel T, Lazzaroni J C, Portalier R, Webster R E. Characterization of the tol-pal region of Escherichia coli K-12: translational control of tolR expression by TolQ and identification of a new open reading frame downstream of pal encoding a periplasmic protein. J Bacteriol. 1996;178:4031–4038. doi: 10.1128/jb.178.14.4031-4038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wiener M, Freymann D, Ghosh P, Stroud R M. Crystal structure of colicin Ia. Nature. 1997;385:461–464. doi: 10.1038/385461a0. [DOI] [PubMed] [Google Scholar]
- 124.Young G M, Postle K. Repression of tonB transcription during anaerobic growth requires Fur binding at the promoter and a second factor binding upstream. Mol Microbiol. 1994;11:943–954. doi: 10.1111/j.1365-2958.1994.tb00373.x. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Y L, Cramer W A. Constraints imposed by protease accessibility on the trans-membrane and surface topography of the colicin E1 ion channel. Protein Sci. 1992;1:1666–1676. doi: 10.1002/pro.5560011215. [DOI] [PMC free article] [PubMed] [Google Scholar]