Abstract
Background
Prior work demonstrates patients with positive (+) electrocardiogram (ECG) but negative (−) echocardiogram wall motion abnormalities (WMAs) on dobutamine stress echocardiography (DSE) testing have an elevated of major adverse cardiovascular events (MACEs). In this study, we aimed to evaluate the long-term prognosis of women with suspected ischemia with no obstructive coronary artery (INOCA) disease by utilizing core lab read DSE, specifically focusing on those with + ECG findings.
Methods
Among women with signs and symptoms of myocardial ischemia undergoing clinically indicated coronary angiography enrolled in the Women’s Ischemia Syndrome Evaluation (WISE) [1997–2001], a prospective cohort study, 99 underwent standardized DSE by site design. Women with positive DSE (n=17), defined as an increase in score based on wall motion scoring index were excluded except for akinetic to dyskinetic (n=10), providing 82 patients in this analysis. ECG was assessed by core laboratory and (+) ECG was defined as >1 mm ST change. Non-obstructive coronary artery disease (CAD) was assessed by core laboratory quantitative coronary angiography and defined as <50% epicardial stenosis. All-cause death follow-up was an average of 8 years, while adjudicated MACE [all-cause mortality, nonfatal myocardial infarction (MI), nonfatal stroke, heart failure hospitalization] was an average of 5.5 years. Comparisons among subject groups [i.e., (+) ECG and (–) ECG] were made using chi-square or Fisher’s exact tests for categorical variables and t-test or Wilcoxon rank-sum test for continuous variables.
Results
Demographic profile included a mean age 59±10 years; 55% had hypertension (HTN), 29% diabetes mellitus (DM), and 72% non-obstructive CAD. Overall, 9/82 women (11%) had (+) ECG in the absence of WMAs. There were significant differences in family history of CAD (P=0.009) and vasodilator (P=0.042) use between the (+) ECG and (−) ECG groups, but otherwise had no significant demographic or clinical differences. At longer-term follow up, patients with (+) ECG had higher risk of MACE [unadjusted hazard ratio (HR): 4.91, 95% confidence interval (CI): 1.83, 13.19, P=0.002].
Conclusions
Abnormal stress ECG findings on dobutamine stress testing with a negative DSE should be viewed as an indicator of longer-term risk in women with signs and symptoms of ischemia.
Keywords: Ischemia with no obstructive coronary artery disease (INOCA disease), ischemic heart disease, dobutamine stress echocardiography (DSE)
Highlight box.
Key findings
• Positive electrocardiogram (ECG) findings in the absence of wall motion abnormalities (WMAs) on dobutamine stress echo was associated with higher risk of major adverse cardiovascular events.
What is known and what is new?
• Positive ECG findings in the absence of WMAs have good specificity for coronary microvascular dysfunction.
• Women have higher prevalence of ischemia with no obstructive disease. Positive ECG findings in the absence of WMA on stress testing may reflect ischemia with no obstructive coronary artery and consequently an increased risk for future cardiac events, particularly in women.
What is the implication, and what should change now?
• Abnormal stress ECG findings on dobutamine stress testing with a negative dobutamine stress echocardiography should be viewed as an indicator of longer-term risk in women with signs and symptoms of ischemia.
Introduction
Background
Dobutamine stress echocardiography (DSE) is a commonly utilized and well-validated procedure for the evaluation of ischemia and suspected coronary artery disease (CAD) (1). This modality has been shown to have a high accuracy (80–90%) in detecting obstructive CAD (>50% stenosis) and good prognostic value following a negative result, with a low risk of overall mortality in the absence of wall motion abnormalities (WMAs) with stress testing (2).
Rationale and knowledge gap
However, the prognostic significance of abnormal electrocardiogram (ECG) findings in the absence of WMA on stress testing remains unclear (3-6). Historically, the diagnostic value of exercise stress testing in women has been questioned to a higher rate of “false positivity” (i.e., ECG changes on exercise stress testing but <50% stenosis seen on coronary angiography) (7), but the prognostic implications of this have been infrequently studied.
Objective
The purpose of our analysis is to study the prognosis of women with ischemic ECG findings on DSE in a well-phenotyped cohort with suspected ischemia with no obstructive coronary arteries (INOCA) with core lab interpretations and long-term follow-up. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-296/rc).
Methods
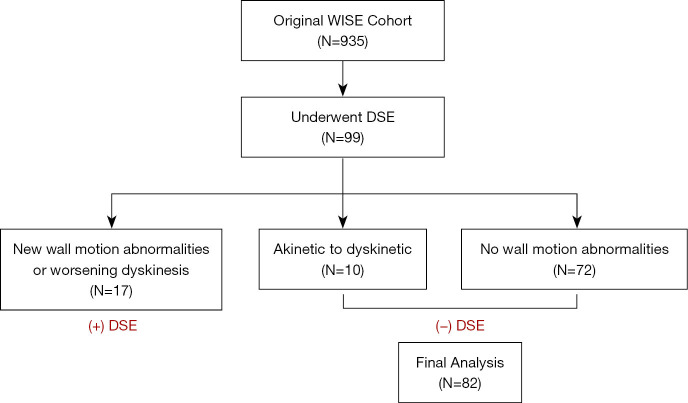
The Women’s Ischemia Syndrome Evaluation (WISE) Study was initiated by the National Institutes of Health-National Heart, Lung, and Blood Institute in 1996 to investigate women with signs and symptoms of ischemia (8). To date, the WISE study has followed more than 900 women for a median follow-up of 9.5 years after clinically indicated invasive coronary angiography. Women 18 years or older were enrolled in the WISE study, a prospective cohort study. Inclusion criteria included chest pain or other symptoms suggestive of myocardial ischemia and clinically indicated coronary angiography. Major exclusion criteria have previously been described and included comorbid conditions that affect one-year follow-up, pregnancy, contraindications to provocative diagnostic testing, cardiomyopathy, recent myocardial infarction (MI) and language barrier to questionnaire completion (8). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional review board approval was obtained from the University of Florida at Gainesville, and written informed consent was obtained from all study participants. A flow diagram showing the cohort study design for this retrospective analysis is shown in Figure 1.
Figure 1.
The flow diagram showing the cohort study design. WISE, Women’s Ischemia Syndrome Evaluation; DSE, dobutamine stress echocardiogram.
DSE protocol
DSE was performed using a protocol modified for the WISE study. A detailed protocol has previously been described (9). In brief, baseline images in standard views were obtained. Dobutamine was initiated at 5 mcg/kg/min, and dosage was increased at 5-minute increments to 10, 20, 30, and 40 mcg/kg/min. Atropine was used to attain target heart rate (HR) when indicated. The left ventricle was divided into 16 segments for analysis. Segments were graded and categorized as normal, hypokinetic, akinetic, or dyskinetic. The DSE echo was considered positive if there was an increase in the score in any segment, except for the transition from akinetic to dyskinetic (10). Studies were categorized as normal if no abnormalities were detected at baseline or peak stress and target HR was achieved. They were categorized as abnormal if there was any occurrence of WMA or increasing dyskinesis developed at peak stress or indeterminate if no abnormality was observed at baseline or peak stress and target HR was not reached. Wall motion analysis was conducted by a single cardiologist experienced in interpretation of DSE studies and remained unaware of clinical and angiogram findings (9). A 12-lead ECG was recorded at baseline, at the end of each 5-min stage and at recovery. The ECG, blood pressure, and echocardiographic images were monitored throughout the procedure. A positive ECG was defined as ≥1-mm ST segment changes, interpreted by the testing cardiologist.
Coronary angiography protocol
Coronary angiography was performed using the WISE study protocol with standard clinical techniques (9). Multiple projections of the left and right coronary arteries were recorded on 35-mm film at ≥30 frames per second. All angiography films were interpreted by the WISE Angiography Core Laboratory without knowledge of clinical or DSE findings. WISE Angiography Core Laboratory methods has previously been published. These angiograms were analyzed qualitatively for the presence or absence of luminal irregularities and stenoses. Stenoses were defined as <20%, 20–49%, and ≥50%. Angiographic coronary disease severity was assessed by the WISE coronary severity score as previously described (11).
Follow-up, mortality and MACEs
A standardized protocol-directed follow-up was conducted by experienced site nurses or physicians via telephone and/or mail communication at 6 weeks, 1 year, and annually thereafter. Subjects were provided with questionnaires regarding symptoms, hospitalizations, and diagnostic or revascularization procedures since last contact. Reported events were adjudicated by the study committee and classified as non-cardiac death, cardiovascular death or MACE including all-cause mortality, nonfatal MI, nonfatal stroke, and heart failure hospitalization. All-cause death had an average of 8 years of follow-up, while MACE had an average of 5.5 years.
Statistical analysis
Clinical variables were summarized as median [interquartile range (IQR)] for continuous variables or count (%) for categorical variables. Comparisons among subject groups [i.e., (+) ECG and (–) ECG] were made using Chi-square or Fisher’s exact tests for categorical variables and t-test or Wilcoxon rank-sum test for continuous variables. All tests were two-sided and statistical significance was considered present at P<0.05. Kaplan-Meier (KM) analysis was used to estimate survival probabilities and the log-rank test was used to compare KM curves. KM curves were stratified by ECG status for two event types: (I) overall mortality; and (II) four-component MACE as defined above. Univariate Cox regression models were constructed to obtain hazard ratio (HR) estimates with respect to ECG status. Analyses were carried out using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
Women with positive DSE (n=17), defined as an increase in score based on wall motion scoring index were excluded except for akinetic to dyskinetic (n=10), providing 82 patients in this analysis (Figure 1). Of the 82 patients included 75 (91.5%) achieved target HR. The baseline characteristics and demographics are summarized in Table 1. There were no significant differences between (−) ECG and (+) ECG groups for race, hypertension (HTN), diabetes mellitus (DM), dyslipidemia, smoking status, or baseline medications.
Table 1. Cohort characteristics (N=82).
| Variable | (−) ECG (N=73) | (+) ECG (N=9) | P value |
|---|---|---|---|
| Age, years (mean ± SD) | 59.3±10.0 | 58.6±13.9 | 0.853 |
| Race: White, n (%) | 56 (77.8) | 7 (77.8) | 0.792 |
| History of diabetes, n (%) | 19 (26.8) | 4 (44.4) | 0.271 |
| History of hypertension, n (%) | 39 (54.9) | 5 (55.6) | 0.999 |
| Family history of coronary disease, n (%) | 41 (60.3) | 1 (11.1) | 0.009 |
| History of dyslipidemia, n (%) | 35 (52.2) | 3 (42.9) | 0.707 |
| Current smoker, n (%) | 13 (18.3) | 2 (22.2) | 0.378 |
| Baseline medications, n (%) | |||
| ACE-I/ARB | 23 (32.4) | 1 (11.1) | 0.266 |
| Beta blockers | 16 (22.5) | 2 (22.2) | 0.999 |
| Calcium channel blockers | 22 (31.0) | 3 (33.3) | 0.999 |
| Nitrates | 14 (19.7) | 3 (33.3) | 0.392 |
| Statins | 22 (31.0) | 2 (22.2) | 0.717 |
| Aspirin | 42 (59.2) | 3 (33.3) | 0.169 |
| Vasodilators | 5 (7.0) | 3 (33.3) | 0.042 |
| WMA on baseline echo | 8 (11.0) | 2 (22.2) | 0.301 |
| Peak HR (mean ± SD) | 142.6±11.2 | 143.2±18.0 | 0.9273 |
| Peak systolic blood pressure (mean ± SD) | 162.5±30.4 | 175.1±34.0 | 0.2506 |
| Sharaf-Gensini Severity Score (mean ± SD) | 10.8±11.0 | 20.4±17.1 | 0.1080 |
| Coronary artery disease (% stenosis), n (%) | 0.672 | ||
| <20% | 28 (38.9) | 2 (22.2) | |
| 20–49% | 24 (33.3) | 4 (44.4) | |
| ≥50% | 20 (27.8) | 3 (33.3) | |
| Mortality, n (%) | 13 (18.1) | 4 (44.4) | 0.087 |
| MACE, n (%) | 19 (26.4) | 6 (66.7) | 0.022 |
Percentages exclude missing data. SD, standard deviation; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; WMA, wall motion abnormalities, HR, heart rate, MACE, major adverse cardiovascular event.
Nine women (11.0%) had (+) ECG findings. Among those with (+) ECG findings, 4 (44.4%) and 3 (33.3%) had 20–49% stenosis and ≥50% stenosis, respectively. Seventy-three (89.0%) patients had (−) ECG findings, and among those, there were 24 (33.3%) and 20 (27.8%) with non-obstructive stenosis (20–49%) and obstructive disease (≥50%), respectively. There was no significant difference between (+) ECG and (−) ECG groups for CAD severity (P=0.672). Overall, the difference between (+) ECG and (−) ECG groups was statistically significant for MACE (P=0.022) but not for all-cause mortality (P=0.087).
A secondary analysis of MACE and death by coronary artery severity demonstrated a statistically significant difference in MACE (P=0.001) and all-cause mortality (P=0.016) when stratified by severity of stenosis (<20%, 20–49% and ≥50%). In women with <20% stenosis, MACE occurred in 2/30 (6.7%) and death in 2/30 (6.7%). Comparatively, in those with non-obstructive CAD (20–49% stenosis), MACE occurred in 12/28 (42.9%) and death in 6/28 (21.4%). In those with obstructive disease, MACE occurred in 11/23 (47.8%) and death in 9/23 (39.1%). When further stratified by ECG response and CAD severity, there were no differences in mortality or MACE.
Long-term adverse outcomes
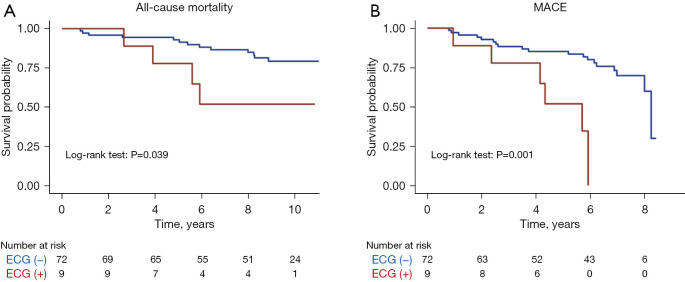
All-cause mortality had an average of 8 years of follow-up, while four-component MACE had an average of 5.5 years. In this long-term follow-up, women with (+) ECG had a significantly higher risk of MACE [unadjusted HR: 4.91, 95% confidence interval (CI): 1.83, 13.19, P=0.002] and all-cause mortality (unadjusted HR: 3.09, 95% CI: 1.00, 9.56, P=0.050) compared to patients with (−) ECG, regardless of echo response. KM curves for freedom from events for MACEs and mortality by ECG findings on DSE are shown in Figure 2.
Figure 2.
Long-term mortality and MACE outcomes by ECG findings on DSE. (A) Kaplan-Meier curves for freedom from events for mortality and (B) MACE by ECG findings on dobutamine stress echocardiography. ECG, electrocardiogram; MACE, major adverse cardiovascular event; DSE, dobutamine stress echocardiogram.
Discussion
Key findings
Our study demonstrates (+) ECG in the absence of WMA during DSE predicts adverse cardiovascular outcomes regardless of echocardiography findings in women undergoing clinically indicated coronary angiography for suspected ischemic heart disease. Notably, a considerable proportion of women with (+) ECG findings (44.4%) had non-obstructive CAD found on invasive angiogram.
Explanations of findings and comparison with similar studies
Prior studies that explored the association between the abnormal ECG findings in the absence of WMA on stress testing and long-term outcomes have yielded mixed results. A contemporary study of 15,077 patients (58.7% women) undergoing exercise stress Echo, patients with (+) ECG and (−) echo had higher rates of MACEs compared to patients with both negative ECG and echo (14.6% vs. 8.5%) over a median follow-up of 7.3 years (3). Historical work has yielded similar findings with a 1999 study of 558 patients (60.6% women) finding overall cardiac event rates of 23% when there was an ischemic ECG response but negative WMA, compared to 7% when both ECG and WMA were negative (4). According to this study, the ability of an ischemic ECG response to predict future cardiac event rates was similar to that of WMAs at rest and a positive stress echo response. Conversely, a study of 1,268 patients (48% women) found that normal findings on stress echo confers a benign prognosis for MACE, independent of ECG response (5). A retrospective study of 677 patients with negative treadmill stress echo results found that (+) ECG result was not predictive of all-cause death, MI or revascularization over a median follow-up of 95 months (6). Notably, in this study, female gender was found to be an independent predictor for events.
WMAs seen on DSE have been shown to reliably detect multi-vessel stenosis (12) but has lower sensitivity in women with single-vessel stenosis and low angiographic severity score (9). Furthermore, a negative noninvasive stress test does not rule out coronary vasomotor dysfunction in symptomatic patients, with dobutamine stress echo having a sensitivity of 29% (95% CI: 8–58%), specificity of 57% (95% CI: 18–90%), and positive predictive value of only 57% (95% CI: 8–58%) for vasomotor dysfunction (13). As such, we postulate that this population of patients with (+) ECG findings but no new or worsening WMA on DSE may represent women with INOCA disease and coronary vasomotor dysfunction.
It has previously been reported that women with INOCA are at risk for long-term adverse outcomes. Of the total WISE cohort undergoing invasive angiogram, 551 were found to have no obstructive CAD. Analysis of these women found that INOCA was associated with considerable morbidity with a 2.5% rate of MACE at 5-year follow up (14). Furthermore, women with stable INOCA and non-obstructive CAD had 10-year all-cause death and cardiac death rates of 17% and 11% respectively, compared to 10% and 6% in women with normal coronaries (15). Females with non-obstructive disease are at increased risk for progression to obstructive CAD (14). Therefore, positive ECG findings in the absence of worsening WMA on stress testing should not be discounted as they may reflect INOCA and consequently an increased risk for future cardiac events, particularly in women. Prior studies that have looked at cohorts of patients with known microvascular dysfunction based on invasive vasoreactivity testing or positron emission tomography (PET) myocardial perfusion study and subsequently underwent non-invasive stress have revealed (+) ECG have good specificity for coronary microvascular dysfunction (13,16). Therefore, further angiographic testing and/or consideration of empiric therapy targeted towards coronary microvascular dysfunction and angina may be warranted in patients with positive ECG findings in the absence of worsening WMAs on stress testing.
Implications of findings
Noninvasive testing for obstructive CAD has generally been thought to have poorer specificity for women compared to men (9). Equivocal or negative stress findings may be a representation of the higher prevalence of INOCA and coronary microvascular dysfunction observed in women (11). Regarding management, it has been shown that women with suspected CAD have fewer additional diagnostic tests than men after an initial abnormal noninvasive stress test result, even when initial diagnostic test positivity rates are similar (17). Cardiac death and MI rates following non-invasive testing have subsequently found to be higher in women during two years of follow-up (6.9% vs. 2.4%) (17). Together, these findings illustrate the importance of recognizing ischemic ECG findings in women undergoing noninvasive testing for signs and symptoms of ischemia to guide appropriate downstream testing and treatments.
Strengths and limitations
Our study adds to the existing literature on prognostic significance of abnormal ECG in stress echo, and to our knowledge is the first study to do so in a cohort of women with suspected ischemia utilizing echo results analyzed in a core lab.
Our results are consistent with a large cohort study showing adverse prognosis in patients with abnormal ECG response on exercise stress testing (3), but is limited by the small sample size of women undergoing DSE at a single WISE site, by design. Specifically, an in-depth analysis of discrepant (+) ECG/(−) echo findings was limited due to the small sample size of subjects with this result. The DSE studies were interpreted by a single experienced cardiologist, and it may be possible that wall motional abnormalities were missed. Furthermore, strain analysis was not available in this data set. Additionally, our results may not be relevant to broader populations of women because the WISE study excluded women with unstable symptoms and as such, may have a lower prevalence of obstructive coronary disease. We hypothesize that women in this cohort with adverse events and non-obstructive disease may represent those with vasomotor dysfunction, but do not report findings of microvascular function or vasospasm. While some women in this cohort did undergo invasive coronary function testing for these purposes, the sample size was small. Future work may also aim to correlate measurements of coronary flow reserve and coronary blood flow to assess whether this population may be phenotypically representative of coronary microvascular dysfunction.
Conclusions
Positive ECG findings in the setting of negative echo findings on dobutamine stress testing may be an indicator of longer-term risk for major adverse cardiovascular outcomes in women with signs and symptoms of ischemia. It is important to recognize this at-risk population of women to target further diagnostics and/or intensify medical management. Larger datasets are needed to further understand and develop sex-specific prognostic metrics for non-invasive stress testing in the evaluation of ischemic heart disease.
Supplementary
The article’s supplementary files as
Acknowledgments
A part of this manuscript (abstract) was previously presented at American College of Cardiology 2021 Scientific Sessions and was published under the title “Association of abnormal electrocardiography response on stress echocardiogram with major adverse cardiovascular events in women: A report from the Women’s Ischemia Syndrome Evaluation (WISE) Study” available at https://doi.org/10.1016/S0735-1097(21)02707-8.
Funding: This work was supported by contracts from the National Heart, Lung and Blood Institutes Nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, K23 HL125941, K23 HL127262, T32 HL69751, R01 HL090957, 1R03 AG032631, R01 HL146158, R01HL124649, PR150224P1 (CDMRP-DoD), U54 AG065141, GCRC grant MO1-RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences Grant UL1TR000124, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, The Society for Women’s Health Research (SWHR), Washington, D.C., the Linda Joy Pollin Women’s Heart Health Program, the Erika Glazer Women’s Heart Health Project, and the Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, California. This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the U.S. Department of Health and Human Services.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional review board approval was obtained from the University of Florida at Gainesville (IRB# 14906), and written informed consent was obtained from all study participants.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-296/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-296/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-296/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-296/coif). J.W. reports receiving honoraria to participate in a Coronary Microvascular Dysfunction Advisory Board for Abbott Vascular, paid to institution. The other authors have no conflicts of interest to declare.
References
- 1.Geleijnse ML, Fioretti PM, Roelandt JR. Methodology, feasibility, safety and diagnostic accuracy of dobutamine stress echocardiography. J Am Coll Cardiol 1997;30:595-606. 10.1016/S0735-1097(97)00206-4 [DOI] [PubMed] [Google Scholar]
- 2.Rachwan RJ, Mshelbwala FS, Bou Chaaya RG, et al. Long-term prognosis and predictors of outcomes after negative stress echocardiography. Int J Cardiovasc Imaging 2020;36:1953-62. 10.1007/s10554-020-01913-6 [DOI] [PubMed] [Google Scholar]
- 3.Daubert MA, Sivak J, Dunning A, et al. Implications of Abnormal Exercise Electrocardiography With Normal Stress Echocardiography. JAMA Intern Med 2020;180:494-502. 10.1001/jamainternmed.2019.6958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krivokapich J, Child JS, Walter DO, et al. Prognostic value of dobutamine stress echocardiography in predicting cardiac events in patients with known or suspected coronary artery disease. J Am Coll Cardiol 1999;33:708-16. 10.1016/S0735-1097(98)00632-9 [DOI] [PubMed] [Google Scholar]
- 5.Mahenthiran J, Bangalore S, Yao SS, et al. Comparison of prognostic value of stress echocardiography versus stress electrocardiography in patients with suspected coronary artery disease. Am J Cardiol 2005;96:628-34. 10.1016/j.amjcard.2005.04.032 [DOI] [PubMed] [Google Scholar]
- 6.Al-Mallah M, Alqaisi F, Arafeh A, et al. Long term favorable prognostic value of negative treadmill echocardiogram in the setting of abnormal treadmill electrocardiogram: a 95 month median duration follow-up study. J Am Soc Echocardiogr 2008;21:1018-22. 10.1016/j.echo.2008.06.005 [DOI] [PubMed] [Google Scholar]
- 7.Kohli P, Gulati M. Exercise stress testing in women: going back to the basics. Circulation 2010;122:2570-80. 10.1161/CIRCULATIONAHA.109.914754 [DOI] [PubMed] [Google Scholar]
- 8.Merz CN, Kelsey SF, Pepine CJ, et al. The Women's Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol 1999;33:1453-61. 10.1016/S0735-1097(99)00082-0 [DOI] [PubMed] [Google Scholar]
- 9.Lewis JF, Lin L, McGorray S, et al. Dobutamine stress echocardiography in women with chest pain. Pilot phase data from the National Heart, Lung and Blood Institute Women's Ischemia Syndrome Evaluation (WISE). J Am Coll Cardiol 1999;33:1462-8. 10.1016/S0735-1097(99)00076-5 [DOI] [PubMed] [Google Scholar]
- 10.Carlos ME, Smart SC, Wynsen JC, et al. Dobutamine stress echocardiography for risk stratification after myocardial infarction. Circulation 1997;95:1402-10. 10.1161/01.CIR.95.6.1402 [DOI] [PubMed] [Google Scholar]
- 11.Sharaf BL, Pepine CJ, Kerensky RA, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women's Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory). Am J Cardiol 2001;87:937-41; A3. [DOI] [PubMed]
- 12.Pellikka PA, Arruda-Olson A, Chaudhry FA, et al. Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. J Am Soc Echocardiogr 2020;33:1-41.e8. 10.1016/j.echo.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 13.Cassar A, Chareonthaitawee P, Rihal CS, et al. Lack of correlation between noninvasive stress tests and invasive coronary vasomotor dysfunction in patients with nonobstructive coronary artery disease. Circ Cardiovasc Interv 2009;2:237-44. 10.1161/CIRCINTERVENTIONS.108.841056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bairey Merz CN, Pepine CJ, Walsh MN, et al. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation 2017;135:1075-92. 10.1161/CIRCULATIONAHA.116.024534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenkre TS, Malhotra P, Johnson BD, et al. Ten-Year Mortality in the WISE Study (Women's Ischemia Syndrome Evaluation). Circ Cardiovasc Qual Outcomes 2017;10:e003863. 10.1161/CIRCOUTCOMES.116.003863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez DM, Divakaran S, Gupta A, et al. Role of Exercise Treadmill Testing in the Assessment of Coronary Microvascular Disease. JACC Cardiovasc Imaging 2022;15:312-21. 10.1016/j.jcmg.2021.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw LJ, Miller DD, Romeis JC, et al. Gender differences in the noninvasive evaluation and management of patients with suspected coronary artery disease. Ann Intern Med 1994;120:559-66. 10.7326/0003-4819-120-7-199404010-00005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as