Abstract
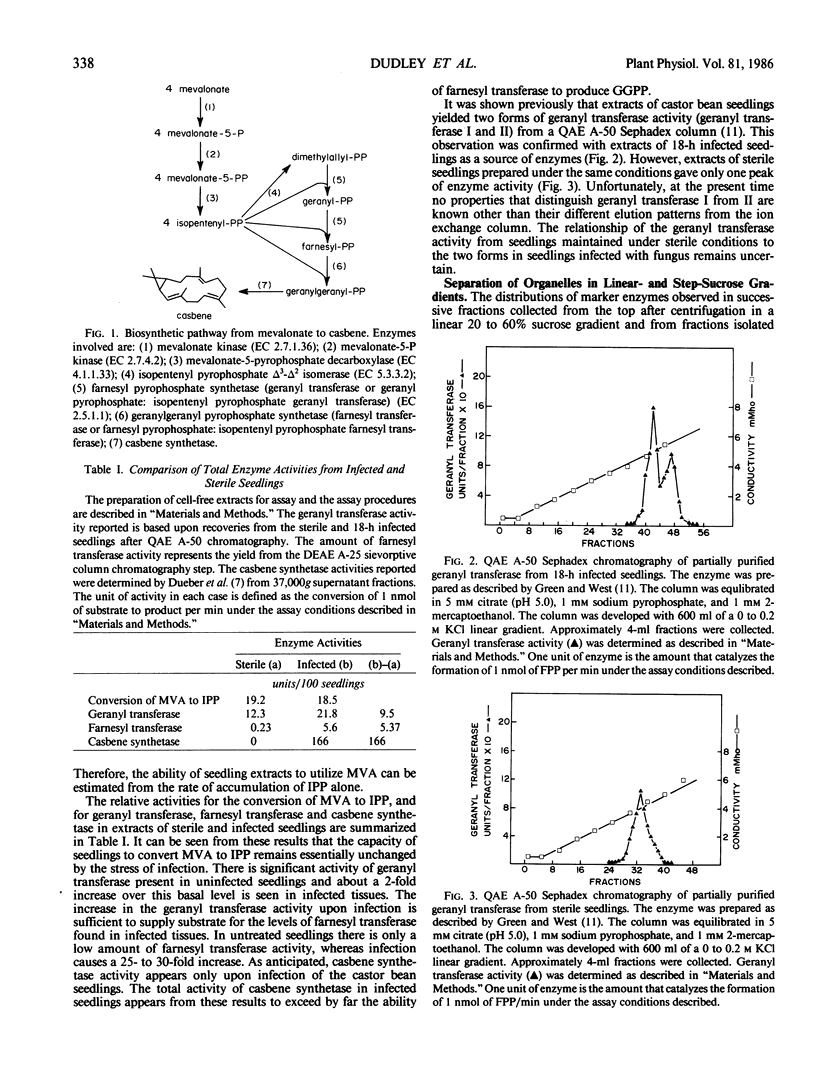
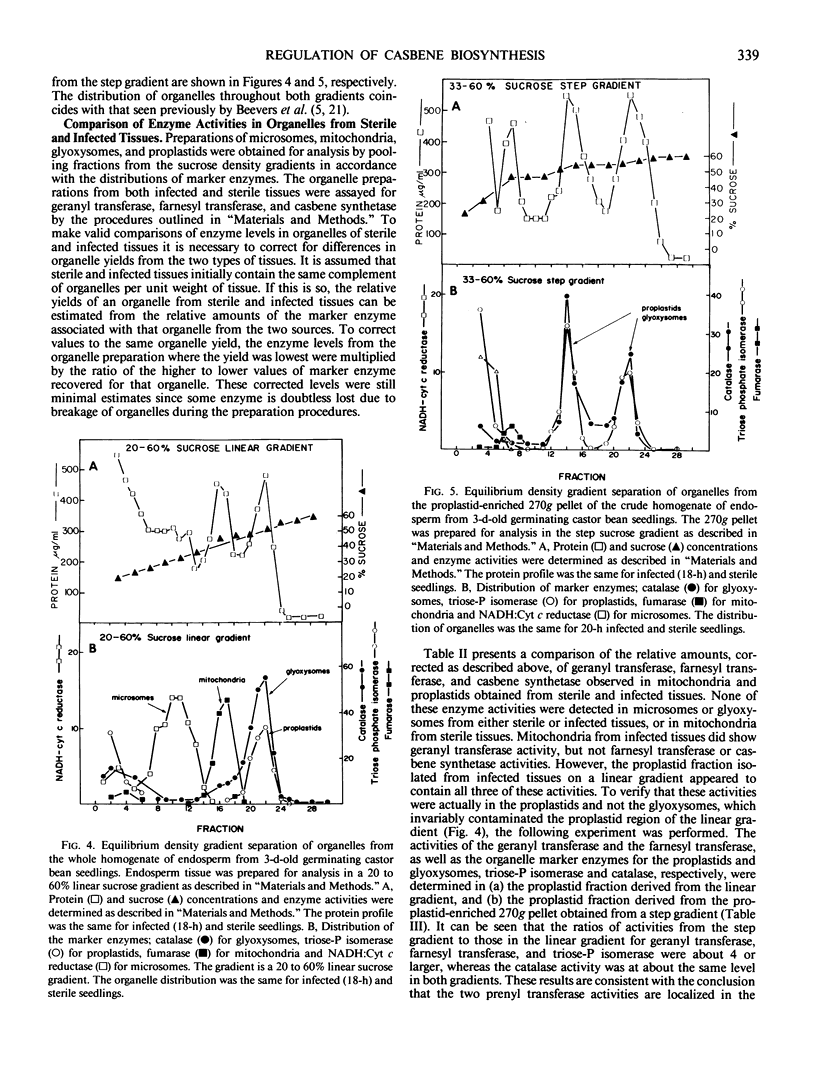
Casbene is a macrocyclic diterpene hydrocarbon that is produced in young castor bean (Ricinus communis L.) seedlings after they are exposed to Rhizopus stolonifer or other fungi. The activities of enzymes that participate in casbene biosynthesis were measured in cell-free extracts of 67-hour castor bean seedlings (a) that had been exposed to R. stolonifer spores 18 hours prior to the preparation of extracts, and (b) that were maintained under aseptic conditions throughout. Activity for the conversion of mevalonate to isopentenyl pyrophosphate does not change significantly after infection. On the other hand, the activities of farnesyl pyrophosphate synthetase (geranyl transferase), geranylgeranyl pyrophosphate synthetase (farnesyl transferase), and casbene synthetase are all substantially greater in infected tissues in comparison with control seedlings maintained under sterile conditions. The subcellular localization of these enzymes of casbene biosynthesis was investigated in preparations of microsomes, mitochondria, glyoxysomes, and proplastids that were resolved by centrifugation in linear and step sucrose density gradients of homogenates of castor bean endosperm tissue from both infected and sterile castor bean seedlings. Isopentenyl pyrophosphate isomerase and geranyl transferase activities are associated with proplastids from both infected and sterile seedlings. Significant levels of farnesyl transferase and casbene synthetase are found only in association with the proplastids of infected tissues and not in the proplastids of sterile tissues. From these results, it appears that at least the last two steps of casbene biosynthesis, geranylgeranyl pyrophosphate synthetase and casbene synthetase, are induced during the process of infection, and that the enzymes responsible for the conversion of isopentenyl pyrophosphate to casbene are localized in proplastids.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruce R. J., West C. A. Elicitation of Casbene Synthetase Activity in Castor Bean : THE ROLE OF PECTIC FRAGMENTS OF THE PLANT CELL WALL IN ELICITATION BY A FUNGAL ENDOPOLYGALACTURONASE. Plant Physiol. 1982 May;69(5):1181–1188. doi: 10.1104/pp.69.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börner H., Grisebach H. Enzyme induction in soybean infected by Phytophthora megasperma f.sp. glycinea. Arch Biochem Biophys. 1982 Aug;217(1):65–71. doi: 10.1016/0003-9861(82)90479-9. [DOI] [PubMed] [Google Scholar]
- Camara B. Terpenoid metabolism in plastids : sites of phytoene synthetase activity and synthesis in plant cells. Plant Physiol. 1984 Jan;74(1):112–116. doi: 10.1104/pp.74.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Dueber M. T., Adolf W., West C. A. Biosynthesis of the Diterpene Phytoalexin Casbene: Partial Purification and Characterization of Casbene Synthetase from Ricinis communis. Plant Physiol. 1978 Oct;62(4):598–603. doi: 10.1104/pp.62.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan R. E., Rasson E., Porter J. W. Separation of water-soluble steroid and carotenoid precursors by DEAE-cellulose column chromatography. Anal Biochem. 1968 Feb;22(2):249–259. doi: 10.1016/0003-2697(68)90314-x. [DOI] [PubMed] [Google Scholar]
- GOODWIN T. W. Studies in carotenogenesis. 25. The incorporation of 14CO2, [2-14C] acetate and [2-14C]mevalonate into beta-carotene by illuminated etiolated maize seedings. Biochem J. 1958 Dec;70(4):612–617. doi: 10.1042/bj0700612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T. R., West C. A. Purification and characterization of two forms of geranyl transferase from Ricinus communis. Biochemistry. 1974 Nov 5;13(23):4720–4729. doi: 10.1021/bi00720a007. [DOI] [PubMed] [Google Scholar]
- Kirkegaard L. H. Gradient sievorptive chromatography. A focusing system for the separation of cellular components. Biochemistry. 1973 Sep 11;12(19):3627–3632. doi: 10.1021/bi00743a009. [DOI] [PubMed] [Google Scholar]
- Kreuz K., Kleinig H. Synthesis of prenyl lipids in cells of spinach leaf. Compartmentation of enzymes for formation of isopentenyl diphosphate. Eur J Biochem. 1984 Jun 15;141(3):531–535. doi: 10.1111/j.1432-1033.1984.tb08225.x. [DOI] [PubMed] [Google Scholar]
- Lawton M. A., Dixon R. A., Hahlbrock K., Lamb C. Rapid induction of the synthesis of phenylalanine ammonia-lyase and of chalcone synthase in elicitor-treated plant cells. Eur J Biochem. 1983 Jan 1;129(3):593–601. doi: 10.1111/j.1432-1033.1983.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Lee S. C., West C. A. Polygalacturonase from Rhizopus stolonifer, an Elicitor of Casbene Synthetase Activity in Castor Bean (Ricinus communis L.) Seedlings. Plant Physiol. 1981 Apr;67(4):633–639. doi: 10.1104/pp.67.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschke D. C., Hadwiger L. A. Effects of Light and of Fusarium solani on Synthesis and Activity of Phenylalanine Ammonia-Lyase in Peas. Plant Physiol. 1981 Sep;68(3):680–685. doi: 10.1104/pp.68.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütke-Brinkhaus F., Liedvogel B., Kleinig H. On the biosynthesis of ubiquinones in plant mitochondria. Eur J Biochem. 1984 Jun 15;141(3):537–541. doi: 10.1111/j.1432-1033.1984.tb08226.x. [DOI] [PubMed] [Google Scholar]
- Miflin B. J., Beevers H. Isolation of intact plastids from a range of plant tissues. Plant Physiol. 1974 Jun;53(6):870–874. doi: 10.1104/pp.53.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oba K., Tatematsu H., Yamashita K., Uritani I. Induction of Furano-terpene Production and Formation of the Enzyme System from Mevalonate to Isopentenyl Pyrophosphate in Sweet Potato Root Tissue Injured by Ceratocystis fimbriata and by Toxic Chemicals. Plant Physiol. 1976 Jul;58(1):51–56. doi: 10.1104/pp.58.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond C. B., Akazawa T., Beevers H. Localization and properties of ribulose diphosphate carboxylase from castor bean endosperm. Plant Physiol. 1975 Feb;55(2):226–230. doi: 10.1104/pp.55.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. R., West C. A. Biosynthesis of cyclic diterpenes in extracts from seedlings of Ricinus communis L. I. Identification of diterpene hydrocarbons formed from mevalonate. Biochemistry. 1970 Jan 6;9(1):70–79. doi: 10.1021/bi00803a010. [DOI] [PubMed] [Google Scholar]
- Robinson D. R., West C. A. Biosynthesis of cyclic diterpenes in extracts from seedlings of Ricinus communis L. II. Conversion of geranylgeranyl pyrophosphate into diterpene hydrocarbons and partial purification of the cyclization enzymes. Biochemistry. 1970 Jan 6;9(1):80–89. doi: 10.1021/bi00803a011. [DOI] [PubMed] [Google Scholar]
- Simcox P. D., Dennis D. T., West C. A. Kaurene synthetase from plastids of developing plant tissues. Biochem Biophys Res Commun. 1975 Sep 2;66(1):166–172. doi: 10.1016/s0006-291x(75)80309-3. [DOI] [PubMed] [Google Scholar]
- Soll J., Kemmerling M., Schultz G. Tocopherol and plastoquinone synthesis in spinach chloroplasts subfractions. Arch Biochem Biophys. 1980 Oct 15;204(2):544–550. doi: 10.1016/0003-9861(80)90066-1. [DOI] [PubMed] [Google Scholar]
- Stekoll M., West C. A. Purification and Properties of an Elicitor of Castor Bean Phytoalexin from Culture Filtrates of the Fungus Rhizopus stolonifer. Plant Physiol. 1978 Jan;61(1):38–45. doi: 10.1104/pp.61.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zähringer U., Ebel J., Grisebach H. Induction of phytoalexin synthesis in soybean. Elicitor-induced increase in enzyme activities of flavonoid biosynthesis and incorporation of mevalonate into glyceollin. Arch Biochem Biophys. 1978 Jun;188(2):450–455. doi: 10.1016/s0003-9861(78)80029-0. [DOI] [PubMed] [Google Scholar]


