Summary
The immune system defines a complex network of tissues and cell types that orchestrate responses across the body in a dynamic manner. The local and systemic interactions between immune and cancer cells contribute to disease progression. Lymphocytes are activated in lymph nodes, traffic through the periphery, and impact cancer progression through their interactions with tumor cells. As a result, therapeutic response and resistance are mediated across tissues, and a comprehensive understanding of lymphocyte dynamics requires a systems-level approach. In this review, we highlight experimental and computational methods that can leverage the study of leukocyte trafficking through an immunomics lens and reveal how adaptive immunity shapes cancer.
Understanding the dynamics of tumor-immune interactions requires high-throughput, system-wide technologies. In this review, Sidiropoulos et al. highlight current methods used to study immune responses at a systems level from the perspective of immunomics.
Introduction
Cells of the immune system originate from hematopoietic precursors in the bone marrow, subsequently forging myeloid and lymphoid lineages. At any given moment in time, available pools of naive and matured immune cells can be circulating in peripheral blood and lymphatic fluid or found to be tissue resident.1 Myeloid cells and lymphoid cells together perform duties that form the basis of adaptive and innate immunity. Throughout their lifespan, these cells will migrate across vessels and tissues, exposing themselves to stimuli and selective pressures that will differentiate them into mature cells with distinct functions and phenotypes.2 The circulation of immune cells across the body culminates in the orchestration of systemic responses as well as in the establishment of immunological niches that shape disease progression. As immune cells transverse through tissue and circulate the lymphatic drainage system, they alter their phenotypes and function.3 As a result, immune cells have heterogeneous resident or migratory states that vary with their cell subtype and function in a site-specific manner during disease progression.4
A hallmark of cancer is its ability to evade anti-tumor immunity, often by suppressing or hijacking immune responses for its benefit.5 Thus, immune responses play an important role in tumorigenesis and metastatic disease progression. Resident immune cells in tumors have been shown to play a crucial role in tumorigenesis by regulating the immune microenvironment and, if phenotypically immunosuppressive, can even facilitate tumor cell survival.6 Immune structures organized to varying degrees, including tertiary lymphoid aggregates (TLAs), mature tertiary lymphoid structures (mTLSs) or ectopic lymphoid structures (ELSs), and immune infiltrates of myeloid origin, can be found modulating the tumor microenvironment across solid tumors.7,8 The location and density of these structures have been proven to have prognostic value, and it is hypothesized that they might contain potential for immunotherapeutic targeting.9 These immune structures can evolve over time and self-organize into highly ordered structures that resemble lymph nodes, particularly with T cell-rich zones and germinal centers that disseminate humoral immunity.8,10 Understanding their development, the trafficking of immune cells to and from them, and the regulatory mechanisms governing their function is critical to immunotherapy because it can provide novel insights into optimizing treatments, identifying new therapeutic targets, and improving patient outcomes.
The mechanisms underlying the development of immune structures throughout the course of the disease, their relationship to immune cell recruitment, and the mediation of immunotherapy response remain largely unresolved. Cutting-edge high-throughput immunomics technologies applied to the tumor microenvironment now achieve the resolution necessary to decipher tumor-immune evolutionary dynamics in cancer.11,12 These technological omics innovations have facilitated an exponential increase in our ability to generate high-dimensional single-cell data, while data science is catching up to this exponential curve.13 Whereas most studies focus on characterizing a single tissue, immune cell trafficking and structures span across tissues and evolve over time. In this review, we highlight considerations in the selection of experimental and computational methodologies and patient samples necessary to examine these systems-level immune processes throughout the body. In this review, our main targets are computational immunology method users and developers who could benefit from an overview of the current technologies and data types available to study leukocyte trafficking contextualized with current research topics in cancer immunology.
Immune cell trafficking and immune structures in cancer
Although interactions between immune and cancer cells likely first occur at the primary tumor site, with increasing disease progression, these interactions become more widespread. As tumor cells continue to invade regional lymph nodes and opportunistically circulate to colonize distant loci, interactions with immune cells greatly influence the course of the disease. Draining lymph nodes are often primary metastatic targets and likely some of the primary sites of acquired immune tolerance.14 After this, tumor tolerance is propagated, promoting tumor cells to colonize more distant lymph nodes and organs.15 As cancer cells continue to interact with immune cells, they influence each other’s evolution in various ways. In one process, known as cancer immunoediting, cancer clonal diversity is modulated by adaptive immune responses targeting cancer antigens.16 In parallel processes, this continuous interaction between immune cells and cancer cells may produce exhausted T cell phenotypes,17 and mechanisms of immune evasion by cancer cells, such as major histocompatibility complex (MHC) downregulation,18 and checkpoint ligand expression.19
Immune cell trafficking refers to the movement of immune cells across tissues in response to various signals, a process that plays a critical role in the immune system’s ability to recognize and respond to threats to the body.20 In this review, we refer to immune cell trafficking in cancer as the physical movement of immune cells through the complex network of lymphoid tissues, vasculature, tumors, and extracellular matrix that govern their navigation. In this network, secreted soluble factors, including cytokines and chemokines, as well as insoluble extracellular vesicles induce or inhibit cellular migration in immune cells.21,22 Many of these factors may be secreted by various cellular regulators, including immune cells, stromal cells, and even tumor cells.23 The 3D spatial plane in tumors, in combination with lymphatic and blood vasculature, together form a complex navigational network for immune cells in cancer. Immune cell trafficking has been studied temporally and mechanistically in many experimental models.24 In humans, however, researchers are limited by snapshots of immune cell dynamics in blood and tissue samples, unable to make direct observations of trafficking or the dynamics of immune structures, like TLSs, over time. Although not a substitute for real-time tracking of cells, recent advances in profiling technologies can help us identify lymphocyte subsets and keep track of their locations (intratumoral, peripheral, lymphatic, etc.), cell signatures (activated, stem like, etc.), and expansion dynamics. By integrating this information, we can infer trafficking in humans, generate hypotheses from clinical correlates, and subsequently validate findings in vitro and in vivo with appropriate models.
Immune structures in cancer refer to collections of immune cells within the tumor microenvironment or in secondary lymphoid organs, such as tumor-draining lymph nodes. These include TLSs and germinal centers, which are composed of highly organized clusters of immune cells, including B cells, T cells, and follicular dendritic cells that propagate humoral responses.25 They also include immune aggregates in zones of immune cell exclusion, including regulatory T cells and immunosuppressive myeloid cells.26,27 Together, these dynamic structures result from immune cell trafficking, facilitating a balancing act between tumor immunity and tumor tolerance.
Others have previously reviewed cell motility and trafficking in cancer with an emphasis on in vitro models and in vivo biological observations.24,28 They described a complex network of extracellular matrix and chemokine regulation for the migration of both tumor and immune cells. Similarly, sophisticated mouse modeling and intravital microscopy have demonstrated trafficking of immune cell subsets from tumors to lymph nodes and synaptic transfer of tumor antigens between these subsets.29 In addition to the biophysical study of cellular dynamics of lymphocyte trafficking in immune system function, a bioinformatic lens can complement in vitro/vivo methods to discover the molecular mechanisms associated with the changing behavior and actions of the immune system. Historically, the field of immunology has relied on isolating cells based on surface multimarker panels for mechanistic molecular investigation. Protein markers of immune cell identity have been extensively experimentally validated by flow cytometry, such as in the case of functional or exhausted T cell markers.30 However, the same type of immune cell can have distinct phenotypes and functions, resulting from complex molecular interactions along gene-regulatory networks. Now, a multitude of newly available technologies allows us to profile the immune system on a larger scale of single-cell and spatial modalities, including proteomics, transcriptomics, epigenomics, and genomics (Table 1). These modalities, when used in conjunction, offer unique and complementary capacities for biological investigation of immune cell function (Figure 1). Computational methods are also being continuously innovated to leverage these high-dimensional technologies in tandem to study immunology in a systems-level approach.
Table 1.
Example high-throughput technologies and protocols available for human immunophenotyping and receptor repertoire studies at single-cell and spatial resolutions
| Resolution | Technologies | Example platforms | Data types | Developers |
|---|---|---|---|---|
| Bulk | RNA-seq | NovaSeq | transcriptome | Nanostring |
| TCR/BCR DNA-seq | immunoSEQ | unpaired TCR α/β, BCR (IGH, IGL, IGK) | Adaptive Biotech | |
| chromatin accessibility sequencing | ATAC-seq | read density peaks in accessible chromatin region | Buenrostro et al.31 | |
| Single cell | Flow Cytometry | CytoFLEX | <21 protein targets | Beckman Coulter |
| Mass Cytometry | HeliosTM | <40 protein targets | Standard BioTools | |
| Single cell RNA-seq ATAC-seq TCR/BCR-seq |
chromium, RAGE-seq (long read) |
transcriptome, paired TCRα/β, BCR (IGH, IGL, IGK), epigenome |
10 Genomics Singh et al.32 |
|
| probed high-plex antibodies combined with scRNA-seq/TCR-seq | CITE-seq, TetTCR- SeqHD |
>150 protein targets, transcriptome, paired TCRα/β | Stoeckius et al.33 Ma et al.34 |
|
| Spatial | 10- to 55-μm spot RNA-seq, chromatin modification profiling | Visium (55 μm), GeoMx TM (>10 μm), Slide-seqV2 (10 μm), Spatial-CUT&Tag –RNA-seq (20–50 μm) |
spatially resolved transcriptome, epigenome | 10 Genomics Nanostring Stickels et al.,35 Deng et al.,36 Zhang et al.37 |
| 10- to 55-μm spot RNA + TCR-seq |
Visium + TCR amplification (55 μm), slide-TCR-seq (10 μm) | spatially resolved transcriptome and TCR repertoire | Hudson et al.38 Liu et al.39 |
|
| 25-μm spot RNA-seq + high-plex protein mapping | spatial CITE-seq | spatially resolved transcriptome and 189 protein targets | Liu et al.40 | |
| 10-μm spot DNA-seq | slide-DNA-seq | spatially resolved DNA sequences | Zhao et al.41 | |
| Spatial single-cell and subcellular resolved | multiplex protein imaging and mass cytometry | CyCIF, COMET, Hyperion, MIBI-TOF | spatial single-cell resolution of ∼30–60 protein targets | Lin et al.,42 Lunaphore Standard BioTools Ionpath |
| probe-based imaging | MERSCOPE, Xenium, PhenoCycler, CosMx | spatial subcellular resolution of 100+ protein & RNA targets | VizGen 10 Genomics Akoya Biosciences Nanostring |
DNA-seq, DNA sequencing; ATAC-seq, assay for transposase-accessible chromatin sequencing; RAGE-seq, repertoire and gene expression by sequencing.
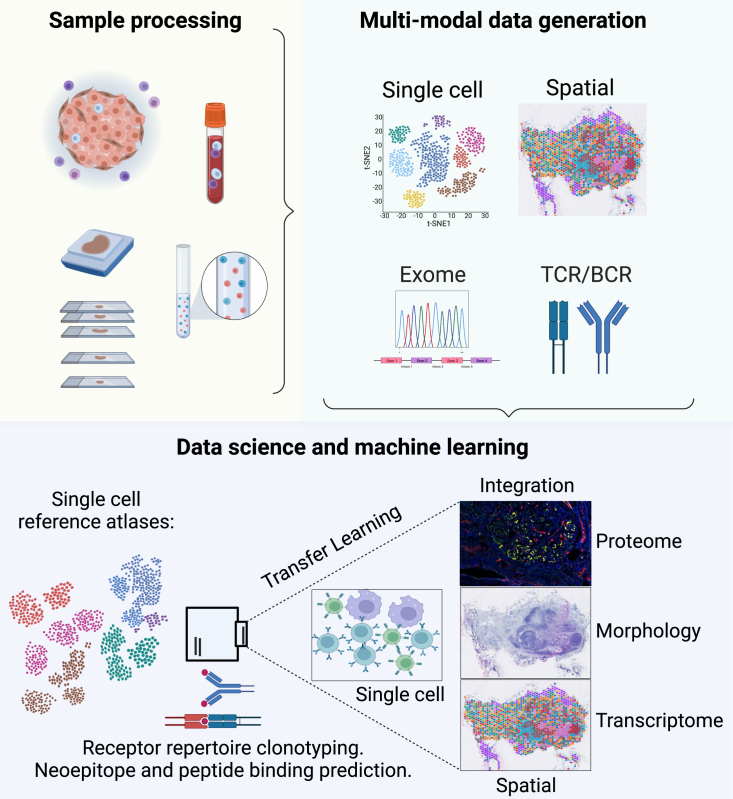
Figure 1.
Overview of samples and tissue structures of focus in leukocyte trafficking, omics data generation, and integration
Lymphatic trafficking refers to the movement of immune cells and other biomolecules through the lymphatic system, and the study of this process often focuses on the tissue structures involved, such as lymph nodes, blood vessels, and lymphatic vessels. Omics data generation is a critical component in this field because it allows comprehensive analysis of the molecular and cellular mechanisms driving leukocyte trafficking.
Monitoring immune cell signatures from the periphery to the tumor with dissociation-based single-cell technologies
Systems immunology and high-throughput profiling technologies have made significant advances in our understanding of the relationship of immune cell phenotypes to immune system function.43 In recent years, considerable work has been published investigating the phenotypes of lymphocytes at various stages of their migration and interactions with cancer at a high resolution.44,45 Historically, flow cytometry techniques have played a pivotal role in analyzing and characterizing lymphocytes.46 By sorting immune cells, researchers can enhance their phenotypic profiling using additional markers or conduct functional experiments to gain deeper insights into their biological behavior. While fluorescence-activated cell sorting (FACS) remains the gold standard for isolating populations for downstream experimentation, single-cell proteomics and transcriptomics technologies are refining the ability to profile intratumoral and peripheral leukocytes.
High-dimensional single-cell technologies have greatly enhanced the ability to profile both intratumoral and peripheral leukocytes. Single-cell proteomics profiling technologies have now greatly improved upon previous methods, such as flow cytometry, by generating protein expression data for dozens of markers and allowing better control over signal bleeding.47 Cytometry by time of flight (CyTOF) applies mass cytometry to quantify antibody-metal conjugates targeting extracellular or intracellular targets in hundreds of thousands of cells per sample.48 These high-dimensional approaches can be used to both phenotype the immune cells within a sample and assess function through the expression of critical protein markers. Because each CyTOF dataset can contain millions of cells, it allows sampling of even relatively rare cell types that constitute less than 1% of a tissue or blood sample using custom-designed panels of up to 40 protein markers. Several studies applying CyTOF to peripheral blood mononuclear cells (PBMCs), tumors, or lymph nodes have reported immune correlates associated with clinical outcomes, including immunotherapy treatment.49,50,51,52,53 CyTOF has also been applied to profile the immune component of multiple tissue sites at once in several mouse models to analyze systemic immune dysfunction and plasticity in an integrated fashion.54 CyTOF profiling of immune cells relies heavily on the design of the panel of proteins used to answer a specific biological hypothesis. Considerations for protein selection in panel design typically include cell type markers for broad clustering as well as functional markers for differential expression between sample groups and conditions. Sequencing-based single-cell proteomics, such as CITE-seq, leveraging antibody-DNA oligonucleotide conjugates increases the number of possible protein targets that can be quantified in tandem, although with lower cell throughput and higher cost per sample.47
While protein-based single-cell approaches mirror the markers used in flow-based functional immunology experiments, RNA sequencing approaches can yield much higher-dimensional data of cellular phenotypes and function. Since the bulk sequencing era, high-throughput transcriptional data have been used to monitor the immune status of tumors.55 These studies have relied on the inference of immune cell states from the population through cellular deconvolution methods.56,57,58,59,60 More recently, high-dimensional single-cell approaches can further resolve the transcriptomic signatures of individual cells to refine the definition of cell types and cell states. Many single-cell RNA sequencing (scRNA-seq) platforms have been implemented to construct genome-wide transcriptomes of individual cells stemming from tumors and peripheral blood, revealing novel immune signatures.61,62 Phenotyping scRNA-seq data often rely on unsupervised clustering analysis to identify cellular groupings and then annotate those clusters based on the marker genes they express. Transfer learning methods, as implemented by software such as projectR63 and cFIT,64 provide powerful means to leverage large-scale atlases to annotate cellular function in new experiments.65 Transfer learning methods have been applied to phenotype intratumoral leukocytes through ProjectTILs.66
The advent of multiomics molecular profiling technologies and complementary data integration methods enables more precise cellular analysis (Figure 2). To refine cell state annotations using canonical protein markers, dual-profiling technologies such as CITE-seq have been developed to assay hundreds of protein markers in conjunction with scRNA-seq in individual cells.33 This has allowed the adaptation of transfer learning computational methods to incorporate protein markers in reference atlases for the annotation of cells in new target scRNA-seq datasets of tumors and PBMCs through Azimuth, for example. Hao et al.67 demonstrated in PBMCs that, for some immune cells, such as B and T cells, protein information is weighed higher than RNA to account for gene dropout, whereas in myeloid cells, information at the RNA level is weighed higher to differentiate between myeloid subsets. This observation suggests that distinct molecular profiling technologies may be required for the study of specific immune cell populations or functions. Current technologies often have a trade-off between the depth of molecular profiling and breadth of cells profiled, which must also be considered in the design of studies of the immune system in cancer. Large-scale consortia, such as the Human Tumor Atlas Network (HTAN) are developing comprehensive multimodal atlases of single-cell datasets from intratumoral and peripheral leukocytes as community resources for analysis of immune cell trafficking at scale.68
Figure 2.
Experimental and bioinformatic workflow for multi-immunomics
Experimental approaches for - omics studies require sample processing to obtain high-quality biomolecules, followed by multimodal data generation using a combination of technologies such as transcriptomics, proteomics, and epigenomics. The integration of data from these different platforms, especially at the single-cell and spatial levels, provides a comprehensive understanding of the molecular mechanisms underlying immunological processes in cancer.
Computationally dissecting immune cell states and trajectories
The new high-dimensional technologies have challenged canonical paradigms that restricted our understanding of the immune system to discrete cell classification using gating strategies on well-annotated marker genes. Often, studies of leukocyte trafficking through single-cell technologies perform analyses by applying clustering or transfer learning methods to annotate discrete leukocyte cell types.53 Such single-cell analyses have led to the discovery of previously unreported immune cell types during immune trafficking. Still, these categorical cell labels can mask the continuously variable nature of immunobiology, such as the differentiation state along the hematopoietic lineage.69 Computational methods for immunomics are emerging alongside single-cell technologies to better capture this complex nature of immune cell function (Table 2). These techniques are critical to capture the dynamic nature of cellular phenotypes both from single-cell proteomics and transcriptomics assays.
Table 2.
Example packages implemented in multimodal immune phenotyping and receptor repertoire analysis
| Data types | Utilities | Algorithms/packages | Developers |
|---|---|---|---|
| Single-cell omics | data management, quality control, standard gene expression data analysis | Seurat Monocle Scanpy FlowSOM | Hao et al.,67 Qiu et al.,70 Wolf et al.,71Van Gassen et al.72 |
| reference immunophenotyping, pattern learning, and transfer learning | ProjectTILs Azimuth SingleR patternMarkers projectR cFIT | Andreatta et al.,66 Hao et al.,67 Aran et al.,73 Stein-O’Brien et al.,74 Sharma et al.,75 Peng et al.76 |
|
| receptor-ligand modeling | Domino LIANA NicheNet CellPhoneDB | Cherry et al.,77 Dimitrov et al.,78 Browaeys et al.,79 Efremova et al.80 | |
| TCR/BCR sequencing | TCR-BCR read alignment to chains, clone read extraction | MiXCR Trust4 10xVDJ ImmunoSEQ | Bolotin et al.,81 Song et al.,82 10 Genomics Adaptive Biotech |
| T/B cell repertoires, clonotypes, peptides, exome | clonotyping, diversity estimation, expansion, compositionality | scRepertoire Immunarch Immcantation pyTCR scirpy | Borcherding et al.,83 Nazarov et al.,84 Vander Heiden et al.,85 Peng et al.,76 Sturm et al.86 |
| neoepitope specificity/MHC prediction | NetTCR-2.0 TCRconv TCRrex NetMHCpan MHCFurry MixMHCpred MixMHC2pred | Montemurro et al.,87 Jokinen et al.,88 Gielis et al.,89 Reynisson et al.,90 O’Donnell et al.,91 Gfeller et al.,92 Racle et al.93 | |
| Spatial imaging & sequencing | profiling of spatially restricted gene expression modules and immune interactions in the tumor microenvironment | Seurat Giotto Squidpy spatialGE | Hao et al.,67 Dries et al.,94 Palla et al.,95 Ospina et al.96 |
| spatial interactions of cellular programs | spaceMarkers COMMOT Ncem Cottrazm CellChat | Deshpande et al.,97 Cang et al.,98 Fischer et al.,99 Xun et al.,100 Jin et al.101 |
Computational algorithms are advancing to extend the study of single-cell datasets from discrete cell types to their continuous phenotypic state. As one example, these continuous states can reflect the dynamic biological process that occurs during cell state transitions. For example, trajectory inference methods such as pseudotime have been used to order cells from a developmental or cell-state origin to a captured transcriptomic terminus.48,102,103,104,105 By focusing on the dynamic and continuous nature of immune cell states, these approaches have been applied to unravel the dynamic interplay of immune cells within the tumor microenvironment and tumor-draining lymph nodes.27,106 Trajectory inference algorithms can be applied to infer transitions between cellular lineages and phenotypes from both single-cell proteomics and scRNA-seq datasets. The higher-dimensional molecular profiling from scRNA-seq data can further enable trajectories to serve as the basis for estimating causal regulatory networks underlying these fate decisions.107 Published trajectory inference methods have been reviewed and benchmarked by others, and comparisons of their performance in different trajectory scenarios can be performed with the package DynVerse.108 Despite the power of these methods, trajectory inference often assumes binary branching in cell fate decisions and relies on a priori knowledge of the cell type point of origin, often necessitating user input to guide the directionality of trajectories. To estimate the maturity of transcripts and infer directionality in the trajectory, RNA velocity algorithms can be applied to compute ratios of spliced and unspliced transcripts.109,110 However, the dependence of these methods on low-dimensional embeddings can introduce biases that can require careful consideration and evaluation.111
Differentiating transient cell phenotypes and distinct cell lineages with these methods still requires a combination of assessing the levels of transcriptional variation after dimension reduction and manual interpretation of the embedding, which may introduce biases and affect reproducibility. Unsupervised machine learning approaches such as matrix factorization provide one tool to evaluate distinct cell lineages and transient phenotypes of immune cell states from both single-cell proteomics and scRNA-seq data. These algorithms have been found to concurrently infer cellular phenotypes, cellular function, and dynamics in a single analysis approach without reliance on a priori knowledge.65 Matrix factorization has been applied to immune cell trafficking in cancer immunology, providing valuable insights into the heterogeneity of immune cell populations, their functional states, and the dynamic interactions that occur during immune responses.66,112 Further advances in topic modeling,113,114 autoencoders,115 and other deep learning methods116,117 are being developed to overcome the linearity assumptions in matrix factorization and thereby model more complex cellular relations for both single-cell proteomics and scRNA-seq technologies.
Both supervised and unsupervised approaches should be considered when working with immunomics data. Supervised learning leverages a combination of prior biological knowledge or reference atlases to annotate cells. When labeled, this facilitates further differential expression analyses or, in the case of multiomics data, regulatory analysis within these cell subtypes. However, a drawback to supervised learning approaches is the ability for de novo discovery of cell subtypes or phenotypes or discovery of novel gene-regulatory programs associated with immune regulation. Unsupervised learning methods can discover the cellular and molecular pathways in a data-driven way, using prior knowledge as a guide to contextualize and annotate these findings. As an example, unsupervised learning methods for matrix factorization and trajectory inference can further provide a dynamic rather than a static method of studying multiple co-expressing features using continuous weights.69 This can be applied to study immune cell trafficking by quantifying state transitions using weighted matrix factors or trajectory inference metrics, such as pseudotime, to compare between tumors and the periphery. However, without tracing the same cell, all of these single-cell assessments of trafficking directionality are limited to correlations between samples. Additionally, profiling with these dissociation-based single-cell technologies disrupts immune structures that are critical for mediating the immune response. These limitations require that single-cell technologies are paired with spatial omics approaches and in vivo preclinical models that allow cell tracing and time course analysis.
Tracing clonal families using receptor repertoires as barcodes
During their development, T cells and B cells undergo germline DNA recombination to generate a diverse repertoire of antigen recognition receptors, T cell receptors (TCRs), and BCRs, respectively.118,119 These receptors have an affinity for non-self antigens with the potential to elicit an adaptive immune response signaling cascade.120 A TCR or BCR recognizes a single or narrow range of antigens. T cells and B cells with shared receptor sequences are considered clonally related to each other. These shared sequences can serve as cellular barcodes to trace clonal families as they clonally expand, migrate between tissue structures, and develop into functional subunits (Figure 3).
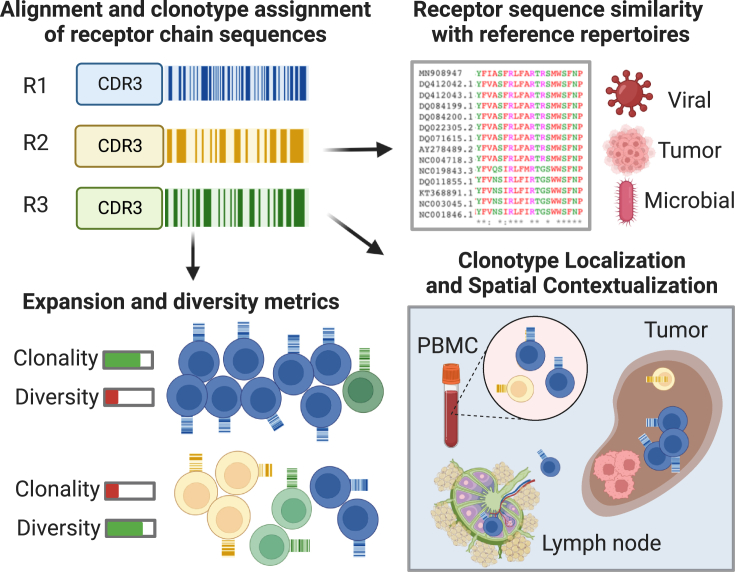
Figure 3.
Tracing clonal families using receptor repertoires as barcodes
Functional assessment of clonal expansion in receptor repertoire sequencing data can shed light on the nature of systemic and localized adaptive immune responses. Clonotype assignments extend measures of repertoire diversity and clonal expansion. Comparison of de novo sequenced receptor chains with reference databases can provide estimates of target antigen origins. Spatial TCR/BCR sequencing or multiplex in situ hybridization methods to spatially resolve hyperexpanded receptor chains of interest help contextualize the trafficking of a B cell or T cell clonal family within the tumor microenvironment and tumor-draining lymph nodes.
Although immune cell states reveal important features of immune niches, allowing inferences about their net-sum effects in the tumor microenvironment, determining TCR and BCR repertoires is a critical step toward revealing important adaptive immune processes in tumors and lymph nodes.121 In principle, each T cell and B cell will have a unique antigen receptor expressed at its surface. The identification of tumor-specific repertoires can be used to track clonally expanded T and B cell families and trace them across tissues and the periphery.122 Immune receptor sequencing to determine TCR or BCR sequences allows a detailed analysis of repertoire diversity and clonality.123 The comprehensive characterization of the immune TCR and BCR repertoire provides the opportunity to understand the mechanisms of immune recognition and response.124 Bulk TCR and BCR approaches, either using DNA-based or mRNA capture-based sequencing, can provide a compilation of T cell receptors TCRα, TCRβ, TCRγ, TCRδ, and immunogbulin (IG) chains IGH, IGK, and IGL. In this bulk sequencing approach, however, paired chains with functional signatures are lost. Parallel transcriptome and TCR/BCR single-cell sequencing can recover paired receptors, which enhances downstream capabilities for clonotyping and clone origin prediction.125 These technologies also provide transcriptional profiles of the cells for each TCR/BCR sequence, further allowing cellular phenotyping. Moreover, multiomics profiling has been extended to concurrently measure cognate antigen specificities, TCR sequences, targeted gene expression, and surface protein expression from tens of thousands of single cells. Tetramer-associated TCR sequencing high dimension (TetTCR-SeqHD) utilizes peptide-encoding DNA oligos to generate corresponding peptides loaded onto MHC molecules with a DNA barcode.34 Tetramer staining, using peptide-loaded MHC tetramers conjugated to DNA-barcoded streptavidin, in combination with AbSeq staining using a panel of 59 DNA-barcoded antibodies and a DNA-barcoded anti-CD50 antibody, facilitated the integration of TCR sequencing, antigen specificity, gene expression, and phenotyping in tens of thousands of single-cells for multiple antigens at once. The inventors applied this technology in a proof-of-concept study in type 1 diabetes patients to identify antigens that preferentially enrich cognate CD8+ T cells and discovered a TCR that cross-reacts with antigens associated with diabetes and microbiotas. These single-cell multi-immunomics platforms elucidate functional measurements and simultaneously track lymphocytes, using receptor repertoires as unique clonal identifiers.
Furthermore, special consideration should be placed on the underlying biology in question as well as costs before choosing between these technologies. Bulk TCR/BCR sequencing approaches, in addition to being the most affordable, typically result in greater overall sequencing depth compared with single-cell TCR/BCR approaches, which are limited by the total viable cell sampling.126 However, gating and enrichment strategies in combination with power calculations to estimate the number of total cells required to adequately sample lymphocytes of interest may improve overall repertoire sampling.127,128 In addition to these specialized TCR/BCR profiling technologies, several tools have been released to extract both TCR and BCR chains from sequencing data, including bulk and single-cell RNA-seq, such as MiXCR81 and Trust4.82 While specialized libraries can improve the depth of sequence recovery, these techniques can serve as valuable estimates of sequences in samples with limited biospecimens for additional profiling or enable assessment of immune characteristics in public domain tumor atlases55,129,130 Application of immune receptor repertoire sequencing technologies and their corresponding data analysis methods have identified an influx of novel T cell clonal families in tumors after immunotherapy, including checkpoint blockade131 and vaccination,132 in humans.
Decoding immune receptors and epitopes
TCRs recognize antigens in the context of MHC presentation and, under co-stimulation, elicit a signaling cascade that may promote anti-tumor immunity.133 TCRs are heterodimers of α and β or γ and δ chains. Although the β chain contains the variable region and, thus, the majority of the diversity, capturing and pairing the two chains together provide the most accurate representation of TCR binding specificity. Similarly, in BCRs, including immunoglobulin G (IgG) antibodies, most of their diversity is from its heavy chain, IGH, but pairing light IGL chain information is likely required for a complete understanding of the potential antigenicity. Although estimates vary, the estimated total possible diversity of immune receptors ranges from 1011 to as high as 1018 different specificity.134 This vast diversity is combined with human leukocyte antigen (HLA) allele diversity, which further complicates the degrees of freedom for antigen presentation and recognition. It is unsurprising, therefore, that there is a lack of information necessary to decipher in silico which interactions between TCRs, BCRs, and their antigens are productive in terms of initiating a robust adaptive immune response (Figure 4). Recent computational approaches that leverage the output of the latest sequencing platforms mentioned previously, such as single-cell or spatial TCR sequencing and whole-exome sequencing, have brought us closer to achieving this goal.135 However, predictability may be limited if the sequence does not reflect the three-dimensional structure of TCR-MHC interactions requiring alternative structural modeling approaches.136
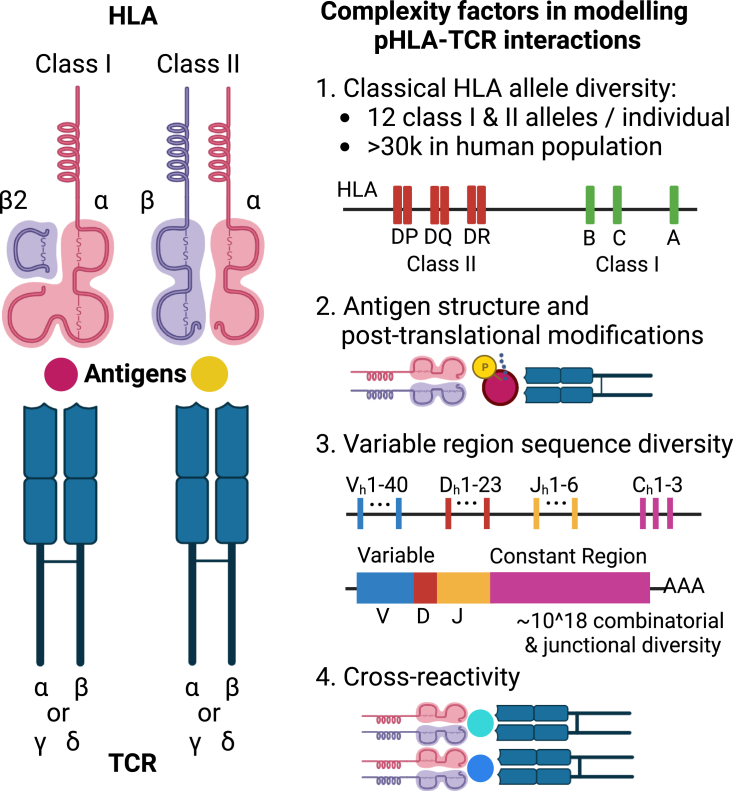
Figure 4.
Variables that affect T cell receptor interactions with presented peptides in humans
HLA-peptide-TCR interactions form the basis of adaptive immune responses, where the major histocompatibility complex (HLA) molecules present antigenic peptides to T cell receptors (TCRs) on T cells. The diverse range of HLA alleles and TCR repertoire sequences enables the immune system to recognize a wide range of antigens, but this complexity also presents a computational challenge to model these interactions and predict them from sequencing data.
Interpretation of immune cell repertoire data can be challenging and requires careful computational analysis and consideration of limitations. Generally, diversity estimation and clonotyping to compute expanded TCR and BCR clonal families are considered helpful steps in receptor repertoire analysis because the expanse itself indicates a productive antigen recognition event that prompted a proliferation of the immune cell. Diversity metrics estimating clonal aspects such as population evenness and richness can assist with the interpretation of repertoire dynamics in the tumor microenvironment. Differences in diversity metrics and repertoire composition profiles between tissues, such as between primary and metastatic tumors, can hint at discordant adaptive immune responses. Packages are available to facilitate these standard analyses, such as Immunarch,137 Immcantation,138 and others (Table 2). For efficient computation of overlap between adaptive immune repertoires, CompAIRR quantifies overlap of convergent immune response patterns using exact and approximate sequence matching.139 If single-cell or spatial transcriptomic experiments were conducted in parallel with receptor repertoire sequencing, the two modalities can be paired using the R/Bioconductor package scRepertoire83 or scirpy86 in Python to extend clonality assessment with functional profiling. It should be noted that sampling bias in most single-cell sequencing approaches influences the estimation of immune receptor repertoire diversity metrics. This bias could also result in missing the observation of clones identified in related samples, such as tumors and adjacent lymph nodes. Taking that bias into consideration, hyperexpanded clonal receptor families located intratumorally may suggest local expansion in response to tumor antigens, self-antigens, or viral/microbial antigens in tumors. If identified hyperexpanded TCRs found intratumorally also show enrichment of signatures related to T cell activation and cytotoxic response, this prompts cloning a TCR for further experimental investigation for its role in T cell-mediated tumor immunity and cytotoxicity.
To extract functional insights from these sequencing data, algorithms have attempted to featurize antigen-specific TCRs. TCRdist calculates distance metrics between TCR sequences, which can be applied to group TCR chains into clusters based on sequence similarity. Each TCR cluster can be inferred to recognize similar MHC-bound peptides under the assumption that a significant sequence overlap translates to a greater probability of binding domain structure similarity.140 GLIPH (grouping of lymphocyte interactions by paratope hotspots) identifies TCR specificity groups based on shared structural features, offering a deeper understanding of T cell recognition and immunity.141 Deep learning methods are now applied to improve TCR-epitope associations. DeepTCR employs deep learning to uncover TCR sequence patterns and their associations with antigens.142 TEIM-Res leverages a deep learning framework to analyze TCR-epitope interactions at a granular level, overcoming data scarcity challenges and providing a valuable understanding of the molecular foundations of binding mechanisms.143
Beyond population-level clonal dynamics of TCRs and BCRs, comparison of de novo-sequenced receptor chains with previously reported, and ideally validated, receptors can greatly improve the interpretation and sophistication of analyses. Differentiating viral from cancer-specific receptors can shed light on the viral pathogenesis of certain cancers or the increased immunogenicity that might occur as a result of receptor promiscuity toward cancer antigens. Web tools such as VDJdb,144 TCRrex,89 and TCR-Explore145 provide visual interfaces to receptor sequence analysis and access to compiled sequencing data. The Immune Epitope Database and Analysis Resource (IEDB) provides a compilation of B cell and T cell epitope prediction tools, including structural analysis, immunogenicity, and MHC binding.146 It should be mentioned that accurate immune receptor epitope inference is challenging to perform without prior neoantigen and HLA allele identification.87,147 MHC class I and class II peptide binding prediction has been attempted through tools leveraging artificial neural networks (ANNs), such as NetMHCpan90 and MHCFurry.91 Gartner et al.,148 however, demonstrated that the use of a random forest classifier on peptides from multiple tumor types outperforms the ANN approaches. Prediction of TCR interactions with MHC-peptide complexes, on the other hand, remains extensively complex. A “shallow” convolutional neural network (CNN) implementation tool, NetTCR, demonstrated that the use of paired TCR sequencing data greatly improved the capacity for TCR specificity prediction.87 A deep CNN, AlphaFold, combined with a classifier also recently demonstrated binding specificity prediction using fine-tuned protein structure prediction on a range of both class I and class II peptide-MHC interactions.149 TCRconv, a deep protein language model, also extracted contextualized motifs that improved TCR-epitope accuracy.88 Altogether, these advancements mark the beginning of an era in which big data of immune receptors are leveraged to model complex interactions between immune receptors and antigens. Eventually, this may lead to a better understanding of the sources of inflammation in immune-enriched tissue structures or whether a therapeutically targeted epitope is indeed inducing an expected immune response.
Spatial technology innovations to molecularly profile immune structures
Single-cell work in systems immunology has answered key questions regarding cell state composition in tumors and the periphery.150 Although revolutionary, the spatial tissue distribution of the profiled dissociated single cells from solid tissue is lost, which limits our understanding of the complex biological architectures in which these cells exist.61 Additionally, molecular profiling methods such as immunofluorescence or immunohistochemistry have been limited in the range of molecular targets that can be assayed simultaneously. However, innovations in multiparameter spatial omics technologies have become an inflection point in systems immunology as advances in proteomic and transcriptomic technologies have allowed highly multiplexed imaging and spatial sequencing. These new technologies offer new opportunities to molecularly characterize immune infiltrates within complex biological architectures, such as tumors and regional draining lymph nodes.
Commercially available high-parameter proteomic platforms, such as highly multiplexed immunofluorescence imaging (e.g., CyCIF;151 PhenoCycler, Akoya; COMET, Lunaphore) and mass-based cytometry imaging (e.g., Hyperion, Standard BioTools; MIBI-TOF, Ionpath) offer low-dimensional (relative to transcriptomic platforms) but fairly high-throughput targeted protein profiling at subcellular resolution. These provide high-fidelity phenotyping and have been applied to query immune infiltrate compositions in specific immunological contexts, including tumors,152,153 as well as regional draining lymph nodes.154 Genome-wide spatial sequencing and probe-based tissue imaging methods spanning biological modalities, including, DNA, RNA, protein, and chromatin, have also been recently developed and implemented to spatially resolve immune signatures within convoluted cellular architectures (Table 1). Spatial transcriptomics platforms, such as genome-wide mRNA-seq (Slide-seqV2;35 Visium, 10 Genomics), digital spatial profiling (GeoMx, Nanostring), and massively multiplexed RNA in situ hybridization (MERFISH; Vizgen; Xenium, 10 Genomics; CosMx, Nanostring), have offered opportunities to conduct high-dimensional molecular characterization of immune-enriched regions of interest in tumors and metastatic lymph nodes.
These emerging spatial molecular technologies offer an unprecedented opportunity to deeply profile the spatial architecture of immune tissue structures. The technical strengths and weaknesses of these spatial omics platforms have been reviewed previously in depth.155,156 Three important considerations should be taken: spatial resolution, sample type compatibility, and molecular depth. Spatial resolution refers to how precisely a quantified molecule, such as RNA or protein, can be pinpointed in the spatial plane to generate multicell, single-cell, or subcellular resolved data. Sample type compatibility mainly refers to whether protocols for formalin-fixed paraffin-embedded (FFPE) or frozen tissue sample processing are available. Molecular coverage refers to the number of molecules that are measured, whether it is across 50 protein markers or genome wide. As listed above regarding the available technologies and in Table 1, some technologies have greater molecular coverage but lower spatial resolution and vice versa. Thus, we advise careful weighing of these factors in addition to consideration of sample size and cost. Overall, deciding which spatial technology to use is dependent on the underlying biology of interest and the cellular and molecular resolutions required to investigate it. Within the scope of immune cell infiltration in tumors, inquiries regarding specific immune cell types of interest and their spatial neighborhoods can often be addressed with proteomics. However, unbiased genome-wide molecular assessment of the immune landscape of tumors necessitates spatial RNA-seq methods, which currently report transcriptomes at a 10- to 55-μm resolution (specific resolutions are reported in Table 1) for Visium or in specified regions of interest for GeoMx. Subsequently, subcellular validation of findings can be implemented with a more targeted probe-based imaging method, including CosMx, Xenium, and MERFISH. For these probe-based imaging approaches, specialized panels must be designed to target signaling pathways, tissue structures, cell types, and cell states of interest. To optimize panel design, single-cell transcriptomics datasets can help refine molecular targets to maximize the number of cell states extracted within a panel’s probe capacity. These spatial technologies can be integrated to expand the capability of immunologists to understand immune cell trafficking and responses against tumors. Applied to intratumoral lymphoid niches following aPD1 therapy in humans, studies leveraging these technologies have been able to demonstrate plasma cell propagation from TLSs in renal cell carcinoma.25 In hepatocellular carcinoma, dendritic cell-CD4+ T helper cell niches have been observed following PD1 blockade, as well as a higher frequency of immunosuppressive niches in non-responders.51,157
To examine the spatial landscape of BCRs and TCRs in cancer, several options are currently being developed to examine the spatial localization and cellular context in which they are found (Figure 3). These new approaches now promise the improved ability to computationally reconstruct the evolution of lymphocytes within and between the tumor microenvironment and secondary and tertiary lymphoid organs. Spatial protocols that locate TCRs and BCRs on tissue slides have emerged. Hudson and Sudmeier38 combined a commercial spatial transcriptomics protocol for frozen tissues that uses 3′ mRNA capture chemistry to recover TCRβ from the cDNA obtained from the tumor samples, thereby generating spatially indexed de novo TCRβ CDR1–CDR3 sequences.38 Modifying the same spatial transcriptomics commercial protocol, Engblom et al.158 combined long-read sequencing and BCR amplification to spatially reconstruct lymphocyte clonal dynamics in human tissue. Both of these protocols rely on commercial slides, which have spatially barcoded spots with a diameter of 55 μm. Applied to metastatic tumors in the human brain, spatially indexed TCR sequencing in parallel with Visium spatial transcriptomics revealed niches of clonally unrelated and phenotypically distinct CD8+ T cells.159
It should be noted that the level of spatial resolution can be limiting when applying spatial receptor repertoire sequencing because lymphocytes can be particularly small (∼8 μm) compared with an average human cell (20–30 μm), making their signal relatively easy to mask by larger cells.160 This could result in mis-estimation of the TCR repertoire and transcriptional profiles of cells within sampled spatial neighborhoods. Notably, Liu et al.39 published the slide-TCR-seq method, which demonstrated higher (10-μm) resolution than Visium combining whole-transcriptomic and TCR spatially indexed in situ sequencing.39 These de novo 3′ mRNA-seq approaches, however, rely on fresh-frozen tissue samples and cannot be applied in FFPE, which is currently only compatible with a probe-based chemistry. Nonetheless, de novo TCR and BCR discovery with bulk approaches can be carried out in FFPE. When clonal families of interest are identified, custom panels of oligonucleotide probes targeting these TCRs and BCRs can be imaged using FFPE-compatible transcriptomic digital spatial profiling technologies. Altogether, spatially mapping hyperexpanded TCR and BCR sequences enables clonal repertoire comparisons between immune-enriched tissue structures to infer immune cell trafficking and local adaptive immunity.
Defining interactomes of diverse cell types involved in immune cell recruitment, organization, and function in cancer
In addition to T and B lymphocytes, many cell types have been identified as key modulators of immune responses in cancer.161,162 Characterized by potent immunosuppressive activity, certain populations of myeloid cells contribute to tumor progression via the inhibition of antitumor immune responses.163,164 These myeloid cells have been demonstrated to contribute to the formation of T cell exclusion zones in tumors through the secretion of various chemokines and cytokines,165 thereby promoting tumor growth and metastasis.166 Monocytes are pivotal in the initiation and maintenance of inflammatory responses.167 Within the tumor microenvironment, monocytes may differentiate into tumor-associated macrophages (TAMs), which display heterogeneous functionality contingent on their cell state.168 TAMs and tumor-infiltrating dendritic cells (DCs) such as mature regulatory DCs (mregDCs) have been shown to regulate the recruitment and function of other immune cells, such as T and B lymphocytes and natural killer (NK) cells, within the tumor microenvironment, thereby directly impacting the immunological response to neoplasms.169,170 As the most abundant leukocyte population in human blood, tumor-associated neutrophils are also integral to the initial stages of immune response to tumors and, depending on the context, can exhibit both protumorigenic and antitumorigenic effects.171,172
The tumor stroma and lymphatic vessels have been found to undergo extensive remodeling and expansion within the tumor microenvironment.7,49 Non-immune cells associated with the tumor stroma, such as cancer-associated fibroblasts (CAFs), also play many crucial roles in cancer progression, including the exclusion of T cells from the tumor core via extracellular matrix (ECM) remodeling or expression of immunosuppressive molecules.173 Overexpression of key vascular endothelial growth factors by CAFs and tumor cells promotes the formation of new vessels and enlargement of lymphatic vessels, which enhances tumor growth and dissemination.174 An enhanced vasculature can also allow more efficient infiltration of leukocytes, such as plasma cells producing tumor-specific immunoglobulins within the tumor microenvironment.25,175
In this era of high-dimensional immunomics, we can now begin to model these complex network interactions in the tumor microenvironment. Single-cell and spatial omics data offer the opportunity to infer intercellular signaling within the tumor microenvironment.176 In scRNA-seq data, tools such as NicheNet,79 Domino,77 CellChat,101 LIANA,78 and CellPhoneDB,80 provide easy unbiased implementation of cell communication modeling. Using these tools in modeling immune signaling networks has revealed interactions among immune cell subsets, such as co-dependent intercellular communication between exhausted T cells and macrophages within tumor regions.177 Extending these capabilities to spatial transcriptomics data is an active and open area of research, but tools such as CellChat are already applicable to the Visium platform. Recent work on spatial data also demonstrated the extraction of gene expression programs at tumor-immune boundaries.97,100,178 However, it should be noted that, although these tools can report putative interactions based on known ligand-receptor pairs, the corresponding cellular communication networks could be inflated and thus warrant orthogonal validation at the protein level.
Future directions and clinical opportunities
Immune cell trafficking is a dynamic and complex process that plays a critical role in cancer biology, autoimmunity, infection, and regenerative processes.24 Thus, the utilization of high-dimensional single-cell and spatial technologies to track the migration of immune cells across the body over time holds immense potential in clinical trials and, eventually, clinical practice. In the context of cancer, cutting-edge approaches allow monitoring of immune cells and their molecular changes over time, providing researchers with a deeper look into the tumor microenvironment and periphery. In this review, we discuss multiomics technologies, from bulk, single-cell, and spatial approaches to profile tissue types involved in mediating response and resistance to immunotherapies. Specific approaches are more appropriate for certain sample types over others. For instance, single-cell approaches are more suited for blood than spatial approaches. Studies leveraging tissue samples such as tumors and lymph, on the other hand, could benefit from both single-cell and spatial approaches. Analyzing multiple sample types requires a multiomics pipeline, including immune receptor repertoire sequencing, to identify clonally and transcriptionally related subpopulations that are either shared or distinct. A systems-level approach provides a comprehensive view of immune response dynamics in cancer and its impact on therapeutic response.
We covered computational methods that can be applied to analyze these data modalities in diverse immunological contexts. These analyses can be used to identify new cell states and markers for therapeutic targeting to develop more effective treatment interventions.179,180 For instance, by molecularly profiling T cells and B cells for phenotypes in tandem with TCRs and BCRs, it is possible to classify B and T cells based on common and disparate characteristics to generate a comprehensive immune model of disease etiology and progression.11 High-dimensional immunomics can also be used to examine immune responses to vaccines, checkpoint blockade, and other immunotherapies in development at high resolution, lending insights into how immune responses are modulated by these treatments.181 Highly resolved cellular and molecular maps can be critically useful in developing and evaluating novel targets for immunotherapies.182,183 Chimeric antigen receptor T cells (CAR-T cells), for example, although having demonstrated great success in non-solid tumors, still lack efficacy in solid tumors.184 One of the significant challenges in solid tumors is the loss of inflammatory effector functions.185 Multiomics data from tumor and non-malignant tissues enables investigation of CAR-T cell persistence, antitumor efficacy, or even related toxicities by examining T cell states.186 The computational frameworks described here, such as using receptor sequences as barcodes, could assist with tracking these engineered cells and uncover patterns that might explain this loss.
Profiling immunomes sampled during cancer treatment can generate patient-specific insights regarding response or resistance to therapy, thereby holding immense clinical utility potential.187 For example, in the case of tumor progression, a more detailed view of changes in immune cell populations can help distinguish true tumor cell growth from immune infiltrate-induced tumor swelling.188 Additionally, in temporal studies, such as longitudinal or time course studies, computational snapshots of the immune system could be stitched together to trace patient responses across time with respect to treatment challenges and disease progression.189 Altogether, clinical and preclinical efforts made to expand and enhance immunotherapies can benefit from synergizing with the computational frameworks discussed here. As many of these technologies become increasingly more mainstream and cost effective, streamlining sample procurement for immunomics while conserving sample quality could massively expand our machine learning training sets aimed toward discovery. To achieve this, sample procurement and processing options should be made compatible with both clinical standards of practice and immunomics. On the technical side, our ability to maximize data depth extracted from valuable clinically sourced samples will rely on integrating multimodal data concurrently using in situ sequencing technologies. Combining whole-exome sequencing with receptor repertoire sequencing, transcriptomics, and proteomics could allow us to feasibly align HLA subtyping, predicted neoantigens, and functionally characterized T cell and B cell receptors within the same patient.
Although many immunomics approaches discussed here help discern clinically and biologically relevant immune correlates, such as immune signatures or repertoires, they do not yet substitute experimental models that physically track individual cells temporally and in situ. Much of our understanding of immune cell trafficking in lymphoid organs has been based on studies leveraging congenic mouse models, fluorescence probes, and in situ imaging.190 Microscopy, especially intravital microscopy, offers a direct window into the dynamics of immune cell interactions and movements within living tissues.191 Intravital imaging, using multiphoton technology and specific near-infrared lasers, has profoundly transformed our understanding of cell dynamics in immune functions, such as the interactions between antigen-specific T cells and dendritic cells within the lymph nodes, shedding light on the kinetics and dynamics of this pivotal immunological interaction.192 Live imaging has had major implications in detailing directionality in highly resolved niches lymphoid organs, such as the bidirectional movement of B cells between the two germinal center (GC) zones.193 In vivo imaging methods with extended imaging windows applying photoactivatable green fluorescent protein (PA-GFP) and multiphoton laser scanning microscopy better reveal dynamics of interzonal migration and affinity-based selection during the humoral response.194 Combining these experimental methods with omics approaches allows expansive hypothesis generation and clinical correlation with mechanistic exploration. While this review focused on technologies and studies in humans, adapting these techniques to mouse models and in vitro culture can enable further temporal tracking with imaging and barcoding as well as mechanistic, experimental validation. Altogether, synergizing methods to hack the adaptive immune repertoire holds immense promise in computationally reconstructing immune processes across tissues, including cancer immunoediting, tumor microenvironment immunomodulation, and lymphocyte trafficking, to ultimately improve immunotherapy.
Acknowledgments
D.N.S. was supported by an NIH T32 training grant awarded to the Cellular and Molecular Medicine Training Program at the Johns Hopkins Medical Institution as well as by a National Cancer Institute (NCI) F31 predoctoral fellowship (F31CA268724-01). This work was also supported by funding from the following sources: NCI grant P01CA247886 (to W.J.H., E.J.F., L.T.K., and E.M.J.), NIH grant P50CA062924 (to W.J.H., L.T.K., E.J.F., and E.M.J.), NCI grant U01 CA253403 (to E.J.F.), NCI grant R21 CA264004 (to W.J.H.), a Stand Up To Cancer – Lustgarten Foundation Pancreatic Cancer Convergence Dream Team Translational Research Grant (SU2C-AACR-DT14-14), the Lustgarten Foundation’s Research Investigator’s Award Program, and the Broccoli Foundation (to E.M.J.). We thank BioRender (BioRender.com) for providing the platform to design our figures.
Declaration of interests
E.M.J. reports other support from Abmeta, other support from Adventris, personal fees from Achilles, personal fees from DragonFly, personal fees from the Parker Institute, personal fees from Surge, grants from Lustgarten, grants from Genentech, personal fees from Mestag, personal fees from Medical Home Group, grants from BMS, and grants from Break Through Cancer outside of the submitted work. E.J.F. is a member of the scientific advisory board of Viscera Therapeutics/ResistanceBio and is a paid consultant for Mestag Therapeutics and Merck. D.N.S. is a paid consultant for Diamond Age Data Science and has previously received speaking honoraria from Standard BioTools. W.J.H. receives patent royalties from Rodeo/Amgen and research grants from Sanofi and NeoTX and has previously received speaking/travel honoraria from Exelixis and Standard BioTools.
Contributor Information
Luciane T. Kagohara, Email: ltsukam1@jhmi.edu.
Elana J. Fertig, Email: ejfertig@jhmi.edu.
References
- 1.Sun H., Sun C., Xiao W., Sun R. Tissue-resident lymphocytes: from adaptive to innate immunity. Cell. Mol. Immunol. 2019;16:205–215. doi: 10.1038/s41423-018-0192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kondo M. Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. Immunol. Rev. 2010;238:37–46. doi: 10.1111/j.1600-065X.2010.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao S., Padera T.P. Lymphatic Function and Immune Regulation in Health and Disease. Lymphat. Res. Biol. 2013;11:136–143. doi: 10.1089/lrb.2013.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heath W.R., Carbone F.R. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat. Immunol. 2013;14:978–985. doi: 10.1038/ni.2680. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 6.Lu Y., Ma S., Ding W., Sun P., Zhou Q., Duan Y., Sartorius K. Resident Immune Cells of the Liver in the Tumor Microenvironment. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.931995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi A., Belmonte B., Carnevale S., Liotti A., De Rosa V., Jaillon S., Piconese S., Tripodo C. Stromal and Immune Cell Dynamics in Tumor Associated Tertiary Lymphoid Structures and Anti-Tumor Immune Responses. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.933113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fridman W.H., Meylan M., Pupier G., Calvez A., Hernandez I., Sautès-Fridman C. Tertiary lymphoid structures and B cells: An intratumoral immunity cycle. Immunity. 2023;56:2254–2269. doi: 10.1016/j.immuni.2023.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Hiraoka N., Ino Y., Yamazaki-Itoh R., Kanai Y., Kosuge T., Shimada K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br. J. Cancer. 2015;112:1782–1790. doi: 10.1038/bjc.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sautès-Fridman C., Lawand M., Giraldo N.A., Kaplon H., Germain C., Fridman W.H., Dieu-Nosjean M.-C. Tertiary Lymphoid Structures in Cancers: Prognostic Value, Regulation, and Manipulation for Therapeutic Intervention. Front. Immunol. 2016;7:407. doi: 10.3389/fimmu.2016.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis-Marcisak E.F., Deshpande A., Stein-O'Brien G.L., Ho W.J., Laheru D., Jaffee E.M., Fertig E.J., Kagohara L.T. From bench to bedside: Single-cell analysis for cancer immunotherapy. Cancer Cell. 2021;39:1062–1080. doi: 10.1016/j.ccell.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonaguro L., Schulte-Schrepping J., Ulas T., Aschenbrenner A.C., Beyer M., Schultze J.L. A guide to systems-level immunomics. Nat. Immunol. 2022;23:1412–1423. doi: 10.1038/s41590-022-01309-9. [DOI] [PubMed] [Google Scholar]
- 13.Moses L., Pachter L. Museum of spatial transcriptomics. Nat. Methods. 2022;19:534–546. doi: 10.1038/s41592-022-01409-2. [DOI] [PubMed] [Google Scholar]
- 14.Pereira E.R., Jones D., Jung K., Padera T.P. The lymph node microenvironment and its role in the progression of metastatic cancer. Semin. Cell Dev. Biol. 2015;38:98–105. doi: 10.1016/j.semcdb.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reticker-Flynn N.E., Zhang W., Belk J.A., Basto P.A., Escalante N.K., Pilarowski G.O.W., Bejnood A., Martins M.M., Kenkel J.A., Linde I.L., et al. Lymph node colonization induces tumor-immune tolerance to promote distant metastasis. Cell. 2022;185:1924–1942.e23. doi: 10.1016/j.cell.2022.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schreiber R.D., Old L.J., Smyth M.J. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 17.Wherry E.J., Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGranahan N., Rosenthal R., Hiley C.T., Rowan A.J., Watkins T.B.K., Wilson G.A., Birkbak N.J., Veeriah S., Van Loo P., Herrero J., et al. Allele-Specific HLA Loss and Immune Escape in Lung Cancer Evolution. Cell. 2017;171:1259–1271.e11. doi: 10.1016/j.cell.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter M.C., Teijeira A., Halin C. T Cell Trafficking through Lymphatic Vessels. Front. Immunol. 2016;7:613. doi: 10.3389/fimmu.2016.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scotton C.J., Wilson J.L., Milliken D., Stamp G., Balkwill F.R. Epithelial cancer cell migration: a role for chemokine receptors? Cancer Res. 2001;61:4961–4965. [PubMed] [Google Scholar]
- 22.Maas S.L.N., Breakefield X.O., Weaver A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017;27:172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roussos E.T., Condeelis J.S., Patsialou A. Chemotaxis in cancer. Nat. Rev. Cancer. 2011;11:573–587. doi: 10.1038/nrc3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du W., Nair P., Johnston A., Wu P.H., Wirtz D. Cell Trafficking at the Intersection of the Tumor-Immune Compartments. Annu. Rev. Biomed. Eng. 2022;24:275–305. doi: 10.1146/annurev-bioeng-110320-110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meylan M., Petitprez F., Becht E., Bougoüin A., Pupier G., Calvez A., Giglioli I., Verkarre V., Lacroix G., Verneau J., et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity. 2022;55:527–541.e5. doi: 10.1016/j.immuni.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Gajewski T.F., Schreiber H., Fu Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sidiropoulos D.N., Rafie C.I., Jang J.K., Castanon S., Baugh A.G., Gonzalez E., Christmas B.J., Narumi V.H., Davis-Marcisak E.F., Sharma G., et al. Entinostat Decreases Immune Suppression to Promote Antitumor Responses in a HER2+ Breast Tumor Microenvironment. Cancer Immunol. Res. 2022;10:656–669. doi: 10.1158/2326-6066.CIR-21-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zundler S., Günther C., Kremer A.E., Zaiss M.M., Rothhammer V., Neurath M.F. Gut immune cell trafficking: inter-organ communication and immune-mediated inflammation. Nat. Rev. Gastroenterol. Hepatol. 2023;20:50–64. doi: 10.1038/s41575-022-00663-1. [DOI] [PubMed] [Google Scholar]
- 29.Ruhland M.K., Roberts E.W., Cai E., Mujal A.M., Marchuk K., Beppler C., Nam D., Serwas N.K., Binnewies M., Krummel M.F. Visualizing Synaptic Transfer of Tumor Antigens among Dendritic Cells. Cancer Cell. 2020;37:786–799.e5. doi: 10.1016/j.ccell.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wherry E.J. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 31.Buenrostro J.D., Giresi P.G., Zaba L.C., Chang H.Y., Greenleaf W.J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods. 2013;10:1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh M., Al-Eryani G., Carswell S., Ferguson J.M., Blackburn J., Barton K., Roden D., Luciani F., Giang Phan T., Junankar S., et al. High-throughput targeted long-read single cell sequencing reveals the clonal and transcriptional landscape of lymphocytes. Nat. Commun. 2019;10:3120. doi: 10.1038/s41467-019-11049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoeckius M., Hafemeister C., Stephenson W., Houck-Loomis B., Chattopadhyay P.K., Swerdlow H., Satija R., Smibert P. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods. 2017;14:865–868. doi: 10.1038/nmeth.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma K.-Y., Schonnesen A.A., He C., Xia A.Y., Sun E., Chen E., Sebastian K.R., Guo Y.-W., Balderas R., Kulkarni-Date M., Jiang N. High-throughput and high-dimensional single-cell analysis of antigen-specific CD8+ T cells. Nat. Immunol. 2021;22:1590–1598. doi: 10.1038/s41590-021-01073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stickels R.R., Murray E., Kumar P., Li J., Marshall J.L., Di Bella D.J., Arlotta P., Macosko E.Z., Chen F. Highly sensitive spatial transcriptomics at near-cellular resolution with Slide-seqV2. Nat. Biotechnol. 2021;39:313–319. doi: 10.1038/s41587-020-0739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng Y., Bartosovic M., Kukanja P., Zhang D., Liu Y., Su G., Enninful A., Bai Z., Castelo-Branco G., Fan R. Spatial-CUT&Tag: Spatially resolved chromatin modification profiling at the cellular level. Science. 2022;375:681–686. doi: 10.1126/science.abg7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang D., Deng Y., Kukanja P., Agirre E., Bartosovic M., Dong M., Ma C., Ma S., Su G., Bao S., et al. Spatial epigenome–transcriptome co-profiling of mammalian tissues. Nature. 2023;616:113–122. doi: 10.1038/s41586-023-05795-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hudson W.H., Sudmeier L.J. Localization of T cell clonotypes using the Visium spatial transcriptomics platform. STAR Protoc. 2022;3 doi: 10.1016/j.xpro.2022.101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu S., Iorgulescu J.B., Li S., Borji M., Barrera-Lopez I.A., Shanmugam V., Lyu H., Morriss J.W., Garcia Z.N., Murray E., et al. Spatial maps of T cell receptors and transcriptomes reveal distinct immune niches and interactions in the adaptive immune response. Immunity. 2022;55:1940–1952.e5. doi: 10.1016/j.immuni.2022.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y., DiStasio M., Su G., Asashima H., Enninful A., Qin X., Deng Y., Nam J., Gao F., Bordignon P., et al. High-plex protein and whole transcriptome co-mapping at cellular resolution with spatial CITE-seq. Nat. Biotechnol. 2023;41:1405–1409. doi: 10.1038/s41587-023-01676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao T., Chiang Z.D., Morriss J.W., LaFave L.M., Murray E.M., Del Priore I., Meli K., Lareau C.A., Nadaf N.M., Li J., et al. Spatial genomics enables multi-modal study of clonal heterogeneity in tissues. Nature. 2022;601:85–91. doi: 10.1038/s41586-021-04217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin J.R., Fallahi-Sichani M., Chen J.Y., Sorger P.K. Cyclic Immunofluorescence (CycIF), A Highly Multiplexed Method for Single-cell Imaging. Curr. Protoc. Chem. Biol. 2016;8:251–264. doi: 10.1002/cpch.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis M.M., Tato C.M., Furman D. Systems immunology: just getting started. Nat. Immunol. 2017;18:725–732. doi: 10.1038/ni.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y.C., Sahoo S., Brien R., Jung S., Humphries B., Lee W., Cheng Y.H., Zhang Z., Luker K.E., Wicha M.S., et al. Single-cell RNA-sequencing of migratory breast cancer cells: discovering genes associated with cancer metastasis. Analyst. 2019;144:7296–7309. doi: 10.1039/c9an01358j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng W., He M., Jiang X., Liu H., Xie T., Qin Z., Huang Q., Liao S., Lin C., He J., et al. Single-Cell RNA Sequencing Reveals the Migration of Osteoclasts in Giant Cell Tumor of Bone. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.715552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Donnell E.A., Ernst D.N., Hingorani R. Multiparameter flow cytometry: advances in high resolution analysis. Immune Netw. 2013;13:43–54. doi: 10.4110/in.2013.13.2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrosius V., Schoof E.M. Recent advances in the field of single-cell proteomics. Transl. Oncol. 2023;27 doi: 10.1016/j.tranon.2022.101556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bendall S.C., Simonds E.F., Qiu P., Amir E.a.D., Krutzik P.O., Finck R., Bruggner R.V., Melamed R., Trejo A., Ornatsky O.I., et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332:687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alitalo K. The lymphatic vasculature in disease. Nat. Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 50.Ho W.J., Yarchoan M., Charmsaz S., Munday R.M., Danilova L., Sztein M.B., Fertig E.J., Jaffee E.M. Multipanel mass cytometry reveals anti-PD-1 therapy-mediated B and T cell compartment remodeling in tumor-draining lymph nodes. JCI Insight. 2020;5 doi: 10.1172/jci.insight.132286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho W.J., Zhu Q., Durham J., Popovic A., Xavier S., Leatherman J., Mohan A., Mo G., Zhang S., Gross N., et al. Neoadjuvant Cabozantinib and Nivolumab Converts Locally Advanced HCC into Resectable Disease with Enhanced Antitumor Immunity. Nat. Cancer. 2021;2:891–903. doi: 10.1038/s43018-021-00234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Subrahmanyam P.B., Dong Z., Gusenleitner D., Giobbie-Hurder A., Severgnini M., Zhou J., Manos M., Eastman L.M., Maecker H.T., Hodi F.S. Distinct predictive biomarker candidates for response to anti-CTLA-4 and anti-PD-1 immunotherapy in melanoma patients. J. Immunother. Cancer. 2018;6:18. doi: 10.1186/s40425-018-0328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hartmann F.J., Babdor J., Gherardini P.F., Amir E.A.D., Jones K., Sahaf B., Marquez D.M., Krutzik P., O'Donnell E., Sigal N., et al. Comprehensive Immune Monitoring of Clinical Trials to Advance Human Immunotherapy. Cell Rep. 2019;28:819–831.e4. doi: 10.1016/j.celrep.2019.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allen B.M., Hiam K.J., Burnett C.E., Venida A., DeBarge R., Tenvooren I., Marquez D.M., Cho N.W., Carmi Y., Spitzer M.H. Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nat. Med. 2020;26:1125–1134. doi: 10.1038/s41591-020-0892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thorsson V., Gibbs D.L., Brown S.D., Wolf D., Bortone D.S., Ou Yang T.-H., Porta-Pardo E., Gao G.F., Plaisier C.L., Eddy J.A., et al. The Immune Landscape of Cancer. Immunity. 2018;48:812–830.e14. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Newman A.M., Liu C.L., Green M.R., Gentles A.J., Feng W., Xu Y., Hoang C.D., Diehn M., Alizadeh A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong T., Szustakowski J.D. DeconRNASeq: a statistical framework for deconvolution of heterogeneous tissue samples based on mRNA-Seq data. Bioinformatics. 2013;29:1083–1085. doi: 10.1093/bioinformatics/btt090. [DOI] [PubMed] [Google Scholar]
- 58.Racle J., de Jonge K., Baumgaertner P., Speiser D.E., Gfeller D. Simultaneous enumeration of cancer and immune cell types from bulk tumor gene expression data. Elife. 2017;6 doi: 10.7554/eLife.26476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li T., Fan J., Wang B., Traugh N., Chen Q., Liu J.S., Li B., Liu X.S. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017;77:e108–e110. doi: 10.1158/0008-5472.CAN-17-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Becht E., Giraldo N.A., Lacroix L., Buttard B., Elarouci N., Petitprez F., Selves J., Laurent-Puig P., Sautès-Fridman C., Fridman W.H., de Reyniès A. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016;17:218. doi: 10.1186/s13059-016-1070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vandereyken K., Sifrim A., Thienpont B., Voet T. Methods and applications for single-cell and spatial multi-omics. Nat. Rev. Genet. 2023;24:494–515. doi: 10.1038/s41576-023-00580-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y., Wang D., Peng M., Tang L., Ouyang J., Xiong F., Guo C., Tang Y., Zhou Y., Liao Q., et al. Single-cell RNA sequencing in cancer research. J. Exp. Clin. Cancer Res. 2021;40:81. doi: 10.1186/s13046-021-01874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma G., Colantuoni C., Goff L.A., Fertig E.J., Stein-O’Brien G. projectR: an R/Bioconductor package for transfer learning via PCA, NMF, correlation and clustering. Bioinformatics. 2020;36:3592–3593. doi: 10.1093/bioinformatics/btaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng M., Li Y., Wamsley B., Wei Y., Roeder K. Integration and transfer learning of single-cell transcriptomes via cFIT. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2024383118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stein-O'Brien G.L., Clark B.S., Sherman T., Zibetti C., Hu Q., Sealfon R., Liu S., Qian J., Colantuoni C., Blackshaw S., et al. Decomposing Cell Identity for Transfer Learning across Cellular Measurements, Platforms, Tissues, and Species. Cell Syst. 2019;8:395–411.e398. doi: 10.1016/j.cels.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andreatta M., Corria-Osorio J., Müller S., Cubas R., Coukos G., Carmona S.J. Interpretation of T cell states from single-cell transcriptomics data using reference atlases. Nat. Commun. 2021;12:2965. doi: 10.1038/s41467-021-23324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hao Y., Hao S., Andersen-Nissen E., Mauck W.M., 3rd, Zheng S., Butler A., Lee M.J., Wilk A.J., Darby C., Zager M., et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587.e29. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rozenblatt-Rosen O., Regev A., Oberdoerffer P., Nawy T., Hupalowska A., Rood J.E., Ashenberg O., Cerami E., Coffey R.J., Demir E., et al. The Human Tumor Atlas Network: Charting Tumor Transitions across Space and Time at Single-Cell Resolution. Cell. 2020;181:236–249. doi: 10.1016/j.cell.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sidiropoulos D.N., Stein-O’Brien G.L., Danilova L., Gross N.E., Charmsaz S., Xavier S., Leatherman J., Wang H., Yarchoan M., Jaffee E.M., et al. Integrated T cell cytometry metrics for immune-monitoring applications in immunotherapy clinical trials. JCI Insight. 2022;7 doi: 10.1172/jci.insight.160398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qiu X., Hill A., Packer J., Lin D., Ma Y.-A., Trapnell C. Single-cell mRNA quantification and differential analysis with Census. Nat. Methods. 2017;14:309–315. doi: 10.1038/nmeth.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolf F., Angerer P., Theis F. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 2018;19:15. doi: 10.1186/s13059-017-1382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Gassen S., Callebaut B., Van Helden M.J., Lambrecht B.N., Demeester P., Dhaene T., Saeys Y. FlowSOM: Using self-organizing maps for visualization and interpretation of cytometry data. Cytometry A. 2015;87:636–645. doi: 10.1002/cyto.a.22625. [DOI] [PubMed] [Google Scholar]
- 73.Aran D., Looney A.P., Liu L., Wu E., Fong V., Hsu A., Chak S., Naikawadi R.P., Wolters P.J., Abate A.R., et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 2019;20:163–172. doi: 10.1038/s41590-018-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stein-O’Brien G.L., Carey J.L., Lee W.S., Considine M., Favorov A.V., Flam E., Guo T., Li S., Marchionni L., Sherman T., et al. PatternMarkers & GWCoGAPS for novel data-driven biomarkers via whole transcriptome NMF. Bioinformatics. 2017;33:1892–1894. doi: 10.1093/bioinformatics/btx058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma G., Colantuoni C., Goff L.A., Fertig E.J., Stein-O’Brien G. projectR: an R/Bioconductor package for transfer learning via PCA, NMF, correlation and clustering. Bioinformatics. 2020;36:3592–3593. doi: 10.1093/bioinformatics/btaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peng K., Moore J., Vahed M., Brito J., Kao G., Burkhardt A.M., Alachkar H., Mangul S. pyTCR: A comprehensive and scalable solution for TCR-Seq data analysis to facilitate reproducibility and rigor of immunogenomics research. Front. Immunol. 2022;13:954078. doi: 10.3389/fimmu.2022.954078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cherry C., Maestas D.R., Han J., Andorko J.I., Cahan P., Fertig E.J., Garmire L.X., Elisseeff J.H. Computational reconstruction of the signalling networks surrounding implanted biomaterials from single-cell transcriptomics. Nat. Biomed. Eng. 2021;5:1228–1238. doi: 10.1038/s41551-021-00770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dimitrov D., Türei D., Garrido-Rodriguez M., Burmedi P.L., Nagai J.S., Boys C., Ramirez Flores R.O., Kim H., Szalai B., Costa I.G., et al. Comparison of methods and resources for cell-cell communication inference from single-cell RNA-Seq data. Nat. Commun. 2022;13:3224. doi: 10.1038/s41467-022-30755-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Browaeys R., Saelens W., Saeys Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat. Methods. 2020;17:159–162. doi: 10.1038/s41592-019-0667-5. [DOI] [PubMed] [Google Scholar]
- 80.Efremova M., Vento-Tormo M., Teichmann S.A., Vento-Tormo R. CellPhoneDB: inferring cell–cell communication from combined expression of multi-subunit ligand–receptor complexes. Nat. Protoc. 2020;15:1484–1506. doi: 10.1038/s41596-020-0292-x. [DOI] [PubMed] [Google Scholar]
- 81.Bolotin D.A., Poslavsky S., Mitrophanov I., Shugay M., Mamedov I.Z., Putintseva E.V., Chudakov D.M. MiXCR: software for comprehensive adaptive immunity profiling. Nat. Methods. 2015;12:380–381. doi: 10.1038/nmeth.3364. [DOI] [PubMed] [Google Scholar]
- 82.Song L., Cohen D., Ouyang Z., Cao Y., Hu X., Liu X.S. TRUST4: immune repertoire reconstruction from bulk and single-cell RNA-seq data. Nat. Methods. 2021;18:627–630. doi: 10.1038/s41592-021-01142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Borcherding N., Bormann N.L., Kraus G. scRepertoire: An R-based toolkit for single-cell immune receptor analysis. F1000Res. 2020;9:47. doi: 10.12688/f1000research.22139.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nazarov V.I., Tsvetkov V.O., Rumynskiy E., Popov A.A., Balashov I., Samokhina M., Lorenc A., Moore D.J., Greiff V., ImmunoMind immunarch: An R Package for Painless Bioinformatics Analysis of T-Cell and B-Cell Immune Repertoires. R Project. 2019 https://cran.r-project.org/web/packages/immunarch/index.html [Google Scholar]
- 85.Vander Heiden J.A., Yaari G., Uduman M., Stern J.N.H., O’Connor K.C., Hafler D.A., Vigneault F., Kleinstein S.H. pRESTO: a toolkit for processing high-throughput sequencing raw reads of lymphocyte receptor repertoires. Bioinformatics. 2014;30:1930–1932. doi: 10.1093/bioinformatics/btu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sturm G., Szabo T., Fotakis G., Haider M., Rieder D., Trajanoski Z., Finotello F. Scirpy: a Scanpy extension for analyzing single-cell T-cell receptor-sequencing data. Bioinformatics. 2020;36:4817–4818. doi: 10.1093/bioinformatics/btaa611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Montemurro A., Schuster V., Povlsen H.R., Bentzen A.K., Jurtz V., Chronister W.D., Crinklaw A., Hadrup S.R., Winther O., Peters B., et al. NetTCR-2.0 enables accurate prediction of TCR-peptide binding by using paired TCRα and β sequence data. Commun. Biol. 2021;4:1060. doi: 10.1038/s42003-021-02610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jokinen E., Dumitrescu A., Huuhtanen J., Gligorijević V., Mustjoki S., Bonneau R., Heinonen M., Lähdesmäki H. TCRconv: predicting recognition between T cell receptors and epitopes using contextualized motifs. Bioinformatics. 2023;39:btac788. doi: 10.1093/bioinformatics/btac788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gielis S., Moris P., Bittremieux W., De Neuter N., Ogunjimi B., Laukens K., Meysman P. Detection of Enriched T Cell Epitope Specificity in Full T Cell Receptor Sequence Repertoires. Front. Immunol. 2019;10:2820. doi: 10.3389/fimmu.2019.02820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reynisson B., Alvarez B., Paul S., Peters B., Nielsen M. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020;48:W449–W454. doi: 10.1093/nar/gkaa379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O'Donnell T.J., Rubinsteyn A., Laserson U. MHCflurry 2.0: Improved Pan-Allele Prediction of MHC Class I-Presented Peptides by Incorporating Antigen Processing. Cell Syst. 2020;11:42–48.e7. doi: 10.1016/j.cels.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 92.Gfeller D., Guillaume P., Michaux J., Pak H.S., Daniel R.T., Racle J., Coukos G., Bassani-Sternberg M. The Length Distribution and Multiple Specificity of Naturally Presented HLA-I Ligands. J. Immunol. 2018;201:3705–3716. doi: 10.4049/jimmunol.1800914. [DOI] [PubMed] [Google Scholar]
- 93.Racle J., Michaux J., Rockinger G.A., Arnaud M., Bobisse S., Chong C., Guillaume P., Coukos G., Harari A., Jandus C., et al. Robust prediction of HLA class II epitopes by deep motif deconvolution of immunopeptidomes. Nat. Biotechnol. 2019;37:1283–1286. doi: 10.1038/s41587-019-0289-6. [DOI] [PubMed] [Google Scholar]
- 94.Dries R., Zhu Q., Dong R., Eng C.-H.L., Li H., Liu K., Fu Y., Zhao T., Sarkar A., Bao F., et al. Giotto: a toolbox for integrative analysis and visualization of spatial expression data. Genome Biol. 2021;22:78. doi: 10.1186/s13059-021-02286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Palla G., Spitzer H., Klein M., Fischer D., Schaar A.C., Kuemmerle L.B., Rybakov S., Ibarra I.L., Holmberg O., Virshup I., et al. Squidpy: a scalable framework for spatial omics analysis. Nat. Methods. 2022;19:171–178. doi: 10.1038/s41592-021-01358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ospina O.E., Wilson C.M., Soupir A.C., Berglund A., Smalley I., Tsai K.Y., Fridley B.L. spatialGE: quantification and visualization of the tumor microenvironment heterogeneity using spatial transcriptomics. Bioinformatics. 2022;38:2645–2647. doi: 10.1093/bioinformatics/btac145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deshpande A., Loth M., Sidiropoulos D.N., Zhang S., Yuan L., Bell A.T.F., Zhu Q., Ho W.J., Santa-Maria C., Gilkes D.M., et al. Uncovering the spatial landscape of molecular interactions within the tumor microenvironment through latent spaces. Cell Syst. 2023;14:285–301.e4. doi: 10.1016/j.cels.2023.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cang Z., Zhao Y., Almet A.A., Stabell A., Ramos R., Plikus M.V., Atwood S.X., Nie Q. Screening cell–cell communication in spatial transcriptomics via collective optimal transport. Nat. Methods. 2023;20:218–228. doi: 10.1038/s41592-022-01728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fischer D.S., Schaar A.C., Theis F.J. Modeling intercellular communication in tissues using spatial graphs of cells. Nature Biotechno. 2023;41:332–336. doi: 10.1038/s41587-022-01467-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xun Z., Ding X., Zhang Y., Zhang B., Lai S., Zou D., Zheng J., Chen G., Su B., Han L., Ye Y. Reconstruction of the tumor spatial microenvironment along the malignant-boundary-nonmalignant axis. Nat. Commun. 2023;14:933. doi: 10.1038/s41467-023-36560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jin S., Guerrero-Juarez C.F., Zhang L., Chang I., Ramos R., Kuan C.-H., Myung P., Plikus M.V., Nie Q. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 2021;12:1088. doi: 10.1038/s41467-021-21246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Trapnell C., Cacchiarelli D., Grimsby J., Pokharel P., Li S., Morse M., Lennon N.J., Livak K.J., Mikkelsen T.S., Rinn J.L. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 2014;32:381–386. doi: 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reid J.E., Wernisch L. Pseudotime estimation: deconfounding single cell time series. Bioinformatics. 2016;32:2973–2980. doi: 10.1093/bioinformatics/btw372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gorin G., Fang M., Chari T., Pachter L. RNA velocity unraveled. PLoS Comput. Biol. 2022;18 doi: 10.1371/journal.pcbi.1010492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ji Z., Ji H. TSCAN: Pseudo-time reconstruction and evaluation in single-cell RNA-seq analysis. Nucleic Acids Res. 2016;44:e117. doi: 10.1093/nar/gkw430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jiao S., Xiong Q., Yan M., Zhan X., Yang Z., Peng C., Sun B., Pang D., Liu T. Intratumor expanded T cell clones can be non-sentinel lymph node derived in breast cancer revealed by single-cell immune profiling. J. Immunother. Cancer. 2022;10 doi: 10.1136/jitc-2021-003325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deshpande A., Chu L.F., Stewart R., Gitter A. Network inference with Granger causality ensembles on single-cell transcriptomics. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2022.110333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saelens W., Cannoodt R., Todorov H., Saeys Y. A comparison of single-cell trajectory inference methods. Nat. Biotechnol. 2019;37:547–554. doi: 10.1038/s41587-019-0071-9. [DOI] [PubMed] [Google Scholar]
- 109.La Manno G., Soldatov R., Zeisel A., Braun E., Hochgerner H., Petukhov V., Lidschreiber K., Kastriti M.E., Lönnerberg P., Furlan A., et al. RNA velocity of single cells. Nature. 2018;560:494–498. doi: 10.1038/s41586-018-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lange M., Bergen V., Klein M., Setty M., Reuter B., Bakhti M., Lickert H., Ansari M., Schniering J., Schiller H.B., et al. CellRank for directed single-cell fate mapping. Nat. Methods. 2022;19:159–170. doi: 10.1038/s41592-021-01346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zheng S.C., Stein-O’Brien G., Boukas L., Goff L.A., Hansen K.D. Pumping the brakes on RNA velocity – understanding and interpreting RNA velocity estimates. bioRxiv. 2022 doi: 10.1186/s13059-023-03065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davis-Marcisak E.F., Fitzgerald A.A., Kessler M.D., Danilova L., Jaffee E.M., Zaidi N., Weiner L.M., Fertig E.J. Transfer learning between preclinical models and human tumors identifies a conserved NK cell activation signature in anti-CTLA-4 responsive tumors. Genome Med. 2021;13:129. doi: 10.1186/s13073-021-00944-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dey K.K., Hsiao C.J., Stephens M. Visualizing the structure of RNA-seq expression data using grade of membership models. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pancheva A., Wheadon H., Rogers S., Otto T.D. Using topic modeling to detect cellular crosstalk in scRNA-seq. PLoS Comput. Biol. 2022;18 doi: 10.1371/journal.pcbi.1009975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Svensson V., Gayoso A., Yosef N., Pachter L. Interpretable factor models of single-cell RNA-seq via variational autoencoders. Bioinformatics. 2020;36:3418–3421. doi: 10.1093/bioinformatics/btaa169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moon K.R., van Dijk D., Wang Z., Gigante S., Burkhardt D.B., Chen W.S., Yim K., Elzen A.v.d., Hirn M.J., Coifman R.R., et al. Visualizing structure and transitions in high-dimensional biological data. Nat. Biotechnol. 2019;37:1482–1492. doi: 10.1038/s41587-019-0336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Burkhardt D.B., Stanley J.S., Tong A., Perdigoto A.L., Gigante S.A., Herold K.C., Wolf G., Giraldez A.J., van Dijk D., Krishnaswamy S. Quantifying the effect of experimental perturbations at single-cell resolution. Nat. Biotechnol. 2021;39:619–629. doi: 10.1038/s41587-020-00803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Davis M.M., Bjorkman P.J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 119.Schroeder H.W., Jr., Cavacini L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010;125:S41–S52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Courtney A.H., Lo W.L., Weiss A. TCR Signaling: Mechanisms of Initiation and Propagation. Trends Biochem. Sci. 2018;43:108–123. doi: 10.1016/j.tibs.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Porciello N., Franzese O., D’Ambrosio L., Palermo B., Nisticò P. T-cell repertoire diversity: friend or foe for protective antitumor response? J. Exp. Clin. Cancer Res. 2022;41:356. doi: 10.1186/s13046-022-02566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Seay H.R., Yusko E., Rothweiler S.J., Zhang L., Posgai A.L., Campbell-Thompson M., Vignali M., Emerson R.O., Kaddis J.S., Ko D., et al. Tissue distribution and clonal diversity of the T and B cell repertoire in type 1 diabetes. JCI Insight. 2016;1 doi: 10.1172/jci.insight.88242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miho E., Yermanos A., Weber C.R., Berger C.T., Reddy S.T., Greiff V. Computational Strategies for Dissecting the High-Dimensional Complexity of Adaptive Immune Repertoires. Front. Immunol. 2018;9:224. doi: 10.3389/fimmu.2018.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sanromán Á.F., Joshi K., Au L., Chain B., Turajlic S. TCR sequencing: applications in immuno-oncology research. Immunooncol. Technol. 2023;17 doi: 10.1016/j.iotech.2023.100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Valkiers S., de Vrij N., Gielis S., Verbandt S., Ogunjimi B., Laukens K., Meysman P. Recent advances in T-cell receptor repertoire analysis: Bridging the gap with multimodal single-cell RNA sequencing. ImmunoInformatics. 2022;5 [Google Scholar]
- 126.Pai J.A., Satpathy A.T. High-throughput and single-cell T cell receptor sequencing technologies. Nat. Methods. 2021;18:881–892. doi: 10.1038/s41592-021-01201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vieth B., Ziegenhain C., Parekh S., Enard W., Hellmann I. powsimR: power analysis for bulk and single cell RNA-seq experiments. Bioinformatics. 2017;33:3486–3488. doi: 10.1093/bioinformatics/btx435. [DOI] [PubMed] [Google Scholar]
- 128.Haque A., Engel J., Teichmann S.A., Lönnberg T. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Med. 2017;9:75. doi: 10.1186/s13073-017-0467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li B., Li T., Wang B., Dou R., Zhang J., Liu J.S., Liu X.S. Ultrasensitive detection of TCR hypervariable-region sequences in solid-tissue RNA-seq data. Nat. Genet. 2017;49:482–483. doi: 10.1038/ng.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hu X., Zhang J., Wang J., Fu J., Li T., Zheng X., Wang B., Gu S., Jiang P., Fan J., et al. Landscape of B cell immunity and related immune evasion in human cancers. Nat. Genet. 2019;51:560–567. doi: 10.1038/s41588-018-0339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yost K.E., Satpathy A.T., Wells D.K., Qi Y., Wang C., Kageyama R., McNamara K.L., Granja J.M., Sarin K.Y., Brown R.A., et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 2019;25:1251–1259. doi: 10.1038/s41591-019-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ellingsen E.B., Bounova G., Kerzeli I., Anzar I., Simnica D., Aamdal E., Guren T., Clancy T., Mezheyeuski A., Inderberg E.M., et al. Characterization of the T cell receptor repertoire and melanoma tumor microenvironment upon combined treatment with ipilimumab and hTERT vaccination. J. Transl. Med. 2022;20:419. doi: 10.1186/s12967-022-03624-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mpakali A., Stratikos E. The Role of Antigen Processing and Presentation in Cancer and the Efficacy of Immune Checkpoint Inhibitor Immunotherapy. Cancers. 2021;13 doi: 10.3390/cancers13010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zarnitsyna V.I., Evavold B.D., Schoettle L.N., Blattman J.N., Antia R. Estimating the diversity, completeness, and cross-reactivity of the T cell repertoire. Front. Immunol. 2013;4:485. doi: 10.3389/fimmu.2013.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hudson D., Fernandes R.A., Basham M., Ogg G., Koohy H. Can we predict T cell specificity with digital biology and machine learning? Nat. Rev. Immunol. 2023;23:511–521. doi: 10.1038/s41577-023-00835-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zaidi N., Soban M., Chen F., Kinkead H., Mathew J., Yarchoan M., Armstrong T.D., Haider S., Jaffee E.M. Role of in silico structural modeling in predicting immunogenic neoepitopes for cancer vaccine development. JCI Insight. 2020;5 doi: 10.1172/jci.insight.136991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.ImmunoMind T. Immunarch: An R Package for Painless Bioinformatics Analysis of T-Cell and B-Cell Immune Repertoires. Zenodo. 2019 [Google Scholar]
- 138.Vander Heiden J.A., Yaari G., Uduman M., Stern J.N.H., O’Connor K.C., Hafler D.A., Vigneault F., Kleinstein S.H. pRESTO: a toolkit for processing high-throughput sequencing raw reads of lymphocyte receptor repertoires. Bioinformatics. 2014;30:1930–1932. doi: 10.1093/bioinformatics/btu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rognes T., Scheffer L., Greiff V., Sandve G.K. CompAIRR: ultra-fast comparison of adaptive immune receptor repertoires by exact and approximate sequence matching. Bioinformatics. 2022;38:4230–4232. doi: 10.1093/bioinformatics/btac505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dash P., Fiore-Gartland A.J., Hertz T., Wang G.C., Sharma S., Souquette A., Crawford J.C., Clemens E.B., Nguyen T.H.O., Kedzierska K., et al. Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature. 2017;547:89–93. doi: 10.1038/nature22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Glanville J., Huang H., Nau A., Hatton O., Wagar L.E., Rubelt F., Ji X., Han A., Krams S.M., Pettus C., et al. Identifying specificity groups in the T cell receptor repertoire. Nature. 2017;547:94–98. doi: 10.1038/nature22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sidhom J.-W., Larman H.B., Pardoll D.M., Baras A.S. DeepTCR is a deep learning framework for revealing sequence concepts within T-cell repertoires. Nat. Commun. 2021;12:1605. doi: 10.1038/s41467-021-21879-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Peng X., Lei Y., Feng P., Jia L., Ma J., Zhao D., Zeng J. Characterizing the interaction conformation between T-cell receptors and epitopes with deep learning. Nat. Mach. Intell. 2023;5:395–407. [Google Scholar]
- 144.Bagaev D.V., Vroomans R.M.A., Samir J., Stervbo U., Rius C., Dolton G., Greenshields-Watson A., Attaf M., Egorov E.S., Zvyagin I.V., et al. VDJdb in 2019: database extension, new analysis infrastructure and a T-cell receptor motif compendium. Nucleic Acids Res. 2020;48 doi: 10.1093/nar/gkz874. D1057-d1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mullan K.A., Zhang J.B., Jones C.M., Goh S.J.R., Revote J., Illing P.T., Purcell A.W., La Gruta N.L., Li C., Mifsud N.A. TCR_Explore: A novel webtool for T cell receptor repertoire analysis. Comput. Struct. Biotechnol. J. 2023;21:1272–1282. doi: 10.1016/j.csbj.2023.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vita R., Mahajan S., Overton J.A., Dhanda S.K., Martini S., Cantrell J.R., Wheeler D.K., Sette A., Peters B. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 2019;47 doi: 10.1093/nar/gky1006. D339-d343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Arunkumar M., Zielinski C.E. T-Cell Receptor Repertoire Analysis with Computational Tools-An Immunologist's Perspective. Cells. 2021;10 doi: 10.3390/cells10123582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gartner J.J., Parkhurst M.R., Gros A., Tran E., Jafferji M.S., Copeland A., Hanada K.-I., Zacharakis N., Lalani A., Krishna S., et al. A machine learning model for ranking candidate HLA class I neoantigens based on known neoepitopes from multiple human tumor types. Nat. Cancer. 2021;2:563–574. doi: 10.1038/s43018-021-00197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Motmaen A., Dauparas J., Baek M., Abedi M.H., Baker D., Bradley P. Peptide-binding specificity prediction using fine-tuned protein structure prediction networks. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2216697120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Steele N.G., Carpenter E.S., Kemp S.B., Sirihorachai V.R., The S., Delrosario L., Lazarus J., Amir E.-a.D., Gunchick V., Espinoza C., et al. Multimodal mapping of the tumor and peripheral blood immune landscape in human pancreatic cancer. Nat. Cancer. 2020;1:1097–1112. doi: 10.1038/s43018-020-00121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lin J.R., Fallahi-Sichani M., Chen J.Y., Sorger P.K. Cyclic Immunofluorescence (CycIF), A Highly Multiplexed Method for Single-cell Imaging. Curr. Protoc. Chem. Biol. 2016;8:251–264. doi: 10.1002/cpch.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Phillips D., Schürch C.M., Khodadoust M.S., Kim Y.H., Nolan G.P., Jiang S. Highly Multiplexed Phenotyping of Immunoregulatory Proteins in the Tumor Microenvironment by CODEX Tissue Imaging. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.687673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hoch T., Schulz D., Eling N., Gómez J.M., Levesque M.P., Bodenmiller B. Multiplexed imaging mass cytometry of the chemokine milieus in melanoma characterizes features of the response to immunotherapy. Sci. Immunol. 2022;7 doi: 10.1126/sciimmunol.abk1692. [DOI] [PubMed] [Google Scholar]
- 154.Rahim M.K., Okholm T.L.H., Jones K.B., McCarthy E.E., Liu C.C., Yee J.L., Tamaki S.J., Marquez D.M., Tenvooren I., Wai K., et al. Dynamic CD8(+) T cell responses to cancer immunotherapy in human regional lymph nodes are disrupted in metastatic lymph nodes. Cell. 2023;186:1127–1143.e18. doi: 10.1016/j.cell.2023.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hu B., Sajid M., Lv R., Liu L., Sun C. A review of spatial profiling technologies for characterizing the tumor microenvironment in immuno-oncology. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.996721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Park J., Kim J., Lewy T., Rice C.M., Elemento O., Rendeiro A.F., Mason C.E. Spatial omics technologies at multimodal and single cell/subcellular level. Genome Biol. 2022;23:256. doi: 10.1186/s13059-022-02824-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Magen A., Hamon P., Fiaschi N., Soong B.Y., Park M.D., Mattiuz R., Humblin E., Troncoso L., D'Souza D., Dawson T., et al. Intratumoral dendritic cell-CD4(+) T helper cell niches enable CD8(+) T cell differentiation following PD-1 blockade in hepatocellular carcinoma. Nat. Med. 2023;29:1389–1399. doi: 10.1038/s41591-023-02345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Engblom C., Thrane K., Lin Q., Andersson A., Toosi H., Chen X., Steiner E., Mantovani G., Hagemann-Jensen M., Saarenpää S., et al. Cold Spring Harbor Laboratory; 2022. Spatial Transcriptomics of T and B Cell Receptors Uncovers Lymphocyte Clonal Dynamics in Human Tissue. [DOI] [PubMed] [Google Scholar]
- 159.Sudmeier L.J., Hoang K.B., Nduom E.K., Wieland A., Neill S.G., Schniederjan M.J., Ramalingam S.S., Olson J.J., Ahmed R., Hudson W.H. Distinct phenotypic states and spatial distribution of CD8(+) T cell clonotypes in human brain metastases. Cell Rep. Med. 2022;3 doi: 10.1016/j.xcrm.2022.100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jiang X., Dudzinski S., Beckermann K.E., Young K., McKinley E., J McIntyre O., Rathmell J.C., Xu J., Gore J.C. MRI of tumor T cell infiltration in response to checkpoint inhibitor therapy. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2019-000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Galon J., Angell H.K., Bedognetti D., Marincola F.M. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 162.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-Rosenberg S., Hedrick C.C., et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bronte V., Brandau S., Chen S.-H., Colombo M.P., Frey A.B., Greten T.F., Mandruzzato S., Murray P.J., Ochoa A., Ostrand-Rosenberg S., et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016;7 doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Umansky V., Blattner C., Gebhardt C., Utikal J. The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines (Basel) 2016;4 doi: 10.3390/vaccines4040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Veglia F., Perego M., Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018;19:108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Italiani P., Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cassetta L., Pollard J.W. Targeting macrophages: therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018;17:887–904. doi: 10.1038/nrd.2018.169. [DOI] [PubMed] [Google Scholar]
- 170.Maier B., Leader A.M., Chen S.T., Tung N., Chang C., LeBerichel J., Chudnovskiy A., Maskey S., Walker L., Finnigan J.P., et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature. 2020;580:257–262. doi: 10.1038/s41586-020-2134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Coffelt S.B., Wellenstein M.D., de Visser K.E. Neutrophils in cancer: neutral no more. Nat. Rev. Cancer. 2016;16:431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 172.Swierczak A., Mouchemore K.A., Hamilton J.A., Anderson R.L. Neutrophils: important contributors to tumor progression and metastasis. Cancer Metastasis Rev. 2015;34:735–751. doi: 10.1007/s10555-015-9594-9. [DOI] [PubMed] [Google Scholar]
- 173.Kalluri R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 174.Stacker S.A., Williams S.P., Karnezis T., Shayan R., Fox S.B., Achen M.G. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer. 2014;14:159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 175.Laumont C.M., Banville A.C., Gilardi M., Hollern D.P., Nelson B.H. Tumour-infiltrating B cells: immunological mechanisms, clinical impact and therapeutic opportunities. Nat. Rev. Cancer. 2022;22:414–430. doi: 10.1038/s41568-022-00466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Stuart T., Butler A., Hoffman P., Hafemeister C., Papalexi E., Mauck W.M., 3rd, Hao Y., Stoeckius M., Smibert P., Satija R. Comprehensive Integration of Single-Cell Data. Cell. 2019;177:1888–1902.e21. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kersten K., Hu K.H., Combes A.J., Samad B., Harwin T., Ray A., Rao A.A., Cai E., Marchuk K., Artichoker J., et al. Spatiotemporal co-dependency between macrophages and exhausted CD8(+) T cells in cancer. Cancer Cell. 2022;40:624–638.e9. doi: 10.1016/j.ccell.2022.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fischer D.S., Schaar A.C., Theis F.J. Modeling intercellular communication in tissues using spatial graphs of cells. Nat. Biotechnol. 2023;41:332–336. doi: 10.1038/s41587-022-01467-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Heath J.R., Ribas A., Mischel P.S. Single-cell analysis tools for drug discovery and development. Nat. Rev. Drug Discov. 2016;15:204–216. doi: 10.1038/nrd.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yao L., Wang J.T., Jayasinghe R.G., O'Neal J., Tsai C.F., Rettig M.P., Song Y., Liu R., Zhao Y., Ibrahim O.M., et al. Single-Cell Discovery and Multiomic Characterization of Therapeutic Targets in Multiple Myeloma. Cancer Res. 2023;83:1214–1233. doi: 10.1158/0008-5472.CAN-22-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jia Q., Chu H., Jin Z., Long H., Zhu B. High-throughput single-сell sequencing in cancer research. Signal Transduct. Target. Ther. 2022;7:145. doi: 10.1038/s41392-022-00990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shalaby N., Dubois V.P., Ronald J. Molecular imaging of cellular immunotherapies in experimental and therapeutic settings. Cancer Immunol. Immunother. 2022;71:1281–1294. doi: 10.1007/s00262-021-03073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zeng J., Zhang Y., Shang Y., Mai J., Shi S., Lu M., Bu C., Zhang Z., Zhang Z., Li Y., et al. CancerSCEM: a database of single-cell expression map across various human cancers. Nucleic Acids Res. 2022;50:D1147–D1155. doi: 10.1093/nar/gkab905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sterner R.C., Sterner R.M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mai D., Johnson O., Reff J., Fan T.-J., Scholler J., Sheppard N.C., June C.H. Combined disruption of T cell inflammatory regulators Regnase-1 and Roquin-1 enhances antitumor activity of engineered human T cells. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2218632120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yang J., Chen Y., Jing Y., Green M.R., Han L. Advancing CAR T cell therapy through the use of multidimensional omics data. Nat. Rev. Clin. Oncol. 2023;20:211–228. doi: 10.1038/s41571-023-00729-2. [DOI] [PubMed] [Google Scholar]
- 187.Arnaout R.A., Prak E.T.L., Schwab N., Rubelt F., Adaptive Immune Receptor Repertoire Community The Future of Blood Testing Is the Immunome. Front. Immunol. 2021;12:626793. doi: 10.3389/fimmu.2021.626793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ma Y., Wang Q., Dong Q., Zhan L., Zhang J. How to differentiate pseudoprogression from true progression in cancer patients treated with immunotherapy. Am. J. Cancer Res. 2019;9:1546–1553. [PMC free article] [PubMed] [Google Scholar]
- 189.Cadot S., Valle C., Tosolini M., Pont F., Largeaud L., Laurent C., Fournie J.J., Ysebaert L., Quillet-Mary A. Longitudinal CITE-Seq profiling of chronic lymphocytic leukemia during ibrutinib treatment: evolution of leukemic and immune cells at relapse. Biomark. Res. 2020;8:72. doi: 10.1186/s40364-020-00253-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.von Andrian U.H., Mempel T.R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 191.Stein J.V., F Gonzalez S. Dynamic intravital imaging of cell-cell interactions in the lymph node. J. Allergy Clin. Immunol. 2017;139:12–20. doi: 10.1016/j.jaci.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 192.Mempel T.R., Henrickson S.E., Von Andrian U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 193.Schwickert T.A., Lindquist R.L., Shakhar G., Livshits G., Skokos D., Kosco-Vilbois M.H., Dustin M.L., Nussenzweig M.C. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007;446:83–87. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- 194.Victora G.D., Schwickert T.A., Fooksman D.R., Kamphorst A.O., Meyer-Hermann M., Dustin M.L., Nussenzweig M.C. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]