Abstract
Eight Caulobacter crescentus flagellar genes, flmA, flmB, flmC, flmD, flmE, flmF, flmG, and flmH, have been cloned and characterized. These eight genes are clustered in pairs (flmAB, flmCD, flmEF, and flmGH) that appear to be structurally organized as operons. Homology comparisons suggest that the proteins encoded by the flm genes may be involved in posttranslational modification of flagellins or proteins that interact with flagellin monomers prior to their assembly into a flagellar filament. Expression of the flmAB, flmEF, and flmGH operons was shown to occur primarily in predivisional cells. In contrast, the flmCD operon was expressed throughout the cell cycle, with only a twofold increase in predivisional cells. The expression of the three temporally regulated operons was subject to positive regulation by the CtrA response regulator protein. Mutations in class II and III flagellar genes had no significant effect on the expression of the flm genes. Furthermore, the flm genes did not affect the expression of class II or class III flagellar genes. However, mutations in the flm genes did result in reduced synthesis of the class IV flagellin proteins. Taken together, these data indicate that the flm operons belong to a new class of flagellar genes.
The life cycle of the aquatic bacterium Caulobacter crescentus provides a model system for the analysis of programmed developmental events. Each cell division produces two morphologically dissimilar progeny cells, a motile swarmer cell and a sessile stalked cell. Flagellum biogenesis is initiated in the predivisional cell and results in the synthesis of a basal body, hook, and flagellar filament at one pole of the developing swarmer cell (reviewed in reference 24). This process involves at least 50 genes (19). Most of the flagellar genes have been organized into a regulatory hierarchy that includes four classes of genes (reviewed in reference 54). In this hierarchy, the expression of genes in an earlier class is required for expression of the genes in subsequent classes. Furthermore, the genes that encode the structural components of the flagellum are transcribed in the order that their gene products are assembled into the structure. For instance, expression of the class II genes is required for expression of the class III hook and basal body genes. Similarly, expression of the class II and III genes and assembly of their products are required for expression of the class IV flagellin genes. One class II gene, rpoN, encodes sigma 54, the sigma factor that binds to class III and class IV promoters (11). In addition, integration host factor and transcriptional activators are required for expression of class III and class IV genes (7, 25, 26, 73). Synthesis of the flagellin subunits encoded by the class IV genes is also subject to posttranscriptional control mechanisms (3, 41, 42). Recently, Quon et al. (53) have shown that mutations in the class I ctrA gene result in altered expression of class II flagellar genes.
The flagellar filament is composed of three distinct flagellin monomers. A 29-kDa flagellin is initially assembled at the hook-proximal portion of the filament (18). Subsequently, the 27- and 25-kDa flagellins are synthesized and assembled consecutively (18, 71). The 25-kDa flagellin constitutes the distal two-thirds of the filament (18). In addition to these structural genes, the expression of the flagellar genes flmA (formerly flaA), flmD (flaR), flmE (flaZ), flmG (flbA), and flmH (flaG) is required for the synthesis of normal flagellin proteins (30). Strains containing mutations in any of these genes have a normal basal body and hook structure but fail to assemble a flagellar filament (30). Mutations in the flmA, flmD, flmG, and flmH genes result in the production of a novel 22-kDa flagellin protein. In addition, the production of the other flagellins is severely decreased (30, 57, 61). Mutations in the flmE gene resulted in the production of flagellins that migrate slightly faster than the wild-type flagellins on sodium dodecyl sulfate (SDS)-polyacrylamide gels. Thus, flagellins from the flmE mutant have apparent molecular masses of 26 and 24 kDa instead of 27 and 25 kDa (30). The 22- and 24-kDa proteins are also produced in flbT mutants when the flagellins are overproduced (57). Recently, we have shown that degradation of the fljK mRNA is regulated by the flbT gene product (42).
To analyze the role of the flm genes in the production of these flagellin proteins, we have cloned and determined the nucleotide sequences of the flmAB, flmCD, flmEF, and flmGH operons. Homology searches revealed that the deduced amino acid sequences of the flmA, flmB, flmC, and flmD genes are similar to sequences of proteins involved in capsular and spore coat polysaccharide biosynthesis. Thus, the flm genes may be involved in glycosylation. To study the regulation of the flm operons, we have constructed fusions of the flmA, flmC, flmE, and flmG promoters to the cat or lacZ reporter gene. We demonstrate that expression of the flmA, flmE, and flmG genes is altered by a mutation in the ctrA gene, a class I flagellar gene. In addition, we show that the flmA, flmE, and flmG genes are expressed primarily in predivisional cells, indicating that these putative operons are temporally regulated. In contrast, the flmC gene is expressed throughout the cell cycle, with a twofold increase in the predivisional cell. These results, along with studies of the flagellar regulatory hierarchy, suggest that the flm genes belong to a new class of flagellar genes.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Tables 1 and 2, respectively. All C. crescentus strains used are derivatives of the wild-type strain CB15. C. crescentus strains were grown at 33°C in peptone-yeast extract (PYE) medium or in defined minimal medium M2 (28). Escherichia coli strains were grown at 37°C in Luria-Bertani (LB) or defined E medium supplemented with 1.0 mM proline (44). Complementation of motility defects was demonstrated in semisolid medium as previously described (59). Plasmids were transferred to the recipient C. crescentus flm mutants by conjugation from their E. coli host S17-1. Antibiotics were added, when appropriate, at the following concentrations: ampicillin, 50 μg/ml; sulfanilamide, 350 μg/ml in E medium; tetracycline, 15 μg/ml in LB and 1 μg/ml in PYE medium; and kanamycin, 50 μg/ml.
TABLE 1.
Bacterial strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| E. coli | ||
| S17-1 | proA recA hsdR hsdM zzz:RP4 (Tc::Mu) (Km::Tn7) | 62 |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac F+ (proAB lacIq M15 Tn10) | Stratagene |
| C. crescentus | ||
| CB15 | Wild type | |
| LS107 | syn-1000 bla-6 | Dickon Alley |
| LS2195 | syn-1000 ctrA401 | 53 |
| PC7070 | recA526 zzz::Tn5 str-30 | 50 |
| SC175 | flmE102 (formerly flaZ102) | 29 |
| SC229 | flmA104 (formerly flaA104) | 29 |
| SC278 | flmH131 (formerly flaG131) | 29 |
| SC305 | flmD148 (formerly flaR148) | 29 |
| SC1029 | flhB195::Tn5 str-152 | 70 |
| SC1030 | flmE618::Tn5 (formerly flaZ618::Tn5) str-152 | 19 |
| SC1032 | flbD198::Tn5 str-152 | 51 |
| SC1055 | rpoN610::Tn5 proA 103 str-140 | 19 |
| SC1066 | fliL179 proA103 str-140 | 19 |
| SC1117 | flbN194::Tn5 str-152 | 19 |
| SC1127 | flmD614::Tn5 (formerly flaR614::Tn5) str-142 | 19 |
| SC1128 | flmA613::Tn5 (formerly flaA613::Tn5) str-142 | 19 |
| SC1132 | flhA608::Tn5 str-152 | 51 |
| SC1134 | flgK603::Tn5 str-152 | 51 |
| SC1135 | flbG602::Tn5 str-152 | 51 |
| SC2663 | fliM667::Tn5-lacI rif-175 | 19 |
| SC3090 | flmD651::Tn5 (formerly flaR651::Tn5) str-152 | 19 |
| SC3809 | fliQR153 zzz::Tn5 recA526 | 19 |
| SC3898 | flmD148 recA526 zzz::Tn5 | φPC7070 × SC305 |
| SC3899 | flmE102 recA526 zzz::Tn5 | φPC7070 × SC175 |
| SC3971 | syn-1000 flmC::pGL20 | pGL20 integrated into LS107 |
| SC3973 | syn-1000 flmE::pGL21 | pGL21 integrated into LS107 |
| SC3975 | syn-1000 flmA::pGL22 | pGL22 integrated into LS107 |
| SC4016 | syn-1000 flmG::pGL41 | pGL41 integrated into LS107 |
| SC4250 | syn-1000 (flmA::lacZ on pRKlac290) | LS107 harboring pSPW1967 |
TABLE 2.
Plasmids used in this study
| Plasmid | Relevant characteristics or construction |
|---|---|
| pBEE302 | Cloning vector (Sulr SMr (derivative of R300B) (58) |
| pBKSII(+/−) | pBluescript II KS +/− phagemid [Ampr ColE1 replicon ori f1 (+/−)] (Stratagene) |
| pBSKII(+/−) | pBluescript II SK +/− phagemid [Ampr ColE1 replicon ori f1 (+/−)] (Stratagene) |
| pKT230 | Cloning vector (Kan Str IncQ replicon) (4) |
| pRKlac290 | Promoterless lacZ gene cloned into pRK290 for transcriptional fusion (26) |
| pRK2L1 | Cloning vector (Tetr IncP replicon, Mob+) (47) |
| pUC19 | Cloning vector (Ampr ColE1 replicon) (76) |
| R300B | Cloning vector (Sulr Smr IncQ replicon, Mob+) (5) |
| pCS91 | rsaA::lacZ in pRKlac290 (65) |
| pGL2 | pSCC33 with 1.6-kb HpaI fragment deleted |
| pGL11 | 0.8-kb SacI fragment from pGL14, containing the flmC promoter cloned into pBKSII(−) |
| pGL14 | 8.6-kb BamHI-EcoRI fragment from pSCC2 into pBKSII(−) |
| pGL14-106 | 1.85-kb deletion from EcoRI site of pGL14 (exonuclease III deletion) |
| pGL14-312 | 4.35-kb deletion from EcoRI site of pGL14 (exonuclease III deletion) |
| pGL15 | pGL14 with 3.2-kb HpaI fragment deleted |
| pGL17 | pGL14-106 cloned into pRK2L1 digested with BamHI |
| pGL18 | pGL14-312 cloned into pRK2L1 digested with BamHI |
| pGL19 | pGL15 cloned into pRK2L1 digested with BamHI |
| pGL20 | Promoterless CAT cartridge inserted into HindIII site of pGL11 (flmC::cat) |
| pGL21 | Promoterless CAT cartridge inserted into HindIII site of pGL24 (flmE::cat) |
| pGL22 | 1.7-kb BamHI-SalI fragment containing flmA promoter cloned into pGL30 (flmA::cat) |
| pGL24 | 0.8-kb SalI fragment from pSCC33 containing flmE promoter cloned into pBSKII(−) in opposite orientation of lacZ promoter |
| pGL30 | Promoterless CAT cartridge inserted into HindIII site of pUC19 in opposite orientation of lacZ promoter |
| pGL36 | 3.16-kb SacI-NcoI fragment from pLSG1 cloned in pBKSII(−) |
| pGL38 | pGL36 cloned into pRK2L1 digested with KpnI |
| pGL39 | 1.5-kb EcoRI-BamHI fragment from pPVS149 containing flmG promoter cloned into pUC19 |
| pGL41 | Promoterless CAT cartridge inserted into HindIII site of pGL39 in opposite orientation of lacZ promoter |
| pGL48 | pGL20 cloned into pRK2L1 digested with KpnI (flmC::cat) |
| pGL49 | pGL21 cloned into pRK2L1 digested with KpnI (flmE::cat) |
| pGL50 | pGL22 cloned into pRK2L1 digested with KpnI (flmA::cat) |
| pGL53 | pGL41 cloned into pRKlac290 digested with BamHI (flmG::lacZ) |
| pLSG1 | pLAFR1-7-derived cosmid containing 25 kb of C. crescentus DNA (flmCD-flmEF region) (58) |
| pLSG6 | pLAFR1-7-derived cosmid containing 20 kb of C. crescentus DNA (flmAB region) (58) |
| pNC1341 | 3.5-kb BamHI-EcoRI from pSCC7 cloned into pKT230 |
| pNC1355 | 1.8-kb SalI-EcoRI subcloned from pNC1341 |
| pNC1356 | 1.7-kb BamHI-SalI subcloned from pNC1341 |
| pPVS149 | 2.4-kb BamHI-SacI fragment cloned into pKT230 (59) |
| pPVS154 | 3.2-kb EcoRI fragment (flmGH region) in R300B (59) |
| pSCC2 | 14-kb EcoRI fragment subcloned from pLSG1 |
| pSCC7 | 5-kb EcoRI fragment from pLSG6 cloned into pBEE302 |
| pSCC33 | 3.7-kb SacI from pLSG1 (flmCD-flmEF region) cloned in R300B |
| pSCW1355 | 1.8-kb SalI-EcoRI fragment from pNC1355 cloned into pBKSII(−) |
| pSCW1356 | 1.7-kb BamHI-SalI fragment from pNC1356 cloned into pBKSII(−) |
| pSCW1967 | 1.7-kb BamHI-SalI fragment from pNC1356 cloned into pRKlac290 (flmA::lacZ) |
| pWZ162 | fliQ::lacZ in pRKlac290 (79) |
Molecular techniques.
General cloning procedures were carried out as described by Sambrook et al. (55). C. crescentus chromosomal DNA was isolated as previously described by Malakooti and Ely (40). All enzymes used in the manipulations of DNA were used according to the specifications of the manufacturer. Transformation of E. coli was carried out as described by Sambrook et al. (55). Transformation of C. crescentus was carried out by electroporation according to the procedure of Gilchrist and Smit (23). The nucleotide sequence of both strands of DNA containing the gene of interest was determined from either double-stranded templates or single-stranded templates by the dideoxy-chain termination method (56) using a Sequenase version 2.0 kit (U.S. Biochemical, Cleveland, Ohio). Nucleotide and amino acid sequence analyses were performed with the Wisconsin Package of the Genetics Computer Group (Madison, Wis.) (16).
Construction of flmA::cat, flmC::cat, flmE::cat, and flmG::cat gene fusions.
For the flmCD, flmEF, and flmGH operons, a promoterless chloramphenicol acetyltransferase (CAT) cartridge contained in a 0.8-kb HindIII fragment was inserted into the unique HindIII sites of pGL39, pGL11, and pGL24, respectively. In the case of the flmAB operon, a 1.7-kb BamHI-SalI fragment containing the flmA promoter region was cloned into pGL30 that contained the cat gene inserted in the orientation opposite that of the lacZ promoter. The resulting plasmids, pGL20, pGL21, pGL22, and pGL41, were introduced in C. crescentus LS107 by electroporation. Transformants were selected for ampicillin resistance, causing the integration of the nonreplicating plasmid into the chromosome. Single-crossover recombinants were identified by Southern blot analysis (data not shown), resulting in strains SC3971, SC3973, SC3975, and SC4016, containing the chromosomal fusions flmA::cat, flmC::cat, flmE::cat, and flmG::cat, respectively.
CAT and β-galactosidase assays.
Strains carrying a transcriptional fusion (plasmid borne or integrated into the chromosome by homologous recombination) were grown to the exponential growth phase (125 Klett units or A600 of 0.5) in PYE medium supplemented with appropriate antibiotics. Cell extracts were prepared by sonic disruption of the cells in 0.1× TE (10 mM Tris–0.1 mM EDTA [pH 8.0]). The cell debris was removed by centrifugation, and the supernatant was assayed to determine the protein concentration (9). CAT activity was assayed by using [3H]acetyl coenzyme A according to the directions of the manufacturer (NEN, Boston, Mass.). Assays of β-galactosidase activity were performed as previously described (44). Totals of 5 and 12 μg of protein were assayed for β-galactosidase and CAT activities, respectively.
Cell synchronization and immunoprecipitation.
Strains containing flmC::cat, flmE::cat, and flmG::cat chromosomal integrated fusions or flmA::lacZ plasmid (pSCW1967)-borne fusion were grown in M2 medium and synchronized by differential centrifugation (6). Swarmer cells were allowed to proceed synchronously through the cell cycle at 30°C. Samples were removed at specific times and pulse-labeled for 10 min with 10 μCi of Tran35S-label (ICN, Costa Mesa, Calif.). Cell extracts were prepared and immunoprecipitated as described by Gomes and Shapiro (27), using antibody to CAT or β-galactosidase protein. Flagellin immunoprecipitation was used as a positive control for cell cycle-dependent expression. The cell cycle was also monitored by light microscopy. The immunoprecipitated proteins were resolved by SDS–10% polyacrylamide gel electrophoresis and visualized by autoradiography.
Nucleotide sequence accession numbers.
The DNA sequences of flmAB, flmCD-flmEF, and flmGH operons described in this report have been assigned GenBank accession no. U27301, U27302, and U28867, respectively.
RESULTS
Isolation and characterization of flmC, flmD, flmE, and flmF genes.
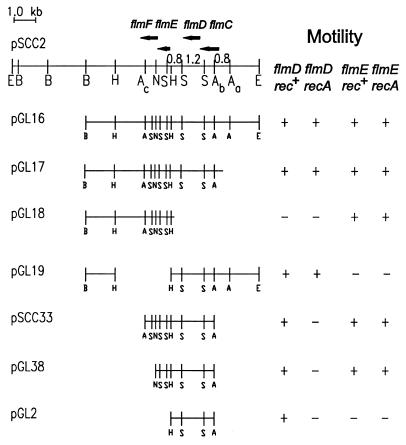
A cosmid, pLSG1, containing about 25 kb of C. crescentus chromosomal DNA was identified by complementation of the motility defect of strains SC1030 (flmE::Tn5), SC1127 (flmD::Tn5), and SC3090 (flmD::Tn5) (58). Plasmid pLSG1 could also complement the SC175 (flmE) and SC305 (flmD) mutants. Deletion analysis of pLSG1 revealed that a 14-kb EcoRI fragment retained in pSCC2 was able to restore motility to the flmD and flmE Tn5 insertion mutants. To define the locations of the flmD and flmE genes in pSCC2, DNA fragments were deleted or subcloned into plasmids that can replicate in C. crescentus (Fig. 1). Plasmids pSCC33, pGL2, and pGL38 could restore motility to the rec+ strains SC305, SC1127, and SC3090, but they were unable to complement the motility defect of the recombination-deficient strain SC3898 (recA526 flmD). These results indicate that the restored motility in the rec+ strains resulted from a recombinational event. Since pGL17 and pGL19 both complemented strain SC3898, and pGL18 and pSCC33 both failed to complement SC3898, we deduced that the flmD gene is contained within a fragment beginning 460 bp to the right of the SacIb site and ending at the HpaI site. By contrast, pSCC33 was able to complement both SC175 (flmE) and SC3899 (flmE recA), indicating that the flmE gene is present entirely within the 3.7-kb SacI fragment. Since both pGL18 and pGL38 were able to complement the recA flmE double-mutant strain SC3899, and since pGL19 failed to complement SC3899, we concluded that the flmE gene is located in a 0.8-kb fragment beginning 410 bp to the right of the HpaI site and ending at the NcoI site. The locations of the flmD and flmE genes were confirmed by Southern analysis of chromosomal DNA from Tn5 insertion mutants. Strains SC1127 and SC3090 contained a flmD::Tn5 insertion located in the 1.2-kb SalI fragment, and strain SC1030 had a flmE::Tn5 insertion located in the 0.8-kb SalI fragment (data not shown).
FIG. 1.
Analysis of the flmCD and flmEF regions. Shown is a restriction map of plasmid pSCC2, which contains a 14-kb EcoRI DNA fragment of the C. crescentus chromosome. Subclones from this region in plasmid pRK2L1 or R300B were tested for the ability to complement the motility defect of strains SC305 (flmD148), SC175 (flmE102), SC3898 (flmC148 recA526 zzz::Tn5), and SC3899 (flmE102 recA526 zzz:Tn5). +, complementation of the motility defect; −, failure to complement. Solid arrows represent predicted open reading frames and direction of transcription. Abbreviations: A, SacI; B, BamHI; E, EcoRI; N, NcoI; P, HpaI; S, SalI.
The nucleotide sequence of approximately 5 kb of DNA from the region containing the flmD and flmE genes was determined from both strands (GenBank accession no. U27302). Four open reading frames were identified as potential coding sequences by using the bias for high GC at the third position of each codon and the frequency of rare codon usage in C. crescentus coding regions (60). The flmD and flmE genes are each structurally organized as an operon with a previously unidentified gene (flmC and flmF, respectively). In both operons, the termination codon of the first gene overlaps the initiation codon of the second gene. Evidence that flmCD is transcribed as an operon is as follows: (i) plasmid pGL2 can correct the motility defect of strain SC305 (flmD) by recombination, and (ii) true complementation of a recA flmD double mutant can be obtained only with pGL17 and pGL19, which both contain the upstream flmC gene in addition to flmD.
Isolation and characterization of the flmA, flmB, flmG, and flmH genes.
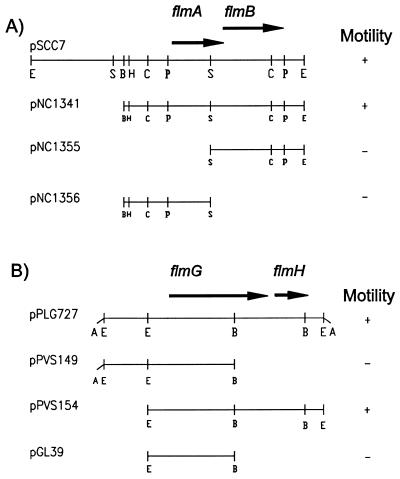
The flmA and flmB genes (formerly designated flaA) were identified by complementation of strains SC229 (flmA104) and SC1128 (flmA::Tn5) by the cosmid clone pLSG6 that contained about 20 kb of C. crescentus chromosomal DNA (58). Pulsed-field gel electrophoresis and Southern analysis of chromosomal DNA from SC1128 revealed that the flmA and flmB genes were present on an 18-kb EcoRI fragment of chromosomal DNA (data not shown). Cosmid pLSG6 contained two EcoRI sites, one in the vector and a second in the cloned C. crescentus DNA. A subclone of the 5-kb EcoRI fragment of pLSG6, pSCC7, could complement the motility defect of both strains SC229 and SC1128. Deletion analysis of pSCC7 revealed that a 3.5-kb BamHI-EcoRI fragment (pNC1341) could fully complement strain SC229 (Fig. 2A). Analysis of additional subclones (pNC1355 and pNC1356) demonstrated that the DNA on both sides of the central SalI site was required for flmA complementation (Fig. 2A). Nucleotide sequence analysis of the entire 3.5-kb BamHI-EcoRI fragment (GenBank accession no. U27301) revealed two potential open reading frames with overlapping termination and initiation codons similar to those found in the flmCD and flmEF operons. The first open reading frame was designated flmA since it spanned the SalI site required for complementation.
FIG. 2.
(A) Analysis of the flmAB region. Genetic organization of plasmid pSCC7 harboring the 5.0-kb EcoRI fragment is represented. Solid arrows represent open reading frames and direction of transcription. The ability to complement strain SC229 (flmA104) is shown. (B) Organization of the flmGH region. Solid arrows represent open reading frames and direction of transcription. + and − denote the ability and inability, respectively to swarm in a semisolid medium. Abbreviations: B, BamHI; C, ClaI; E, EcoRI; H, HindIII; P, HpaI; S, SalI.
Previous studies (59) resulted in the isolation and characterization of the flmG (formerly flbA) and flmH (formerly flaG) genes. Using complementation analysis, Schoenlein et al. (59) demonstrated by that flmG and flmH were organized as an operon. Both genes were present on a 3.2-kb EcoRI fragment borne by pPVS154 (Fig. 2B) (59). The nucleotide sequence of the entire 3.2-kb EcoRI fragment was determined on both strands (GenBank accession no. U28867). Examination of the DNA sequence confirmed that flmG and flmH genes are organized as an operon (Fig. 2B). However, in this case, the two coding regions were separated by 152 bp.
Database comparisons.
The deduced amino acid sequences of the eight Flm proteins were compared to entries in the GenBank database. As shown in Table 3, FlmA, FlmB, FlmC, and FlmD show significant levels of identity (23 to 41% identity) with proteins involved in capsular, lipopolysaccharide (LPS), and spore coat polysaccharide biosynthesis from Bacillus subtilis, Methanococcus jannaschii, and other bacteria. Furthermore, FlmC also shows homology with the CMP-KDO syn- thetase (3-deoxy-manno-octulosonate cytidylyltransferase) involved in LPS biosynthesis in E. coli (8), Chlamydia trachomatis (67), and Haemophilus influenzae (21). In Helicobacter pylori, a FlmA homolog, FlaA1, has been sequenced and found to show 61% identity over a 321-amino-acid overlap (GenBank accession no. AE00595) (68). Since flm mutants produce flagellins with altered migration in SDS-polyacrylamide gels (30), these results suggest that FlmA, FlmB, FlmC, and FlmD could be involved in the glycosylation of flagellin monomers or other proteins involved in flagellin biogenesis.
TABLE 3.
Homology comparisons of the FlmA, FlmB, FlmC, FlmD, FlmE, FlmF, FlmG, and FlmH proteins
| Protein (amino acids) | Homolog/organism | % Identity/length of amino acid overlapa | Function (accession no.) |
|---|---|---|---|
| FlmA (332) | D protein/Methanococcus jannaschii | 41/330 | Capsular polysaccharide biosynthesis (U67549) |
| Cap8E/Staphylococcus aureus | 38/327 | Capsular polysaccharide biosynthesis (U73374) | |
| Cap5E/Staphylococcus aureus | 38/314 | Capsular polysaccharide biosynthesis (U81973) | |
| BpIL/Bordetella pertussis | 34/331 | LPS biosynthesis (X90711) | |
| CapD/Staphylococcus aureus | 34/327 | Capsular polysaccharide biosynthesis (P39853) | |
| TrsG/Yersinia enterocolitica | 31/330 | LPS biosynthesis (S51266) | |
| LpsB/Rhizobium etli | 34/227 | Putative dTDP-glucose 4,6-dehydratase (U56723) | |
| YveM/Bacillus subtilis | 35/270 | Hypothetical protein (Z71928) | |
| SpsJ/Bacillus subtilis | 23/313 | Spore coat polysaccharide biosynthesis (P39630) | |
| FlmB (386) | SpsC/Bacillus subtilis | 41/383 | Spore coat polysaccharide biosynthesis (P39623) |
| BplF/Bordetella pertussis | 39/383 | LPS biosynthesis (X90711) | |
| SpsC/Escherichia coli | 37/382 | Similar to B. subtilis SpsC protein (D90856) | |
| C protein/Methanococcus jannaschii | 35/386 | Spore coat polysaccharide biosynthesis (U67549) | |
| LmbS/Streptomyces lincolnensis | 35/384 | Lincomycin production (X79146) | |
| BplC/Bordetella pertussis | 36/353 | LPS biosynthesis (X90711) | |
| DegT/Bacillus stearothermophilus | 34/372 | Regulator of protease (M29002) | |
| SpsC/Synechocystis sp. | 35/345 | Spore coat polysaccharide biosynthesis (D90911) | |
| RfbE/Escherichia coli | 31/342 | Perosamine synthetase homolog (S83460) | |
| FlmC (238) | SpsF/Bacillus subtilis | 35/237 | Spore coat polysaccharide (P39626) |
| F protein/Methanococcus jannaschii | 31/238 | Spore coat polysaccharide biosynthesis (U67549) | |
| KdsB/Escherichia coli | 45/59 | CMP-KDO synthetase (P04951) | |
| KDO/Chlamydia trachomatis | 33/110 | CMP-KDO synthetase (U15192) | |
| KDO/Haemophilus influenzae | 24/109 | CMP-KDO synthetase (U32691) | |
| FlmD (330) | MurG/Mycobacterium tuberculosis | 29/170 | UDP-N-acetylglucosamine transferase (Z95388) |
| SpsH/Bacillus subtilis | 25/112 | Spore coat polysaccharide (P39628) | |
| G protein/Methanococcus jannaschii | 23/124 | Spore coat polysaccharide biosynthesis (U67549) | |
| FlmE (216) | TCMO/Streptomyces glaucescens | 39/51 | Tetracenomycin methyltransferase (M80674) |
| CobL/Rhodococcus sp. | 28/113 | Methyltransferase/Decarboxylase (L21196) | |
| ORFb/Mycobacterium tuberculosis | 22/206 | Unknown (Z80226) | |
| ORF/Erwinia herbicola | 32/56 | Similar to methyltransferase (AF006625) | |
| FlmF (421) | IaaM/Erwinia herbicola | 51/45 | Tryptophan monooxygenase (L33867) |
| Aux1/Agrobacterium rhizogenes | 41/42 | Tryptophan monooxygenase (Q09109) | |
| IaaM/Pseudomonas syringae pv. savastanoi | 41/37 | Tryptophan monooxygenase (P06617) | |
| IaaM/Pseudomonas syringae pv. syringae | 39/38 | Tryptophan monooxygenase (U04538) | |
| FlmG (597) | ORF MJ1345/Methanococcus jannaschii | 26/182 | Predicted coding sequence (U67574) |
| OGT/Homo sapiens | 25/165 | O-linked acetylglucosamine transferase (U77413) | |
| OGT/Rattus norvegicus | 25/165 | O-linked acetylglucosamine transferase (U76557) | |
| OGT/Caenorhabditis elegans | 24/165 | O-linked acetylglucosamine transferase (U77412) | |
| ORF/Synechocystis sp. | 26/185 | Hypothetical protein (D64003) | |
| FlmH (197) | SpeG/Escherichia coli | 25/180 | Diamine acetyltransferase (spermidine) (P37354) |
| YP20/Bacillus licheniformis | 27/172 | Unknown (PO5332) | |
| YdaF/Bacillus subtilis | 27/110 | Probable acetyltransferase (AB001488) | |
| AacA4/Serratia sp. | 21/171 | Aminoglycoside transferase (JC1322) | |
| RimJ/Escherichia coli | 20/174 | Ribosomal alanine acetyltransferase (P09454) | |
| AacA4/Klebsiella pneumoniae | 20/173 | Aminoglycoside acetyltransferase (P19650) | |
| AacA4/Serratia marcescens | 19/168 | Aminoglycoside acetyltransferase (P20092) | |
| ORF/Mycobacterium tuberculosis | 25/101 | Similar to E. coli RimJ (Z94752) | |
| AacA4/Pseudomonas aeruginosa | 18/173 | Acetyltransferase (X60321) |
The percentage of gap is less than 3% of the length of the amino acids sequences compared.
ORF, open reading frame.
The predicted FlmH protein shows significant levels of homology with acetyltransferases from several bacteria (Table 3) (8, 22, 49, 69, 78). Similarly, the FlmE gene product shows a low level of homology with several methyltransferases (Table 3) (15, 66). Also, the deduced amino acid sequence of the flmF gene shows a high level of homology (39 to 51% over a 38- to 45-amino-acid overlap) with tryptophan monooxygenases from Erwinia herbicola, Agrobacterium rhizogenes (13), and Pseudomonas syringae (43, 75). This homology extends from positions 4 to 48. More interestingly, a motif search revealed that FlmF contains a sugar transport signature—(LIVMSTAG) (LIVMFSAG) ×2 (LIVMSA) (DE) × (LIVMFYWA) G R (RK) ×6 (GSTA)—at residues 92 to 109 (Wisconsin Package version 9.0; Genetics Computer Group). Finally, the deduced FlmG product shows 24 to 25% identity over 165 amino acids to O-linked N-acetylglucosaminyltransferases from Homo sapiens, Rattus norvegicus, and Caernorhabditis elegans (Table 3) (33, 39). It is believed that this enzyme adds O-linked N-acetylglucosamine to transcription factors and nuclear pore proteins (39). A FlmG homolog has also been identified in H. pylori (GenBank accession no. AE000550) (68). Taken together, these results suggest that FlmA, FlmB, FlmC, FlmD, FlmE, FlmF, FlmG, and FlmH could be involved in glycosylation, acetylation, and/or methylation of flagellin subunits or proteins that interact with flagellins monomers prior to their assembly into a flagellar filament.
Effect of flagellar mutations on the expression of flmA, flmC, flmE, and flmG fused to cat.
Quon et al. (53) have proposed that CtrA binds directly to promoters containing the (TTAA-N7-TTAAC) consensus site to activate the flagellar regulatory hierarchy, to prevent replication of DNA, and to control DNA methylation and cell division. To test whether CtrA regulates the expression of the flmAB, flmCD, flmEF, and flmGH operons, plasmids pGL48 (flmC::cat), pGL49 (flmE::cat), pGL50 (flmA::cat), pGL53 (flmG::lacZ), pCS91 (rsaA::lacZ), and pWZ162 (fliQ::lacZ) were mated into strain LS2195, which contains a temperature-sensitive ctrA401 mutation. The resulting constructs were grown in PYE medium under the permissive condition (28°C) and then shifted to the restrictive condition (37°C) for 6 h. Cell extracts were prepared, and CAT and β-galactosidase activities were assayed (Table 4). As previously reported (53), expression of the fliQ::lacZ decreased about twofold in the ctrA401 background at the restrictive temperature, and the crystalline surface array protein promoter, rsaA::lacZ, was relatively unaffected by the ctrA401 mutation. However, the level of transcription of the rsaA::lacZ gene fusion showed a 2- to 2.5-fold increase at 37°C in both LS107 and LS2195 backgrounds (data not shown), suggesting that its expression is heat induced. More importantly, expression of the flmA::cat, flmE::cat, and flmG::lacZ gene fusion products was significantly (2.4- to 3.6-fold) reduced in the strain LS2195 (ctrA401) at the restrictive temperature. In contrast, flmC expression was relatively unaffected by the ctrA401 mutation at either temperature. These results suggest that CtrA positively regulates the flmA, flmE, and flmG promoters either directly or indirectly.
TABLE 4.
Effects of ctrA401 on flmA, flmC, flmE, and flmG transcription
| Plasmid | Promoter fusion | Activitya
|
|
|---|---|---|---|
| 28°C | 37°C | ||
| pGL50 | flmA::cat | 102 ± 11 | 28 ± 13 |
| pGL48 | flmC::cat | 95 ± 15 | 81 ± 33 |
| pGL49 | flmE::cat | 78 ± 12 | 30 ± 9 |
| pGL53 | flmG::lacZ | 117 ± 3 | 41 ± 2 |
| pWZ162 | fliQ::lacZ | 218 ± 2 | 133 ± 0.4 |
| pCS91 | rsaA::lacZ | 85 ± 5 | 119 ± 2 |
Strain LS2195 cells were grown to mid-log phase (120 to 150 Klett units) in PYE medium supplemented with tetracycline (1 μg/ml) under permissive (28°C) and restrictive (37°C) conditions. CAT and β-galactosidase specific activities were assayed and normalized to the level of activity found for control strain LS107. Data represent the mean ± standard deviation of duplicate samples from two independent experiments.
To determine the effect of class II and class III flagellar mutations on transcription of the flmAB, flmCD, flmEF, and flmGH promoters, various Tn5 insertion mutations in flagellar genes were introduced by transduction into strains SC3971, SC3973, SC3975, and SC4016, containing the integrated chromosomal fusions flmC::cat, flmE::cat, flmA::cat, and flmG::cat, respectively (see Materials and Methods). The expression of flmA, flmC, flmE, and flmG fused to cat was not altered more than twofold by a mutation in the rpoN gene (Table 5). Since the rpoN gene codes for the RNA polymerase sigma 54 subunit, these results indicate that the flm promoters are not transcribed by the sigma 54 holoenzyme. Therefore, they are not regulated like class III or IV flagellar genes. This conclusion is supported by the fact that mutations in other class II genes that greatly reduce class III and IV flagellar gene expression (3, 48, 74) cause only minor changes in the level of expression of the four genes (Table 5).
TABLE 5.
CAT activities of chromosomal flm-cat fusions in different fla mutants
| Genetic backgrounda | Flagellar mutation (class) | Relative sp actb
|
|||
|---|---|---|---|---|---|
| flmA::cat | flmC::cat | flmE::cat | flmG::cat | ||
| SC1029 | flhB (II) | 64 ± 27 | 46 ± 6 | 34 ± 3 | 121 ± 2 |
| SC1032 | flbD (II) | 77 ± 2 | 57 ± 4 | 46 ± 9 | 116 ± 2 |
| SC1055 | rpoN (II) | 142 ± 34 | 52 ± 5 | 75 ± 1 | 118 ± 8 |
| SC1066 | fliL (II) | 47 ± 8 | 52 ± 16 | 36 ± 3 | 100 ± 11 |
| SC1132 | flhA (II) | 107 ± 42 | 45 ± 22 | 44 ± 17 | 134 ± 24 |
| SC2663 | fliM (II) | 188 ± 25 | 89 ± 26 | ND | 207 ± 14 |
| SC3809 | flaS (II) | 89 ± 26 | 55 ± 12 | 42 ± 11 | 136 ± 6 |
| SC1117 | flgH (III) | 77 ± 32 | 120 ± 20 | 55 ± 7 | 85 ± 2 |
| SC1134 | flgK (III) | 80 ± 29 | 90 ± 15 | 60 ± 9 | 93 ± 25 |
| SC1135 | flbG (III) | 88 ± 20 | 53 ± 8 | 71 ± 12 | 125 ± 18 |
| SC1128 | flmA | ND | 84 ± 24 | 82 ± 5 | 92 ± 3 |
Strains were grown in PYE medium supplemented with kanamycin (50 μg/ml) at 30°C.
Normalized to a value of 100 for wild-type strain LS107. CAT activities were 498 ± 227, 197 ± 92, 500 ± 201, and 171 ± 58 cpm/μg of protein for flmA::cat, flmC::cat, flmE::cat, and flmG::cat gene fusions, respectively. CAT background activity for wild-type strain SC3844 was 7 ± 4 cpm/μg of protein. Values represent the mean ± standard deviation of duplicate samples from two or more independent experiments. ND, not determined.
It has been reported that the transcriptional activity of class II gene promoters increased about twofold in the presence of other class II mutations (64). In our study, the only significant increases in flm promoter expression were the flmA and flmG promoters in a fliM mutant background. Furthermore, in contrast to class II genes, mutations in the flmAB, flmCD, flmEF, and flmGH operons do not show defects in cell division. Previous studies have shown that mutations in the flmA, flmD, flmE, and flmH genes do not regulate class II (fliF and flhA), class III (flgE, flgK, and flbG), or class IV (fljK and fljL) flagellar genes (3, 48, 74). Thus, the four flm operons do not have the properties of the previously studied class II genes even though the expression of three of these flagellar operons is affected by a ctrA mutation. Taken together, these results indicate that the four flagellar operons represent a new class or classes of flagellar genes.
To test whether flmA, flmC, flmE, and flmG genes are autoregulated or involved in the same regulatory pathway, we measured their transcription in each of the flm mutant backgrounds (Table 6). Plasmids carrying transcriptional fusions of the flmA, flmC, flmE, and flmG promoters to cat or lacZ were introduced into flmA, flmD, flmE, and flmH mutant strains. Cell extracts of mid-logarithmic-phase cultures were prepared and assayed for cat or lacZ activity. Mutations in flmA, flmD, flmE, and flmH have no significant effect on flmA, flmC, flmE, and flmG gene expression. Identical results were observed when the expression of chromosomal flmC::cat, flmE::cat, and flmG::cat gene fusions was measured in the presence of the flmA (flaA104) mutation (Table 5). These results indicate that there is no autoregulation or regulatory interactions among the flmAB, flmCD, flmEF, and flmGH operons.
TABLE 6.
CAT activities of flm-cat fusions in various flm mutant backgrounds
| Genetic backgrounda | Relative sp actb
|
|||
|---|---|---|---|---|
| pGL50 (flmA::cat) | pGL48 (flmC::cat) | pGL49 (flmE::cat) | pGL53 (flmG::lacZ) | |
| flmA | 108 ± 2 | 97 ± 5 | 97 ± 1 | 125 ± 4 |
| flmD | 112 ± 3 | 93 ± 8 | 88 ± 24 | 145 ± 1 |
| flmE | 95 ± 15 | 100 ± 11 | 67 ± 25 | 124 ± 17 |
| flmH | 111 ± 14 | 92 ± 12 | 92 ± 16 | 115 ± 1 |
Strains were grown in PYE with tetracycline (1 μg/ml) at 30°C.
Normalized to a value of 100 for wild-type strain LS107. CAT activities were 945, 382, 686, and 14 cpm/μg of protein for strain LS107 harboring plasmids pGL48, pGL49, pGL50, and pGL53, respectively. β-Galactosidase activities were 795 and 45 Miller units for LS107(pGL53) and LS107(pRKlac290), respectively. Data represent the mean ± standard deviation of duplicate samples from two or more independent experiments.
Temporal regulation of the flmA, flmC, flmE, and flmG genes.
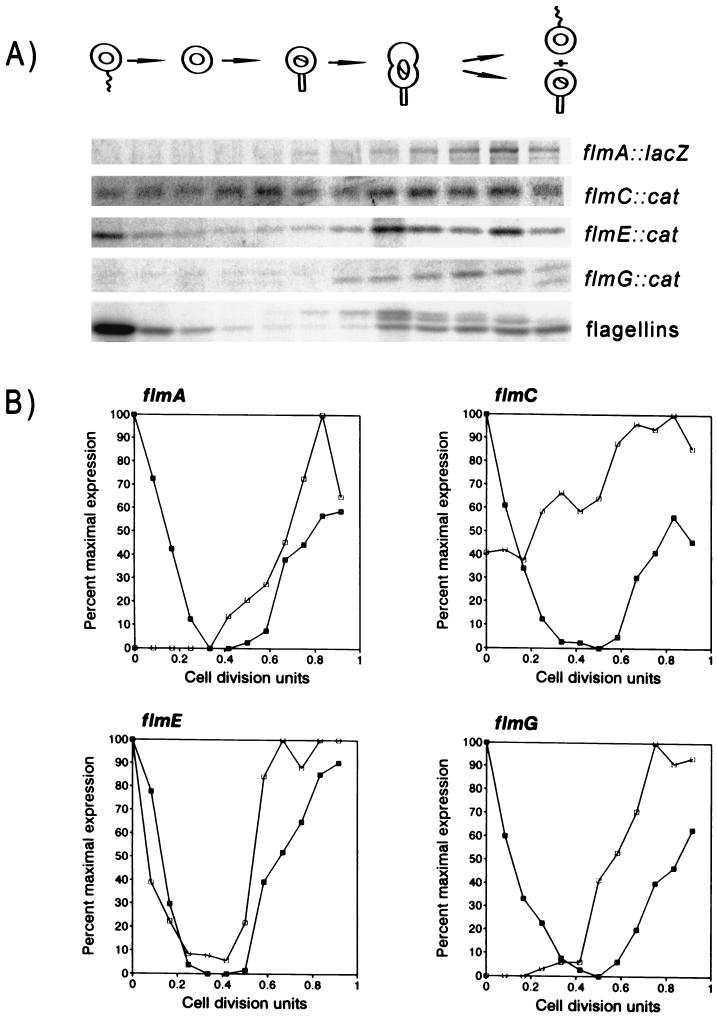
To determine whether expression of the flmA, flmC, flmE, and flmG genes is temporally regulated, strains containing a chromosomally inserted transcriptional cat fusion or a plasmid-borne lacZ fusion were synchronized and analyzed throughout the cell cycle. Expression of the flmA, flmE, and flmG operons occurred primarily in predivisional cells (Fig. 3). Transcription of both the flmA and flmG genes is very low or absent in swarmer cells and during the stalk-to-predivisional cell transition (0 to 0.5 cell division unit). It reaches a peak of expression in late predivisional cells (0.8 cell division unit) that coincides with the completion of filament assembly and the appearance of motility. The pattern of flmE expression is similar, but it shows a periodicity analogous to that observed for the 25-kDa flagellin where transcription continues in swarmer cells. In contrast, transcription of the flmC gene occurred throughout the cell cycle, with only twofold increase in predivisional cells (Fig. 3).
FIG. 3.
Cell cycle expression of the flmA, flmC, flmE, and flmG operons. Synchronized populations of Caulobacter strains SC3971, SC3973, SC4016, and SC4250 were pulse-labeled with [35S]methionine at 15-min intervals during the cell cycle. (A) Immunoprecipitation of labeled proteins with CAT, β-galactosidase, or flagellin antibodies. A cartoon showing progress through the cell cycle is shown at the top. The cell cycle-dependent expression of the flagellin genes is shown as a control. (B) Quantification of these data by using a Alpha Innotech photodocumentation system. Percentage of maximal expression of each sample is shown as a function of cell division units. One cell division unit is equivalent to a generation time of 180 min. Closed squares represent 25-kDa flagellin expression (recovered from SC3973 cells carrying the flmE::cat fusion); open squares represent expression of the flmA::lacZ, flmC::cat, flmE::cat, or flmG::cat fusion.
DISCUSSION
The regulation of flagellum biogenesis is complex, and many details remain to be elucidated. The results presented above demonstrate that the flm genes represent a new class of flagellar genes. DNA sequence analysis revealed that the flmAB, flmCD, and flmEF genes are structurally organized as operons. In each operon, the termination codon of the first gene overlaps the initiation codon of the second gene. DNA sequence analysis of the flmG and flmH genes confirmed that they also are organized in an operon as reported by Schoenlein et al. (59). However, in this case, the two coding regions are separated by 152 bp. The close spacing of the genes in the flmAB, flmCD, and flmEF operons suggests that translation of these operons may be governed by a translational coupling mechanism. In E. coli, there are many examples of translational coupling where the interruption of translation of the first gene causes a severe decrease in the expression of the translationally coupled distal gene (1, 52, 77). For translational coupling to occur, the efficient expression of the distal genes would be dependent on both translation of the first gene and termination of this translation in close proximity to the start codon for the second gene. Thus, translational coupling could be a mechanism to ensure equimolar synthesis of both proteins.
Homology searches of the deduced amino acid sequences revealed that FlmA, FlmB, FlmC, and FlmD have significant levels of identity with proteins involved in capsular, LPS, and spore coat polysaccharide biosynthesis from B. subtilis, M. jannaschii, and other bacteria. However, since FlmC also shared homology to the CMP-KDO synthetase from E. coli, C. trachomatis, and H. influenzae, these results suggested that these proteins could be involved in LPS biosynthesis. To test this hypothesis, we measured the KDO synthetase enzyme activity in flmA, flmD, flmE, flmG, and flmH mutants. Each mutant had wild-type levels of KDO synthetase activity and appeared to have wild-type LPS profiles (37). Thus, it does not appear that mutations in the flm genes affect LPS biosynthesis. The other Flm (FlmEFGH) proteins show homology to proteins involved in glycosylation, methylation, and/or acetylation in several bacteria (Table 3). Mutations in flmA, flmD, flmE, flmG, and flmH genes result in the production of a 22-kDa flagellin. Furthermore, we have shown that the 22-kDa protein results from a modification or a breakdown product of the 25-kDa flagellin proteins (20). Glycosylation of flagellin proteins has been reported for Campylobacter (17), Spirochaeta aurantia (10), some archaea (35, 63, 72), and Azospirillum brasilense (46). Azospirillum contains flmAB homologs, and a mutation in one of these genes prevents assembly of the flagellar filament (45). In addition, Wieland et al. (72) have suggested that in halobacteria, glycosylation of the flagellins was necessary for proper incorporation of the flagella into the cell envelope and that overproduction of flagellins resulted in subunits with lower molecular weights. In Caulobacter, the 22-kDa flagellin is present in a flbT mutant that overproduces flagellins (57). Flagellins also can be modified by methylation (2, 12, 36, 38), phosphorylation (31), and sulfation (36, 72). Strains containing mutations in flmA, flmD, flmE, flmG, and flmH genes have a normal basal body and hook structure but fail to assemble a flagellar filament (30). Therefore, our current hypothesis is that modification of the flagellin subunits or some other flagellar proteins by glycosylation, acetylation, and methylation is required for proper assembly of flagellin subunits into the filament. Clearly, this hypothesis has important implications for the structure and mechanism of assembly of the flagellar filament. Recently, we have determined the nucleotide sequences of five of the six flagellin genes in C. crescentus (20). Analysis of deduced amino acids indicated that there is a discrepancy between the calculated molecular weight and the actual mass determined by mass spectroscopy (37).
It has been demonstrated in E. coli (32), Salmonella typhimurium (34), and C. crescentus (14, 48, 74) that a cascade of positive and negative transcriptional control regulates the temporal expression of flagellar genes. Previously, the flmAB, flmCD, flmEF, and flmGH operons had been placed in class III in the flagellar gene regulatory hierarchy (48). However, the experiments presented in this report demonstrate that the flm operons represent a new class of flagellar genes. First, we have shown that none of the flm operons require the RNA polymerase sigma factor 54 for transcription, indicating that they are not class III or IV genes (Table 5). Second, we have shown that flmAB, flmEF, and flmGH are positively regulated by CtrA (Table 4), a transcriptional response regulator that controls class II flagellar genes (53). However, in contrast to class II flagellar genes, mutations in flmAB, flmCD, flmEF, and flmGH operons do not cause defects in cell division. In addition, previous studies (3, 48, 74) have shown that the flmA, flmD, flmE, and flmG genes do not regulate transcription of genes from class II (fliF and flhA), class III (flgE, flgK, and flbG), or class IV (fljK and fljL). Taken together, we conclude that the flmAB, flmCD, flmEF, and flmGH operons belong to a new class of flagellar genes.
It has been shown that synthesis of the flagellin subunits encoded by the class IV genes is subject to posttranscriptional control mechanisms (3, 41, 42). Anderson and Newton (3) showed that both fljK::lacZ transcriptional and translational fusions were expressed at nearly wild-type levels in strains carrying mutations in flmA, flmD, or flmH. Nevertheless, immunoprecipitation experiments measuring short (30-s or 1-min) pulses of flagellin protein synthesis demonstrated that mutations in these genes do result in reduced levels of flagellin synthesis (30). Our current hypothesis is that this reduced level of flagellin synthesis may be due to a feedback mechanism involving unassembled flagellin subunits rather than any direct action involving the flm gene products. Furthermore, since we have shown that the FlbT product regulates flagellin synthesis by altering mRNA stability (42), it is likely that the effects of flm mutations on flagellin gene expression involve mRNA stability as well.
ACKNOWLEDGMENTS
We thank Gilles Leclerc for stimulating and helpful discussion. We also thank William B. Crymes for critical reading of the manuscript and Nelida Caballero and Bonsung Koo for expert technical assistance.
This work was supported by NIH grants GM50547 and GM34765 to B. Ely.
REFERENCES
- 1.Aksoy S, Squires C L, Squires C. Translational coupling of the trpB and trpA genes in the Escherichia coli tryptophan operon. J Bacteriol. 1984;157:363–367. doi: 10.1128/jb.157.2.363-367.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambler R P, Rees M W. ɛ-N-Methyl-lysine in bacterial flagellar protein. Nature. 1959;184:56–57. doi: 10.1038/184056b0. [DOI] [PubMed] [Google Scholar]
- 3.Anderson D K, Newton A. Posttranscriptional regulation of Caulobacter flagellin genes by a late flagellum assembly checkpoint. J Bacteriol. 1997;179:2281–2288. doi: 10.1128/jb.179.7.2281-2288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagdasarian M, Lurz R, Ruckert B, Franklin F C, Bagdasarian M M, Frey J, Timmis K N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981;16:237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- 5.Barth P T. RP4 and R300B as wide host-range plasmid cloning vehicles. In: Timmis K N, Puhler A, editors. Plasmids of medical, environmental and commercial importance. Amsterdam: Elsevier/North-Holland Biomedical Press; 1979. pp. 399–410. [Google Scholar]
- 6.Bender R A, Refson C M, O’Neill E A. Role of the flagellum in the cell-cycle-dependent expression of bacteriophage receptor activity in Caulobacter crescentus. J Bacteriol. 1989;171:1035–1046. doi: 10.1128/jb.171.2.1035-1040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benson A K, Wu J, Newton A. The role of FlbD in regulation of flagellar gene transcription in Caulobacter crescentus. Res Microbiol. 1994;12:420–430. doi: 10.1016/0923-2508(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 8.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, et al. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 9.Bradford M M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Brahamsha B, Greenberg E P. A biochemical and cytological analysis of the complex periplasmic flagella from Spirochaeta aurantia. J Bacteriol. 1988;170:4023–4032. doi: 10.1128/jb.170.9.4023-4032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brun Y V, Shapiro L. A temporally controlled sigma-factor is required for polar morphogenesis and normal cell division in Caulobacter. Genes Dev. 1992;6:2395–2408. doi: 10.1101/gad.6.12a.2395. [DOI] [PubMed] [Google Scholar]
- 12.Burnens A P, Stanley J, Sack R, Hunziker P, Brodard I, Nicolet J. The flagellin N-methylase gene fliB and an adjacent serovar-specific IS200 element in Salmonella typhimurium. Microbiology. 1997;143:1539–1547. doi: 10.1099/00221287-143-5-1539. [DOI] [PubMed] [Google Scholar]
- 13.Camilleri C, Jouanin L. The TR-DNA region carrying the auxin synthesis genes of the Agrobacterium rhizogenes agropine-type plasmid pRiA4: nucleotide sequence analysis and introduction into tobacco plants. Mol Plant Microbe Interact. 1991;4:155–162. doi: 10.1094/mpmi-4-155. [DOI] [PubMed] [Google Scholar]
- 14.Champer R A, Dingwall A, Shapiro L. Cascade regulation of Caulobacter flagellar and chemotaxis genes. J Mol Biol. 1987;194:71–80. doi: 10.1016/0022-2836(87)90716-9. [DOI] [PubMed] [Google Scholar]
- 15.De Mot R, Nagy I, Schoofs G, Vanderleyden J. Sequences of the cobalamin biosynthetic genes cobK, cobL and cobM from Rhodococcus sp. NI86/21. Gene. 1994;143:91–93. doi: 10.1016/0378-1119(94)90610-6. [DOI] [PubMed] [Google Scholar]
- 16.Devereux D, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doig P, Kinsella N, Guerry P, Trust T J. Characterization of a post-translational modification of Campylobacter flagellin: identification of a sero-specific glycosyl moiety. Mol Microbiol. 1996;19:379–387. doi: 10.1046/j.1365-2958.1996.370890.x. [DOI] [PubMed] [Google Scholar]
- 18.Driks A, Bryan R, Shapiro L, DeRosier D J. The organization of the Caulobacter crescentus flagellar filament. J Mol Biol. 1989;206:627–636. doi: 10.1016/0022-2836(89)90571-8. [DOI] [PubMed] [Google Scholar]
- 19.Ely B, Ely T. Use of pulsed field gel electrophoresis and transposon mutagenesis to estimate number of genes required for motility in Caulobacter crescentus. Genetics. 1989;123:649–654. doi: 10.1093/genetics/123.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ely, B., S. Minnich, and T. Ely. Unpublished data.
- 21.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 22.Galimand M, Lambert T, Gerbaud G, Courvalin P. Characterization of the aac(6′)-lb gene encoding an aminoglycoside 6′-N-acetyltransferase in Pseudomonas aeruginosa BM2656. Antimicrob Agents Chemother. 1993;37:1456–1462. doi: 10.1128/aac.37.7.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilchrist A, Smit J. Transformation of fresh water and marine caulobacters by electroporation. J Bacteriol. 1991;173:921–925. doi: 10.1128/jb.173.2.921-925.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gober J W, Marques M V. Regulation of cellular differentiation in Caulobacter crescentus. Microbiol Rev. 1995;59:31–47. doi: 10.1128/mr.59.1.31-47.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gober J W, Shapiro L. Integration host factor is required for the activation of developmentally regulated genes in Caulobacter. Genes Dev. 1990;4:1494–1504. doi: 10.1101/gad.4.9.1494. [DOI] [PubMed] [Google Scholar]
- 26.Gober J W, Shapiro L. A developmentally regulated Caulobacter flagellar promoter is activated by 3′ enhancer and IHF binding elements. Mol Biol Cell. 1992;3:913–926. doi: 10.1091/mbc.3.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomes S L, Shapiro L. Differential expression and positioning of chemotaxis methylation proteins in Caulobacter. J Mol Biol. 1984;177:551–568. doi: 10.1016/0022-2836(84)90238-9. [DOI] [PubMed] [Google Scholar]
- 28.Johnson R C, Ely B. Isolation and spontaneously-derived mutants from Caulobacter crescentus. Genetics. 1977;86:25–32. doi: 10.1093/genetics/86.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson R C, Ely B. Analysis of nonmotile mutants of the dimorphic bacterium Caulobacter crescentus. J Bacteriol. 1979;137:627–634. doi: 10.1128/jb.137.1.627-634.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson R C, Ferber D M, Ely B. Synthesis and assembly of flagellar components by Caulobacter crescentus motility mutants. J Bacteriol. 1983;154:1137–1144. doi: 10.1128/jb.154.3.1137-1144.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly-Wintenberg K, South S L, Montie T C. Tyrosine phosphate in a- and b-type flagellins of Pseudomonas aeruginosa. J Bacteriol. 1993;175:2458–2461. doi: 10.1128/jb.175.8.2458-2461.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komeda Y. Transcriptional control of flagellar genes in Escherichia coli K-12. J Bacteriol. 1986;168:1315–1318. doi: 10.1128/jb.168.3.1315-1318.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreppel L K, Blomberg M A, Hart G W. Dynamic glycosylation of nuclear and cytosolic protein. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem. 1997;272:9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 34.Kutsukake K, Nakao T, Iino T. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J Bacteriol. 1990;172:741–747. doi: 10.1128/jb.172.2.741-747.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lechner J, Wieland F. Structure and biosynthesis of prokaryotic glycoproteins. Annu Rev Biochem. 1989;58:173–194. doi: 10.1146/annurev.bi.58.070189.001133. [DOI] [PubMed] [Google Scholar]
- 36.Lechner J, Wieland F, Sumper M. Transient methylation of dolichyl oligosaccharides is an obligatory step in halobacterial sulfated glycoprotein biosynthesis. J Biol Chem. 1985;260:8984–8989. [PubMed] [Google Scholar]
- 37.Leclerc, G., and B. Ely. Unpublished data.
- 38.Logan S M, Trust T J, Guerry P. Evidence for posttranslational modification and gene duplication of Campylobacter flagellin. J Bacteriol. 1989;171:3031–3038. doi: 10.1128/jb.171.6.3031-3038.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lubas W A, Frank D W, Krause M, Hanover J A. O-linked GlcNActransferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J Biol Chem. 1997;272:9316–9324. doi: 10.1074/jbc.272.14.9316. [DOI] [PubMed] [Google Scholar]
- 40.Malakooti J, Ely B. Identification and characterization of the ilvR gene encoding a LysR-type regulator of Caulobacter crescentus. J Bacteriol. 1994;176:1275–1281. doi: 10.1128/jb.176.5.1275-1281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangan E K, Bartamian M, Gober J W. A mutation that uncouples flagellum assembly from transcription alters the temporal pattern of flagellar gene expression in Caulobacter crescentus. J Bacteriol. 1995;177:3176–3184. doi: 10.1128/jb.177.11.3176-3184.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mangan, E. K., J. Malakooti, A. Caballero, P. Anderson, B. Ely, and J. W. Gober. Unpublished data. [DOI] [PMC free article] [PubMed]
- 43.Mazzola M, White F F. A mutation in the indole-3-acetic acid biosynthesis pathway of Pseudomonas syringae pv. syringae affects growth in Phaseolus vulgaris and syringomycin production. J Bacteriol. 1994;176:1374–1382. doi: 10.1128/jb.176.5.1374-1382.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 45.Moens, S. Personal communication.
- 46.Moens S, Michiels K, Vanderleyden J. Glycosylation of the flagellin of the polar flagellum of Azospirillum brasilense, a Gram-negative nitrogen-fixing bacterium. Microbiology. 1995;141:2651–2657. [Google Scholar]
- 47.Mullin D A, Newton A. Ntr-like promoters and upstream regulatory sequence ftr are required for transcription of a developmentally regulated Caulobacter crescentus flagellar gene. J Bacteriol. 1989;171:3218–3227. doi: 10.1128/jb.171.6.3218-3227.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newton A, Ohta N, Ramakrishnan G, Mullin D, Raymond G. Genetic switching in the flagellar gene hierarchy of Caulobacter requires negative as well as positive regulation of transcription. Proc Natl Acad Sci USA. 1989;86:6651–6655. doi: 10.1073/pnas.86.17.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nobuta K, Tolmasky M E, Crosa L M, Crosa J H. Sequencing and expression of the 6′-N-acetyltransferase gene of transposon Tn1331 from Klebsiella pneumoniae. J Bacteriol. 1988;170:3769–3773. doi: 10.1128/jb.170.8.3769-3773.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohta N, Mullin D A, Tarleton J, Ely B, Newton A. Identification, distribution, and sequence analysis of new insertion elements in Caulobacter crescentus. J Bacteriol. 1990;172:236–242. doi: 10.1128/jb.172.1.236-242.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohta N, Swanson E, Ely B, Newton A. Physical mapping and complementation analysis of transposon Tn5 mutations in Caulobacter crescentus: organization of transcriptional units in the hook gene cluster. J Bacteriol. 1984;158:897–904. doi: 10.1128/jb.158.3.897-904.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oppenheim D S, Yanofsky C. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics. 1980;95:785–795. doi: 10.1093/genetics/95.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quon K C, Marczynski G T, Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell. 1996;84:83–93. doi: 10.1016/s0092-8674(00)80995-2. [DOI] [PubMed] [Google Scholar]
- 54.Roberts R C, Mohr C D, Shapiro L. Developmental programs in bacteria. Curr Top Dev Biol. 1996;43:207–257. doi: 10.1016/s0070-2153(08)60712-7. [DOI] [PubMed] [Google Scholar]
- 55.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 56.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schoenlein P V, Ely B. Characterization of strains containing mutations in the contiguous flaF, flbT, or flbA-flaG transcription units and the identification of a novel Fla phenotype in Caulobacter crescentus. J Bacteriol. 1989;171:1554–1561. doi: 10.1128/jb.171.3.1554-1561.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schoenlein P V, Gallman L S, Ely B. Use of transmissible plasmids as cloning vectors in Caulobacter crescentus. Gene. 1988;70:321–329. doi: 10.1016/0378-1119(88)90204-1. [DOI] [PubMed] [Google Scholar]
- 59.Schoenlein P V, Gallman L, Ely B. Organization of the flaFG gene cluster and identification of two additional genes involved in flagellum biogenesis in Caulobacter crescentus. J Bacteriol. 1989;171:1544–1553. doi: 10.1128/jb.171.3.1544-1553.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schoenlein P V, Gallman L S, Winkler M E, Ely B. Nucleotide sequence analysis of the Caulobacter crescentus flaF and flbT genes and an analysis of the codon usage in organisms with G + C-rich genomes. Gene. 1990;92:17–25. doi: 10.1016/0378-1119(90)90130-j. [DOI] [PubMed] [Google Scholar]
- 61.Schoenlein P V, Lui J, Gallman L, Ely B. The Caulobacter crescentus flaFG region regulates synthesis and assembly of flagellin proteins encoded by two genetically unlinked gene clusters. J Bacteriol. 1992;174:6046–6053. doi: 10.1128/jb.174.19.6046-6053.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simon R, Preifer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 63.Southam G, Kalmokoff M L, Jarell K F, Koval S F, Beveridge T J. Isolation, characterization, and cellular insertion of the flagella from two strains of the archaebacterium Methanospirillum hungatei. J Bacteriol. 1990;172:3221–3228. doi: 10.1128/jb.172.6.3221-3228.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stephens C, Mohr C, Boyd C, Maddock J, Gober J, Shapiro L. Identification of the fliI and fliJ components of the Caulobacter flagellar type III protein secretion system. J Bacteriol. 1997;179:5355–5365. doi: 10.1128/jb.179.17.5355-5365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stephens C M, Shapiro L. An unusual promoter controls cell-cycle regulation and dependence on DNA replication of the Caulobacter fliLM early flagellar operon. Mol Microbiol. 1993;9:1169–1179. doi: 10.1111/j.1365-2958.1993.tb01246.x. [DOI] [PubMed] [Google Scholar]
- 66.Summers R G, Wendt-Pienkowski E, Motamedi H, Hutchinson C R. Nucleotide sequence of the tcmII-tcmIV region of the tetracenomycin C biosynthetic gene cluster of Streptomyces glaucescens and evidence that the tcmN gene encodes a multifunctional cyclase-dehydratase-O-methyl transferase. J Bacteriol. 1992;174:1810–1820. doi: 10.1128/jb.174.6.1810-1820.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tipples G, McClarty G. Cloning and expression of the Chlamydia trachomatis gene for CTP synthetase. J Biol Chem. 1995;270:7908–7914. doi: 10.1074/jbc.270.14.7908. [DOI] [PubMed] [Google Scholar]
- 68.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 69.Tran van Nhieu G, Collatz E. Primary structure of an aminoglycoside 6′-N-acetyltransferase AAC(6′)-4, fused in vivo with the signal peptide of the Tn3-encoded beta-lactamase. J Bacteriol. 1987;169:5708–5714. doi: 10.1128/jb.169.12.5708-5714.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang S P, Sharma P L, Schoenlein P V, Ely B. A histidine protein kinase is involved in polar organelle development in Caulobacter crescentus. Proc Natl Acad Sci USA. 1993;90:630–634. doi: 10.1073/pnas.90.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weissborn A H, Steinman H M, Shapiro L. Characterization of the proteins of the Caulobacter crescentus flagellar filament. J Biol Chem. 1982;257:2066–2074. [PubMed] [Google Scholar]
- 72.Wieland F, Paul G, Sumper M. Halobacterial flagellins are sulfated glycoproteins. J Biol Chem. 1985;260:15180–15185. [PubMed] [Google Scholar]
- 73.Wingrove J A, Mangan E K, Gober J W. Spatial and temporal phosphorylation of a transcriptional activator regulates pole-specific gene expression in Caulobacter. Genes Dev. 1993;7:1979–1992. doi: 10.1101/gad.7.10.1979. [DOI] [PubMed] [Google Scholar]
- 74.Xu H, Dingwall A, Shapiro L. Negative transcriptional regulation in the Caulobacter flagellar hierarchy. Proc Natl Acad Sci USA. 1989;86:6656–6660. doi: 10.1073/pnas.86.17.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamada T, Palm C J, Brooks B, Kosuge T. Nucleotide sequences of the Pseudomonas savastanoi indoleacetic acid genes show homology with Agrobacterium tumefaciens T-DNA. Proc Natl Acad Sci USA. 1985;82:6522–6526. doi: 10.1073/pnas.82.19.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 77.Yates J L, Nomura M. Feedback regulation of ribosomal protein synthesis in E. coli: localization of the mRNA target sites for repressor action of ribosomal protein L1. Cell. 1981;24:243–249. doi: 10.1016/0092-8674(81)90520-1. [DOI] [PubMed] [Google Scholar]
- 78.Yoshikawa A, Isono S, Sheback A, Isono K. Cloning and nucleotide sequencing of the genes rimI and rimJ which encode enzymes acetylating ribosomal proteins S18 and S5 of Escherichia coli K12. Mol Gen Genet. 1987;209:481–488. doi: 10.1007/BF00331153. [DOI] [PubMed] [Google Scholar]
- 79.Zhuang W Y, Shapiro L. Caulobacter FliQ and FliR membrane proteins, required for flagellar biogenesis and cell division, belong to a family of virulence factor export proteins. J Bacteriol. 1995;177:343–356. doi: 10.1128/jb.177.2.343-356.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]