Summary
Background
Interferon-γ release assays (IGRAs), which are widely used to diagnose tuberculosis (TB), cannot effectively discriminate latent TB infection (LTBI) from active TB (ATB). This study aimed to identify potential antigen-specific biomarkers for differentiating LTBI cases from ATB cases.
Methods
Ongoing recruitment was conducted of individuals meeting study inclusion criteria at Beijing Chest Hospital from May 2020 to April 2022; 208 participants were enrolled and assigned to three groups: HC (60 healthy controls), LTBI (52 subjects with LTBI) and ATB (96 ATB patients). After participants were assigned to the discovery cohort (20 or 21 subjects/group), all others were assigned to the verification cohort. Discovery cohort blood levels of 40 chemokines were measured using Luminex assays to identify chemokines that could be used to discriminate LTBI cases from ATB cases; candidate biomarkers were verified using enzyme-linked immunosorbent assay-based testing of validation cohort samples.
Results
Luminex results revealed highest ATB group levels of numerous cytokines, growth factors and chemokines. Receiving operating characteristic curve-based analysis of 40 biomarkers revealed CCL8 (AUC = 0.890) and CXCL9 (AUC = 0.883) effectively discriminated between LTBI and TB cases; greatest diagnostic efficiency was obtained using both markers together (AUC = 0.929). Interpretation of CCL8 and CXCL9 levels for validation cohort IGRA-positive subjects (based on a 0.658-ng/ml cutoff) revealed ATB group CCL8-based sensitivity and specificity rates approaching 90.79% and 100.00%, respectively.
Conclusion
TB-specific chemokines hold promise as ATB diagnostic biomarkers. Additional laboratory confirmation is needed to establish whether CCL8-based assays can differentiate between ATB and LTBI cases, especially for bacteriologically unconfirmed TB cases.
Introduction
Tuberculosis (TB), a human disease caused by Mycobacterium tuberculosis (MTB) complex, remains one of the most common causes of death from an infectious disease.1 In fact, 2022 World Health Organisation (WHO) report findings indicate that in 2021 there were 10.6 million incident TB cases and 1.6 million deaths from the disease worldwide.2 Of great concern, results of another study suggest that an estimated 4.2 million active TB (ATB) cases escaped detection by global health systems and thus are likely undermining global TB control efforts by preventing control of TB transmission within communities.3 A contributing cause of this issue is delayed diagnosis, which has been mainly attributed to inadequate patient access to diagnostic services and deficient diagnostic detection of ATB cases.4 Thus, more effective service delivery and novel diagnostic tools are urgently needed to meet global goals for eliminating TB by 2035.
Current TB diagnostic tests include mycobacterial culture, which requires weeks or months for completion,5 as well as more recently developed nucleic acid amplification-based rapid tests (e.g. Xpert MTB/RIF, also known as Xpert), which are widely used to supplement conventional methods for diagnosing ATB cases.6 Despite Xpert’s high accuracy rate and endorsement by the WHO,7 Xpert sensitivity is low when used to test paucibacillary specimens, including those obtained from patients with smear-negative pulmonary TB and various forms of extrapulmonary TB.8,9 To address this issue, interferon-γ release assays (IGRAs), immunological tests that detect MTB antigen-specific T cells, have been used to assist clinicians in achieving accurate TB diagnoses, especially for suspected TB cases without aetiological evidence of MTB infection.10 However, IGRAs cannot effectively differentiate between latent TB infection (LTBI) and ATB and thus are viewed as inadequate tools for detecting ATB cases from a clinical standpoint.
Numerous attempts have been made to identify new high-performing diagnostic biomarkers that can stratify TB cases according to active vs. latent disease status.11,12 Consequently, blood-based gene transcriptional signatures have been identified that hold great promise as biomarkers for use in diagnosing ATB cases and predicting progression of LTBI to ATB disease.13 However, most of these biomarker candidates participate not only in the host defence against invasive tubercle bacilli, but also in host defences against numerous other pathogens, thus raising the concern that individuals with non-MTB infections will be at increased risk of receiving false-positive results when tested using TB diagnostic assays based on detection of the abovementioned biomarkers. To address this issue, alternate strategies based on differences in MTB-specific immune responses between LTBI cases and ATB cases may be useful for discriminating between patients with latent and ATB. In this study, we investigated the abilities of 40 potential antigen-specific biomarkers to differentiate between LTBI and ATB cases. Further experimental tests were conducted using specimens obtained from an independent cohort to validate diagnostic accuracies of biomarker candidates when used for this purpose.
Materials and methods
Study design
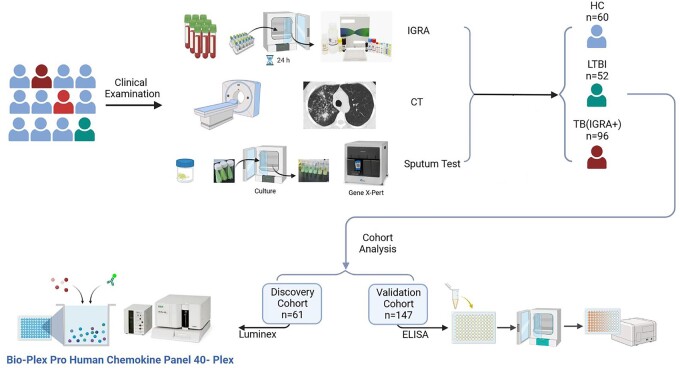
Ongoing enrolment of patients with ATB symptoms was conducted at Beijing Chest Hospital from May 2020 to April 2022. As shown in Figure 1, prospective study subjects were screened for ATB disease based on results of comprehensive testing that included chest computed tomography (CT) scans, sputum MTB culture, sputum Xpert (Cepheid, Sunnyvale, USA) and IGRA-based testing of blood plasma samples (Leide, Guangzhou, China). After IGRA-negative TB patients were excluded, participants were assigned to three groups designated HC (healthy control subjects), LTBI and ATB (Table 1). Next, 20 or 21 participants randomly selected from the three groups were assigned to the discovery cohort and the remaining participants were assigned to the validation cohort. Thereafter, blood plasma cytokine levels of discovery cohort participants were measured using Luminex assays to tentatively identify cytokine-based biomarkers that could be used to discriminate between LTBI and ATB cases. Candidate cytokines were next verified for the abovementioned discriminatory ability using enzyme-linked immunosorbent assay (ELISA)-based testing of validation cohort blood samples.
Figure 1.
The workflow of this study. CT, computed tomography; IGRA, interferon-γ release assays; TB, tuberculosis; LTBI, latent tuberculosis infection; ELISA, enzyme-linked immunosorbent assay.
Table 1.
Participant grouping definition
| Classification | Definitions |
|---|---|
| HC | Normal chest radiography without clinical symptoms suggestive of active TB; IGRA-negative |
| LTBI | Normal chest radiography without clinical symptoms suggestive of active TB; IGRA-positive |
| TB | Chest CT with clinical symptoms suggestive of active TB; Sputum Mtb culture positive; Sputum GeneXpert MTB/RIF positive; IGRA-positive |
HC, healthy control; LTBI, latent TB infection; TB, tuberculosis; IGRA, IFN-γ release assays; Mtb, Mycobacterium tuberculosis.
IGRA method
Fresh peripheral whole blood specimens were collected in blood collection tubes containing heparin anticoagulant (Becton, Dickinson and Co., USA) and processed according to the manufacturer’s instructions within 2 h (Leide, Guangzhou, China). Thereafter, processed samples were added to stimulation tubes containing MTB antigen then tubes were incubated at 37°C in an incubator containing 5% CO2 for 20 ± 2 h. Next, tubes were centrifuged (1000 × g) for 5 min then the upper plasma layer was collected. Plasma IFN-γ concentrations were next measured using an ELISA kit (Leide, Guangzhou, China), with interpretation of test results conducted based on the manufacturer’s suggested cut-off value for a positive result (≥20 pg/ml).
Multiplex chemokine assay
Plasma chemokine concentrations were measured using the Bio-Plex Pro Human Chemokine Panel 40-plex assay kit (Bio-Rad, CA, USA). The 40-plex panel included 6Ckine/CCL21, BCA-1/CXCL13, CTACK/CCL27, ENA-78/CXCL5, Eotaxin/CCL11, Eotaxin-2/CCL24, Eotaxin-3/CCL26, Fractalkine/CX3CL1, GCP-2/CXCL6, GM-CSF, Gro-α/CXCL1, Gro-β/CXCL2, I-309/CCL1, IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8/CXCL8, IL-10, IL-16, IP-10/CXCL10, I-TAC/CXCL11, MCP-1/CCL2, MCP-2/CCL8, MCP-3/CCL7, MCP-4/CCL13, MDC/CCL22, MIF, MIG/CXCL9, MIP-1α/CCL3, MIP-1δ/CCL15, MIP-3α/CCL20, MIP-3β/CCL19, MPIF-1/CCL23, SCYB16/CXCL16, SDF-1α + β/CXCL12, TARC/CCL17, TECK/CCL25 and TNF-α. Samples were incubated in 96-well plates containing corresponding substrates for 1 h. Next, detection antibody was added to wells then plates were incubated for an additional 30 min. Thereafter, streptavidin coupled with phycoerythrin (PE) fluorescence marker were added to wells then plates were incubated for an additional 10 min. Finally, fluorescence levels of experimental and standard samples were measured using the Bio-Plex 200 system (Luminex Corp., Austin, TX, USA).
Plasma CCL8 and CXCL9 testing
Plasma CCL8 and CXCL9 levels were measured using commercial sandwich ELISAs (QuantiCyto® Human MCP-2/CCL8 and QuantiCyto® Human MIG/CXCL9 ELISA kits, NeoBioscience, Guangzhou, China) according to the manufacturer’s instructions.
Statistical analysis
All statistical analyses were performed using SPSS version 22.0 and GraphPad Prism 9.0 software. Continuous variables were expressed as median (range) values and categorical variables were expressed as percent values (%). One-way ANOVA was conducted to compare differences in chemokine levels between pairs of groups then the diagnostic performance of each chemokine was evaluated based on analysis of receiving operating characteristic (ROC) curves. Optimal sensitivity and specificity rates obtained for the various chemokines were estimated using Youden’s index. The proportion of correctly diagnosed patients for each chemokine was calculated based on proportionality to the area-under-the-curve (AUC) value. Differences between groups were declared significant for results with two-sided P-values of <0.05.
Results
Study population
A total of 208 participants were enrolled in the study. Participants were classified into three groups that included 60 subjects in the HC group, 52 subjects in the LTBI group and 96 subjects in the ATB group. Next, 20–21 subjects from each group were assigned to the discovery cohort and the remaining subjects (40 HC, 32 LTBI and 76 ATB subjects) were assigned to the validation cohort. The average age of HC group members was 35 years and most members were female. Similarly, the number of LTBI group females, who were assigned to this group based on IGRA results and clinical examination findings, slightly exceeded the number of males, while the average age of LTBI group members was 40 years. For the ATB group, the average age of members was 42 years and males outnumbered females. Clinical examination findings revealed that 53 ATB group patients with positive IGRA results had ATB, as based on CT scan findings indicative of ATB disease, while 51 of these patients were culture positive for MTB. The most common comorbid diseases afflicting ATB patients were liver disease and diabetes mellitus (Table 2).
Table 2.
Clinical and demographic characteristics of participants
| Patient characteristics | HC (n = 60) | LTBI (n = 52) | TB (n = 96) | Total (n = 208) |
|---|---|---|---|---|
| Age in years, median (IQR) | 35 (30–40) | 40 (36–46) | 42 (26–56) | 40 (30–48) |
| Sex, n (%) | ||||
| Male | 23 (38.46) | 23 (44.44) | 52 (53.95) | 98 (41.12) |
| Female | 37 (61.54) | 29 (55.56) | 44 (46.05) | 110 (52.88) |
| Clinical examination | ||||
| IGRA positive | 60 | 52 | 96 | 208 |
| CT positive | – | – | 53 | 53 |
| Culture positive | – | – | 51 | 51 |
| Xpert positive | – | – | 71 | 71 |
| Complication | ||||
| Diabetes mellitus | – | – | 15 | 15 |
| Liver disease | – | – | 27 | 27 |
| Kidney disease | – | – | 1 | 1 |
| Hypertension/CHD | – | – | 7 | 7 |
| Cerebral infarction | – | – | 3 | 3 |
HC, healthy control; LTBI, latent TB infection; TB, tuberculosis; CT, computed tomography; IGRA, IFN-γ release assays.
Comparisons of discovery cohort chemokines levels
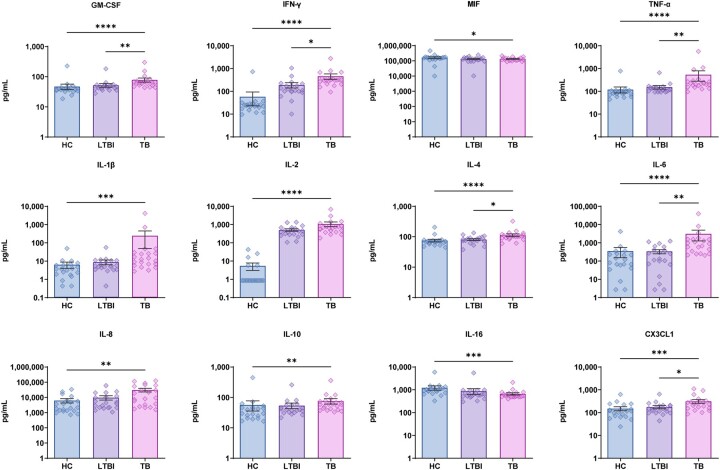
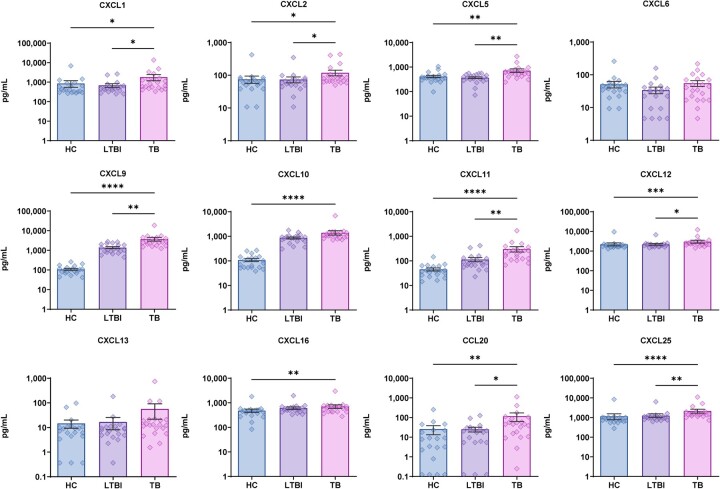
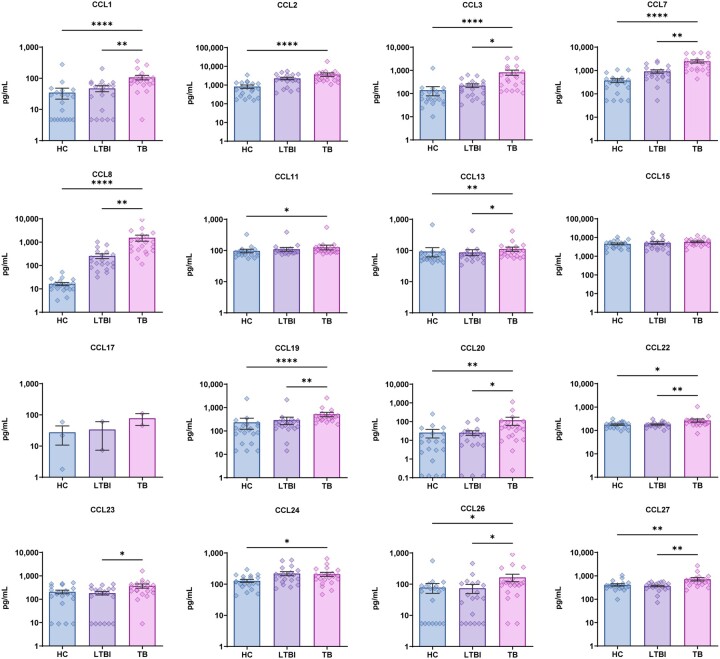
We first measured discovery cohort plasma levels of chemokines after specific stimulation of patient peripheral white blood cells with MTB antigen (Figures 2–4). Pairwise intergroup comparisons of results revealed that only ATB group levels of anti-inflammatory cytokines MIF and IL-10 were significantly lower than corresponding HC group cytokine levels, while all other ATB cytokine levels were significantly higher than corresponding HC levels. Meanwhile, comparisons of LTBI group cytokine (with chemotactic effect) levels to those of the ATB group revealed higher ATB group levels of only GM-CSF, IFN-γ, TNF-α, IL-4, IL-6 and CX3CL1 and no significant intergroup differences in levels of other cytokines (with chemotactic effect) (Figure 2). Moreover, comparisons of growth factor (with chemotactic effect) levels (Figure 3) among the three groups revealed only significant intergroup differences in CXCL6 and CXCL13 levels, while ATB group levels of other growth factors were significantly higher than corresponding HC and LTBI levels (except for CXCL10 and CXCL16). Similarly, ATB group levels of a large number of chemokines were significantly higher than corresponding HC levels, although no significant differences in levels of CCL15, CCL17 and CCL23 were observed. Meanwhile, comparisons of ATB and LTBI chemokine levels revealed no significant intergroup differences in levels of almost half of the chemokines, while levels of CCL1, CCL3, CCL7, CCL8, CCL13, CCL19, CCL20, CCL22, CCL23, CCL26 and CCL27 were significantly greater than corresponding LTBI group levels (Figure 4). In addition, almost all CCL17 levels fell below the limit of detection of the assay as an explanation for why the number of samples for this chemokine (as indicated in Figure 4) is lower than sample numbers indicated for other chemokines.
Figure 2.
Difference in plasma cytokines (with chemotactic effect) levels between tuberculosis (TB), latent tuberculosis infection (LTBI) and healthy control (HC) by Luminex. Data denoting means ± SME. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Figure 3.
Difference in plasma growth factors (with chemotactic effect) levels between tuberculosis (TB), latent tuberculosis infection (LTBI) and healthy control (HC) by Luminex. Data denoting means ± SME. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Figure 4.
Difference in plasma chemokines levels between tuberculosis (TB), latent tuberculosis infection (LTBI) and healthy control (HC) by Luminex. Data denoting means ± SME. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
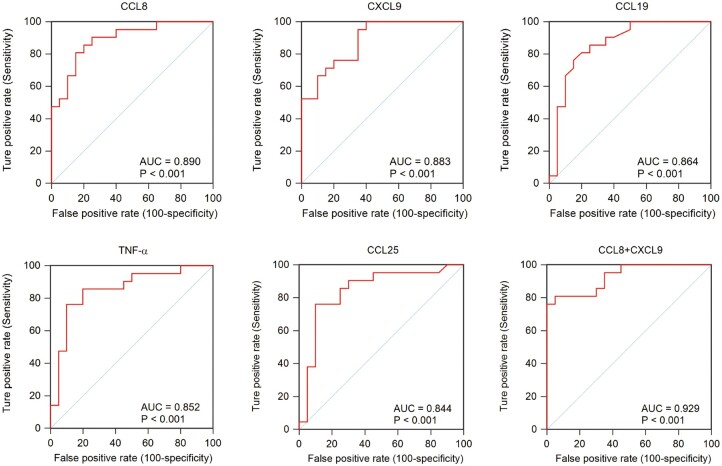
ROC analyses
Given that ATB group plasma levels of numerous cytokines, growth factors and chemokines were significantly higher than corresponding LTBI group levels, we conducted ROC analysis to assess the ability of these factors to detect ATB disease in members of the IGRA-positive population (Figure 5). The results revealed that the highest AUC value was obtained for CCL8 (0.890), followed by values obtained for CXCL9 (0.883), CCL19 (0.864), TNF-α (0.852) and CCL25 (0.844). After repeating the ROC analysis based on levels of both CCL8 and CXCL9 chemokines, an AUC value of 0.929 was obtained that suggested that CCL8 and CXCL9 are potential biomarkers that may facilitate detection of ATB cases within the IGRA-positive patient population.
Figure 5.
Receiving operating characteristic (ROC) curve analysis between tuberculosis (TB) and latent tuberculosis infection (LTBI). AUC, area under the curve.
Performance of CCL8 and CXCL9 biomarkers in achieving rapid identification of LTBI and TB cases
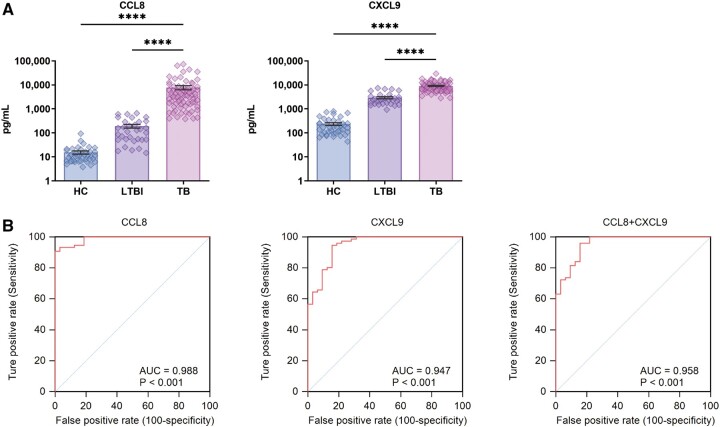
We next used ELISA kits to measure CCL8 and CXCL9 levels in supernatants of MTB antigen-stimulated cells in validation cohort blood samples. As shown in Figure 6A, ELISA results aligned with Luminex results showing that levels of CCL8 and CXCL9 in ATB group samples were significantly higher than corresponding LTBI and HC group levels. Notably, results of ROC analysis-based comparisons of ATB and LTBI group chemokine levels (Figure 6B) revealed that CCL8 possessed a remarkable ability to discriminate between ATB and LTBI samples, as reflected by AUC values as high as 0.988 (95% CI, 0.944–0.999; sensitivity, 90.79%; specificity, 100.00%). Notably, the ATB-detection ability of the CCL8+CXCL9 assay (AUC = 0.958; 95% CI, 0.902–0.987; sensitivity, 96.05%; specificity, 84.37%) exceeded the ATB-detection ability of the CXCL9 assay (AUC = 0.947; 95% CI, 0.886–0.981; sensitivity, 94.74%; specificity, 84.37%), but did not exceed that of the CCL8 assay.
Figure 6.
(A) Plasma CCL8 and CXCL9 levels between tuberculosis (TB), latent tuberculosis infection (LTBI) and healthy control (HC) by ELISA. (B) Receiving operating characteristic (ROC) curve analysis between tuberculosis (TB) and latent tuberculosis infection (LTBI). AUC, area under the curve.
Discussion
IGRAs show acceptable performance for use in detecting LTBI cases and thus are useful auxiliary tools for diagnosing ATB in adults and children.14 However, low IGRA performance in differentiating LTBI cases from ATB cases diminishes the clinical value of this test.15 Here, our results demonstrated significant intergroup differences in blood plasma levels of several chemokines (i.e. CCL8, CXCL9 and CCL19) after MTB antigen stimulation of blood cells collected from subjects of LTBI and ATB groups. Therefore, these chemokines may serve as a new type of biomarker for use in differentiating ATB from LTBI cases, even though results of several studies have indicated variable performance of multiple cytokines and chemokines when used for this purpose.16 For example, results of a preliminary study conducted by Chegou et al.17 revealed that levels of CXCL10 in combination with EGF and MIP-1β levels could be used to differentiate IGRA-positive ATB cases from IGRA-positive LTBI cases, with an overall accuracy rate of 86.0%. Similarly, an assay based on a combination of IFN-γ and CXCL10 markers yielded a sensitivity rate of 89.6% and a specificity rate of 71.1% for discriminating ATB from LTBI cases,16 thus emphasizing the potential value of CXCL10 as a diagnostic biomarker for ATB cases. Nevertheless, CXCL10 levels did not differ between ATB and LTBI groups within our patient population and thus our results contradict the results of the abovementioned study.16 This contradiction may stem from the fact that CXCL10 level is strongly induced by IFN-γ, whereby excessive IFN-γ secretion may stimulate CXCL10 production not only by T cells of subjects with ATB, but also by monocytes of individuals with LTBI that reduce CSCL10 diagnostic specificity in discriminating between LTBI and ATB cases.
As an alternative marker, CCL8 provided greater sensitivity and specificity rates than other cytokines and chemokines when used to differentiate between ATB and LTBI cases; in fact, interpretation of CCL8 assay results based on a cut-off concentration of 0.658 ng/ml led to assay sensitivity and specificity rates for detection of ATB cases in the IGRA-positive population that reached 90.79% and 100.00%, respectively. As consistent with our results, results of a previous study by Yu et al.18 demonstrated markedly different antigen-stimulated blood cell supernatant CCL8 levels between the ATB group and the lung cancer control group. Furthermore, our results also aligned with results of a smaller study comparing chemokine levels in pleural effusion samples obtained from tuberculous pleurisy patients to corresponding control levels via protein array analysis that revealed significantly greater CCL8 levels in tuberculous pleurisy patient samples vs. controls.19 Importantly, expression of CCL, a chemokine produced by activated antigen-presenting cells, is inducible by IFN-γ, IFN-α and IL-1 and mainly chemoattracts granulocytes, monocytes and T cells.17 Indeed, despite similar MTB antigen-induced blood cell IFN-γ levels observed in ATB and LTBI groups in this study and in a previously reported study, significantly greater MTB antigen-induced IFN-α and IL-1β levels were found in ATB patient samples than LTBI subject samples11 as a plausible explanation for increased CCL8 levels observed in our ATB group patients. Taken together, the abovementioned results support the use of assays based on MTB antigen-specific triggering of CCL8 production as a promising strategy for achieving differential diagnosis of TB cases with positive IGRA results as ATB vs. LTBI cases.
We also observed significant upregulation of ATB group levels of CCL19, a chemokine shown by Kahnert et al.20 in an animal model-based study to play an in vivo role in the spatial organization of cells involved in the anti-MTB granulomatous response. Moreover, in a recent cohort study from India, decreased CCL19 levels were found in close contacts of ATB patients who were IGRA-positive before developing ATB during the 2-year follow-up period.21 These results indicate that reduced CCL19 levels noted in individuals with LTBI who progress to ATB status may be attributed to impaired granuloma formation that supports disease progression by permitting in vivo multiplication of tubercle bacilli in ATB lesions; conversely, individuals with unimpaired granuloma formation produce appropriate chemokines that recruit T cells to MTB-infected tissues to control the infection and maintain LTBI status.
We acknowledge several obvious limitations of the present study. First, our conclusions were drawn from results obtained from a small number of subjects and thus should be confirmed using a larger number of subjects obtained from multiple centres. Second, despite CCL8’s demonstrated capability in discriminating between ATB and LTBI cases in general populations, results of previous studies suggest that CCL8 is less suitable for use as an independent immunodiagnostic marker for assessing TB infection status of HIV-infected subjects.17 Therefore, further investigations are needed to determine CCL8 assay diagnostic performance in assessing TB status of immunocompromised patients. Third, release of chemokines is affected by various factors, including host immune status and antigen concentration. For example, antigen concentration has been shown to be positively correlated with bacterial burden, such that levels of chemokines might be useful indicators of clinical TB patient outcomes, warranting further study. Finally, about one-fifth of ATB patients yield false-negative IGRA results in clinical practice,22 as consistent with results obtained in a preliminary experiment conducted by our research group (data not shown) indicating impaired secretion of CCL8 in IGRA-negative TB patients relative to that of IGRA-positive TB patients. Hence, additional experiments to confirm this result are urgently needed before CCL8 can serve as a biomarker for differentially diagnosing individuals with TB who belong to this special population. In addition, CCL19 also shows a good discrimination effect in the discovery cohort, but due to the limitations of the clinical sample volume, we have to focus on the CCL8 and CXCL9. At the same time, in our follow-up multiple centres verification experiment, CCL19 test is being carried out actively.
To conclude, results of this study obtained for the discovery cohort demonstrated that TB-specific chemokines may serve as biomarkers for diagnosing ATB disease. Further validation of chemokine candidates through testing of a larger cohort generated results confirming that the CCL8 biomarker-based assay had higher sensitivity and specificity rates than assays based on other biomarkers when used to differentiate between ATB and LTBI. These results will likely pave the way for the future development of a simple IGRA-like assay based on multiple biomarkers to facilitate early clinical diagnosis of ATB cases, especially in resource-challenged clinical settings.
Acknowledgements
We would like to thank all the staffs participating this study from Beijing Chest Hospital.
Contributor Information
H Li, Department of Bacteriology and Immunology, Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis and Thoracic Tumor Research Institute, Postal No. 9, Beiguan Street, Tongzhou District, Beijing 101149, People’s Republic of China.
W Ren, Department of Bacteriology and Immunology, Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis and Thoracic Tumor Research Institute, Postal No. 9, Beiguan Street, Tongzhou District, Beijing 101149, People’s Republic of China.
Q Liang, Department of Tuberculosis, Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing, People’s Republic of China.
X Zhang, Department of Bacteriology and Immunology, Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis and Thoracic Tumor Research Institute, Postal No. 9, Beiguan Street, Tongzhou District, Beijing 101149, People’s Republic of China.
Q Li, Department of Tuberculosis, Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing, People’s Republic of China.
Y Shang, Department of Bacteriology and Immunology, Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis and Thoracic Tumor Research Institute, Postal No. 9, Beiguan Street, Tongzhou District, Beijing 101149, People’s Republic of China.
L Ma, Department of Tuberculosis, Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing, People’s Republic of China.
S Li, Department of Bacteriology and Immunology, Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis and Thoracic Tumor Research Institute, Postal No. 9, Beiguan Street, Tongzhou District, Beijing 101149, People’s Republic of China.
Y Pang, Department of Bacteriology and Immunology, Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis and Thoracic Tumor Research Institute, Postal No. 9, Beiguan Street, Tongzhou District, Beijing 101149, People’s Republic of China.
Ethics approval and consent to participate
This study was approved by the Ethics committee of Beijing Chest Hospital, Capital Medical University (approval number: YJS-2019-016). The guidelines outlined in the Declaration of Helsinki were followed.
Consent for publication
Written informed consent was obtained from the patient.
Funding
This work was supported by the National Key Research and Development Program of China (Grant No. 2022YFC2302900) and Science and Technology Plan Project of Guangxi Zhuang Autonomous Region, China (AA22096027). The funders had no role in study design, data collection, analysis, interpretation or writing of the report.
Conflict of interest
The authors declare that they have no conflict of interest.
Authors’ contributions
Haoran Li (Data curation [equal], Resources [equal], Validation [equal], Writing—original draft [lead], Writing—review & editing [equal]), Weicong Ren (Data curation [lead], Methodology [equal], Software [lead], Validation [equal]), Qingtao Liang (Investigation [equal], Methodology [equal], Resources [lead]), Xuxia Zhang (Data curation [equal], Methodology [equal], Visualization [equal]), Qing Li (Resources [equal]), Yuanyuan Shang (Data curation [equal], Formal analysis [equal], Methodology [equal]), Liping Ma (Methodology [equal], Resources [equal]), Shanshan Li (Data curation [equal], Formal analysis [equal], Software [equal]) and Yu Pang (Funding acquisition [lead], Methodology [lead], Writing—review & editing [lead]).
Data availability
All data contained in this study can be obtained from the corresponding author under reasonable request.
References
- 1. Koegelenberg CFN, Schoch OD, Lange C.. Tuberculosis: the past, the present and the future. Respiration 2021; 100:553–6. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organisation. Global Tuberculosis Report 2022. Geneva: World Health Organization, 2022. [Google Scholar]
- 3. McNerney R, Maeurer M, Abubakar I, Marais B, McHugh TD, Ford N, et al. Tuberculosis diagnostics and biomarkers: needs, challenges, recent advances, and opportunities. J Infect Dis 2012; 205(Suppl 2):S147–58. [DOI] [PubMed] [Google Scholar]
- 4. Labuda SM, McDaniel CJ, Talwar A, Braumuller A, Parker S, McGaha S, et al. Tuberculosis outbreak associated with delayed diagnosis and long infectious periods in rural Arkansas, 2010-2018. Public Health Rep 2022; 137:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rojano B, Caminero JA, Hayek M.. Curving tuberculosis: current trends and future needs. Ann Glob Health 2019; 85:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Opota O, Mazza-Stalder J, Greub G, Jaton K.. The rapid molecular test Xpert MTB/RIF ultra: towards improved tuberculosis diagnosis and rifampicin resistance detection. Clin Microbiol Infect 2019; 25:1370–6. [DOI] [PubMed] [Google Scholar]
- 7. Shi H, Wang X, Li D, Tang W, Wang H, Xu W, et al. Molecular characterization of cotton 14-3-3L gene preferentially expressed during fiber elongation. J Genet Genomics 2007; 34:151–9. [DOI] [PubMed] [Google Scholar]
- 8. Kohli M, Schiller I, Dendukuri N, Yao M, Dheda K, Denkinger CM, et al. Xpert MTB/RIF ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2021; 1:CD012768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maynard-Smith L, Larke N, Peters JA, Lawn SD.. Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary and pulmonary tuberculosis when testing non-respiratory samples: a systematic review. BMC Infect Dis 2014; 14:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diel R, Goletti D, Ferrara G, Bothamley G, Cirillo D, Kampmann B, et al. Interferon-gamma release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur Respir J 2011; 37:88–99. [DOI] [PubMed] [Google Scholar]
- 11. Frahm M, Goswami ND, Owzar K, Hecker E, Mosher A, Cadogan E, et al. Discriminating between latent and active tuberculosis with multiple biomarker responses. Tuberculosis (Edinb) 2011; 91:250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu J, Wang S, Lu C, Shao L, Gao Y, Zhou Z, et al. Multiple cytokine responses in discriminating between active tuberculosis and latent tuberculosis infection. Tuberculosis (Edinb) 2017; 102:68–75. [DOI] [PubMed] [Google Scholar]
- 13. Moreira FMF, Verma R, Pereira Dos Santos PC, Leite A, da Silva Santos A, de Araujo RCP, et al. Blood-based host biomarker diagnostics in active case finding for pulmonary tuberculosis: a diagnostic case-control study. EClinicalMedicine 2021; 33:100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Auguste P, Tsertsvadze A, Pink J, Court R, McCarthy N, Sutcliffe P, et al. Comparing interferon-gamma release assays with tuberculin skin test for identifying latent tuberculosis infection that progresses to active tuberculosis: systematic review and meta-analysis. BMC Infect Dis 2017; 17:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gong W, Wu X.. Differential diagnosis of latent tuberculosis infection and active tuberculosis: a key to a successful tuberculosis control strategy. Front Microbiol 2021; 12:745592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nonghanphithak D, Reechaipichitkul W, Namwat W, Naranbhai V, Faksri K.. Chemokines additional to IFN-γ can be used to differentiate among Mycobacterium tuberculosis infection possibilities and provide evidence of an early clearance phenotype. Tuberculosis (Edinb) 2017; 105:28–34. [DOI] [PubMed] [Google Scholar]
- 17. Chegou NN, Heyckendorf J, Walzl G, Lange C, Ruhwald M.. Beyond the IFN-γ horizon: biomarkers for immunodiagnosis of infection with Mycobacterium tuberculosis. Eur Respir J 2014; 43:1472–86. [DOI] [PubMed] [Google Scholar]
- 18. Yu Y, Zhang Y, Hu S, Jin D, Chen X, Jin Q, et al. Different patterns of cytokines and chemokines combined with IFN-γ production reflect Mycobacterium tuberculosis infection and disease. PLoS One 2012; 7:e44944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu H, Liu Z, Chen J, Chen L, He X, Zheng R, et al. Induction of CCL8/MCP-2 by mycobacteria through the activation of TLR2/PI3K/akt signaling pathway. PLoS One 2013; 8:e56815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kahnert A, Höpken UE, Stein M, Bandermann S, Lipp M, Kaufmann SH.. Mycobacterium tuberculosis triggers formation of lymphoid structure in murine lungs. J Infect Dis 2007; 195:46–54. [DOI] [PubMed] [Google Scholar]
- 21. Daniel EA, Thiruvengadam K, Rajamanickam A, Chandrasekaran P, Pattabiraman S, Bhanu B, et al. QuantiFERON supernatant-based host biomarkers predicting progression to active tuberculosis disease among household contacts of tuberculosis patients. Clin Infect Dis 2023; 76:1802–13. [DOI] [PubMed] [Google Scholar]
- 22. Li Q, Ren W, Yuan J, Guo H, Shang Y, Wang W, et al. Significant difference in Th1/Th2 paradigm induced by tuberculosis-specific antigens between IGRA-positive and IGRA-negative patients. Front Immunol 2022; 13:904308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data contained in this study can be obtained from the corresponding author under reasonable request.