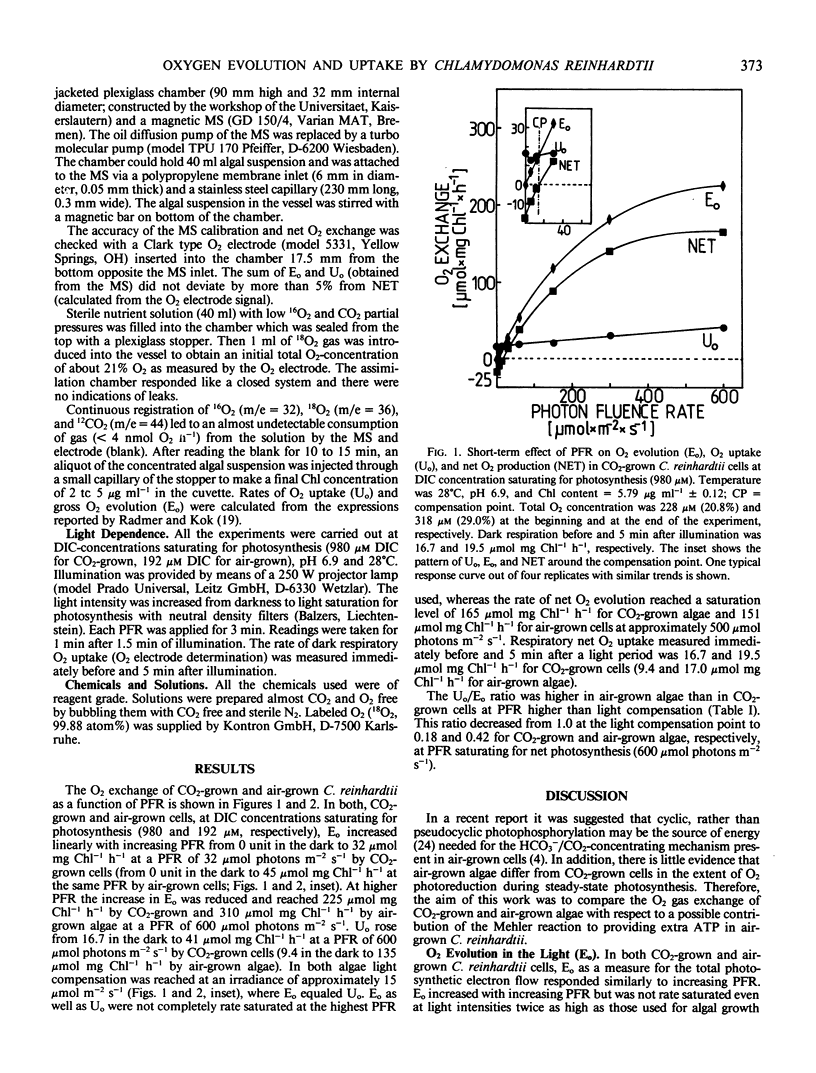
Abstract
A closed system consisting of an assimilation chamber furnished with a membrane inlet from the liquid phase connected to a mass spectrometer was used to measure O2 evolution and uptake by Chlamydomonas reinhardtii cells grown in ambient (0.034% CO2) or CO2-enriched (5% CO2) air. At pH = 6.9, 28°C and concentrations of dissolved inorganic carbon (DIC) saturating for photosynthesis, O2 uptake in the light (Uo) equaled O2 production (Eo) at the light compensation point (15 micromoles photons per square meter per second). Eo and Uo increased with increasing photon fluence rate (PFR) but were not rate saturated at 600 micromoles photons per square meter per second, while net O2 exchange reached a saturation level near 500 micromoles photons per square meter per second which was nearly the same for both, CO2-grown and air-grown cells. Comparison of the Uo/Eo ratios between air-grown and CO2-grown C. reinhardtii showed higher values for air-grown cells at light intensities higher than light compensation. For both, air-grown and CO2-grown algae the rates of mitochondrial O2 uptake in the dark measured immediately before and 5 minutes after illumination were much lower than Uo at PFR saturating for net photosynthesis. We conclude that noncyclic electron flow from water to NADP+ and pseudocyclic electron flow via photosystem I to O2 both significantly contribute to O2 exchange in the light. In contrast, mitochondrial respiration and photosynthetic carbon oxidation cycle are regarded as minor O2 consuming reactions in the light in both, air-grown and CO2-grown cells. It is suggested that the “extra” O2 uptake by air-grown algae provides ATP required for the energy dependent CO2/HCO3− concentrating mechanism known to be present in these cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro E. M., Gerbaud A., André M. CO(2) and O(2) Exchange in Two Mosses, Hypnum cupressiforme and Dicranum scoparium. Plant Physiol. 1984 Oct;76(2):431–435. doi: 10.1104/pp.76.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. A., Osmond C. B., Lorimer G. H. Fixation of O(2) during Photorespiration: Kinetic and Steady-State Studies of the Photorespiratory Carbon Oxidation Cycle with Intact Leaves and Isolated Chloroplasts of C(3) Plants. Plant Physiol. 1978 Dec;62(6):954–967. doi: 10.1104/pp.62.6.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham B. C., Coleman J. R., Colman B. Measurement of photorespiration in algae. Plant Physiol. 1982 Jan;69(1):259–262. doi: 10.1104/pp.69.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechignac F., Andre M. Oxygen Uptake and Photosynthesis of the Red Macroalga, Chondrus crispus, in Seawater: Effects of Light and CO(2) Concentration. Plant Physiol. 1984 Aug;75(4):919–923. doi: 10.1104/pp.75.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canvin D. T., Berry J. A., Badger M. R., Fock H., Osmond C. B. Oxygen exchange in leaves in the light. Plant Physiol. 1980 Aug;66(2):302–307. doi: 10.1104/pp.66.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egneus H., Heber U., Matthiesen U., Kirk M. Reduction of oxygen by the electron transport chain of chloroplasts during assimilation of carbon dioxide. Biochim Biophys Acta. 1975 Dec 11;408(3):252–268. doi: 10.1016/0005-2728(75)90128-0. [DOI] [PubMed] [Google Scholar]
- Furbank R. T., Badger M. R., Osmond C. B. Photosynthetic oxygen exchange in isolated cells and chloroplasts of c(3) plants. Plant Physiol. 1982 Oct;70(4):927–931. doi: 10.1104/pp.70.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOCH G., OWENS O. V., KOK B. Photosynthesis and respiration. Arch Biochem Biophys. 1963 Apr;101:171–180. doi: 10.1016/0003-9861(63)90547-2. [DOI] [PubMed] [Google Scholar]
- MEHLER A. H. Studies on reactions of illuminated chloroplasts. II. Stimulation and inhibition of the reaction with molecular oxygen. Arch Biochem Biophys. 1951 Dec;34(2):339–351. doi: 10.1016/0003-9861(51)90012-4. [DOI] [PubMed] [Google Scholar]
- Peltier G., Thibault P. Light-Dependent Oxygen Uptake, Glycolate, and Ammonia Release in l-Methionine Sulfoximine-Treated Chlamydomonas. Plant Physiol. 1985 Feb;77(2):281–284. doi: 10.1104/pp.77.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier G., Thibault P. O(2) uptake in the light in chlamydomonas: evidence for persistent mitochondrial respiration. Plant Physiol. 1985 Sep;79(1):225–230. doi: 10.1104/pp.79.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R. J., Kok B. Photoreduction of O(2) Primes and Replaces CO(2) Assimilation. Plant Physiol. 1976 Sep;58(3):336–340. doi: 10.1104/pp.58.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N. MITOTIC REPLICATION OF DEOXYRIBONUCLEIC ACID IN CHLAMYDOMONAS REINHARDI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. C. Evidence for Cyclic Photophosphorylation during CO(2) Fixation in Intact Chloroplasts: Studies with Antimycin A, Nitrite, and Oxaloacetate. Plant Physiol. 1983 Jun;72(2):313–320. doi: 10.1104/pp.72.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]


