This randomized clinical trial investigates if combination paramagnetic seed and superparamagnetic iron oxide is equivalent to guidewire plus superparamagnetic iron oxide for breast cancer localization and sentinel lymph node detection.
Key Points
Question
Is the combination of paramagnetic seed and superparamagnetic iron oxide (SPIO) equivalent to guidewire and SPIO for breast cancer localization and sentinel lymph node detection (SLND)?
Findings
This randomized clinical trial including 426 patients from 3 hospitals in Sweden found that a totally magnetic technique was equivalent to the combination of guidewire and SPIO in re-excision frequency, specimen volumes, and SLND. In addition, seed and SPIO resulted in shorter operative times and increased satisfaction among health care practitioners.
Meaning
A totally magnetic technique is an effective option for breast cancer localization and SLND.
Abstract
Importance
Guidewires have been the standard for breast lesion localization but pose operative and logistic challenges. Paramagnetic seeds have shown promising results, but to the authors’ knowledge, no randomized comparison has been performed.
Objective
To determine whether the combination of a paramagnetic seed and superparamagnetic iron oxide (SPIO) is equivalent to guidewire and SPIO for breast cancer localization and sentinel lymph node detection (SLND).
Design, Setting, and Participants
This was a phase 3, pragmatic, equivalence, 2-arm, open-label, randomized clinical trial conducted at 3 university and/or community hospitals in Sweden from May 2018 to May 2022. Included in the study were patients with early breast cancer planned for breast conservation and SLND. Study data were analyzed July to November 2022.
Interventions
Participants were randomly assigned 1:1 to a paramagnetic seed or a guidewire. All patients underwent SLND with SPIO.
Main Outcomes and Measures
Re-excision rate and resection ratio (defined as actual resection volume / optimal resection volume).
Results
A total of 426 women (median [IQR] age, 65 [56-71] years; median [IQR] tumor size, 11 [8-15] mm) were included in the study. The re-excision rate was 2.90% (95% CI, 1.60%-4.80%), and the median (IQR) resection ratio was 1.96 (1.15-3.44). No differences were found between the guidewire and the seed in re-excisions (6 of 211 [2.84%] vs 6 of 209 [2.87%]; difference, −0.03%; 95% CI, −3.20% to 3.20%; P = .99) or resection ratio (median, 1.93; IQR, 1.18-3.43 vs median, 2.01; IQR, 1.11-3.47; P = .70). Overall SLN detection was 98.6% (95% CI, 97.1%-99.4%) with no differences between arms (203 of 207 [98.1%] vs 204 of 206 [99.0%]; difference, −0.9%; 95% CI, −3.6% to 1.8%; P = .72). More failed localizations occurred with the guidewire (21 of 208 [10.1%] vs 4 of 215 [1.9%]; difference, 8.2%; 95% CI, 3.3%-13.2%; P < .001). Median (IQR) time to specimen excision was shorter for the seed (15 [10-22] minutes vs 18 [12-30] minutes; P = .01), as was the total operative time (69 [56-86] minutes vs 75.5 [59-101] minutes; P = .03). The experience of surgeons, radiologists, and surgical coordinators was better with the seed.
Conclusions and Relevance
The combination of SPIO and a paramagnetic seed performed comparably with SPIO and guidewire for breast cancer conserving surgery and resulted in more successful localizations, shorter operative times, and better experience.
Trial Registration
ISRCTN.org Identifier: ISRCTN11914537
Introduction
Breast cancer screening, along with the improvement of imaging, have led to an increase in breast cancer diagnosis at a presymptomatic stage.1 In the majority of these cases, breast-conserving surgery is feasible, but preoperative tumor localization is required.
The guidewire has been the most extensively used method of breast tumor localization due to its low cost and ease of use.2,3 However, complications such as dislocation, migration, and patient discomfort have been described.4,5,6,7 Apart from these complications, guidewire localization is restricted to the day of surgery, posing logistical challenges. These issues have led to the development of novel, wire-free localization devices8 such as radioiodine seeds,9,10,11 radar reflectors,12,13 radiofrequency tags,14,15 and paramagnetic/magnetic seeds.16,17
Most of these patients are clinically node negative and undergo sentinel lymph node dissection (SLND), which has traditionally been performed with a radioisotope (RI) with or without blue dye (BD). Although highly reliable, this combination poses challenges due to restricted access to nuclear medicine facilities, strict regulations, and risk of allergic reaction to BD, whereas the short half-life of the RI limits administration on the day of surgery or the day before, complicating logistics. Superparamagnetic iron oxide (SPIO) nanoparticles have shown comparable performance with an RI with or without BD with the additional advantage of a wider time frame of preoperative administration.18,19,20 Perceived drawbacks of the method are skin staining and artifacts on postoperative magnetic resonance imaging (MRI)21,22; a recent meta-analysis,20 however, suggests that peritumoral SPIO administration could address these concerns, without any compromise of SLN detection outcomes.
Previous large cohort studies have shown that paramagnetic seeds are advantageous in terms of operating time and ease of logistics compared with the guidewire and with comparable re-excision rates and specimen sizes; this, however, has not been validated in randomized clinical trials (RCTs).16,23 At the same time, combining seeds with SPIO for a totally magnetic technique encompassing tumor localization and SLN detection has been investigated in small studies.24,25 The technique was found feasible with the possible advantages of simplified logistics, as the localization procedure and tracer injection are detached from the day of surgery and, possibly, increased patient and physician satisfaction. Furthermore, both seed and SPIO are detectable by the same probe, avoiding multiple equipment in the operating room. Therefore, an RCT would elucidate these questions.
Methods
In the interest of higher external validity, the Magnetic Marker to Detect Primary Lesion and Sentinel Node in Breast Cancer (MAGTOTAL) trial was designed as a phase 3, open-label, pragmatic trial including centers with different levels of experience with the magnetic technique (Supplement 1). The trial was approved by the Uppsala Regional ethics committee and registered to a publicly available database. Enrollment took place between May 1, 2018, and May 1, 2022, at 3 hospitals in Sweden (Akademiska University Hospital, Uppsala; Västmanlands Hospital, Västerås; and Sahlgrenska University Hospital, Gothenburg). Adult patients with nonpalpable ductal cancer in situ (DCIS) or T1 to T3 invasive breast cancer who were scheduled to receive breast-conserving surgery and SLND were eligible for inclusion in the trial. Patients with small, diffusely palpable lesions requiring preoperative localization or multifocal/multicentric lesions amenable to breast conservation were also included. Exclusion criteria included intolerance or hypersensitivity to iron or dextran compounds, iron overload disease, pregnancy and lactation, inability to provide informed consent, and pacemakers or implantable devices in the ipsilateral chest-wall or shoulder. Participant race and ethnicity were not collected because there is not any known interaction between these and the outcomes examined in the trial. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines for pragmatic trials.26
After oral and written informed consent, participants were randomly assigned with an allocation ratio of 1:1 in blocks of 8. The randomization was performed using the randomizeR package of R statistical software, version 3.5.1 (R Project for Statistical Computing).27 The sequence was concealed in opaque envelopes until the intervention was assigned. During the COVID-19 pandemic, the protocol was amended to allow for tolerance and ensure that scheduled surgery would not be affected by randomization.
In the experimental arm, lesion localization was performed with the Magseed marker (Endomag), a 5-mm paramagnetic seed used for the localization of breast cancer lesions, and in the control arm, with a guidewire (Bard Peripheral Vascular Inc). Regardless of randomization, because SPIO dose and injection timing do not affect SLN detection, patients received 1 to 1.5 mL of Magtrace (Endomag), a nonradioactive liquid tracer containing iron oxide nanoparticles, dorsally to the tumor, at any point between the preoperative visit for surgical planning to the day of surgery, either simultaneously with lesion localization or not.20 Following trial pragmatism, the placement of the marker and the administration of SPIO were to be performed according to local routines or case-by-case convenience, meaning that surgeons or radiologists could insert the paramagnetic marker with or without simultaneous injection of the liquid tracer preoperatively, whereas guidewires were exclusively inserted by a breast radiologist on the day of the surgery or the day before. Both methods of localization were performed under local anesthesia, and accurate localization was verified radiologically. There were no prerequisites such as medical professional level (resident, fellow, consultant), minimum experience, or a completed learning curve for participating radiologists and surgeons. Specimen radiography was performed as per routine, and SLND was performed with the SentiMag probe (Endomag), a probe that can detect both the paramagnetic marker and the liquid tracer, adhering to the 10% of the maximum signal cutoff rule, to complete the procedure. Due to the nature of the intervention, masking was not possible.
The primary outcome measure was resection ratio for each marker in patients with negative margins. The resection ratio was defined as the actual resection volume (ARV) divided by the optimal resection volume (ORV), the latter being the assessed volume needed to excise the lesion with 1-cm margins. The ARV was derived from the fresh specimen weight with concomitant volume calculation, and the ORV was calculated based on preoperative radiology; in cases of discordance between different modalities, the largest measurement was used. Negative margins were defined as “no tumor on ink” for invasive cancer and 2 mm for DCIS. Secondary outcomes included SLN detection rate, adverse events, time to specimen excision, operative time, and ease of implementation by all involved health care practitioners (surgeons, radiologists, surgical coordinators), assessed by Likert scales (scored 0-10, with a higher score denoting higher satisfaction). A prespecified longitudinal analysis of patient-reported outcomes and quality of life evaluation as well as patient-reported experience measures and cost-effectiveness analyses will be reported elsewhere.
Statistical Analysis
According to the Swedish Breast Cancer Registry, the 3 participating sites had comparable re-excision frequencies, with a documented average between 4% and 7%. Therefore, a clinically meaningful improvement based solely on a new device was not expected. However, placing the paramagnetic marker and injecting SPIO in the same location could cause an overlapping signal, possibly leading to excision of larger specimens, a concern that would not apply with the guidewire. Available literature suggests that the resection ratio for guidewire-based excision ranges between 1.9 and 2.8.23,28 The MAGTOTAL pilot study suggested that the totally magnetic technique for nonpalpable tumor localization and magnetic SLND used in the trial had a resection ratio of 1.5,25 whereas a nonrandomized comparison of guidewires and paramagnetic seeds with isotope-based SLND found comparable ratios (1.92 vs 1.67) with comparable re-excision rates (14 vs 16%).23 In the absence of established reference values, we assumed a 2-sided equivalence of 0.3 difference in resection ratio as clinically meaningful (corresponding to a 30% difference in excised volume), with a 2-sided P value set at .05 and power of 80%, corresponding to 191 patients per arm. This population also satisfied the hypothesis of noninferiority in re-excision rates for a standard of 4% by a 5% margin, and an additional 10% was included per arm.
Continuous variables were summarized as means with SD or medians with IQR, depending on data distribution. Comparisons were performed using a t test for means and the Mann-Whitney U test or the Kruskal-Wallis test for medians. Likert items were analyzed as ordinal data (median, IQR) and compared with nonparametric tests, as appropriate. Categorical variables were summarized as numbers and proportions with 95% CIs and comparisons were performed with Fisher exact test for unpaired data (Wald test for differences) and McNemar test for paired data. Multivariable regression analysis was performed if significant univariate associations of clinically relevant variables were demonstrated. Intention-to-treat and per-protocol analyses were performed for the primary end points, and per-protocol analyses were performed for the secondary end points. Effect sizes (odds ratios [ORs] for logistic regression and β coefficients for linear regression) were reported with 95% CIs. Analyses were performed with Stata 17 (StataCorp) and SPSS, version 28 (IBM Corp).
Results
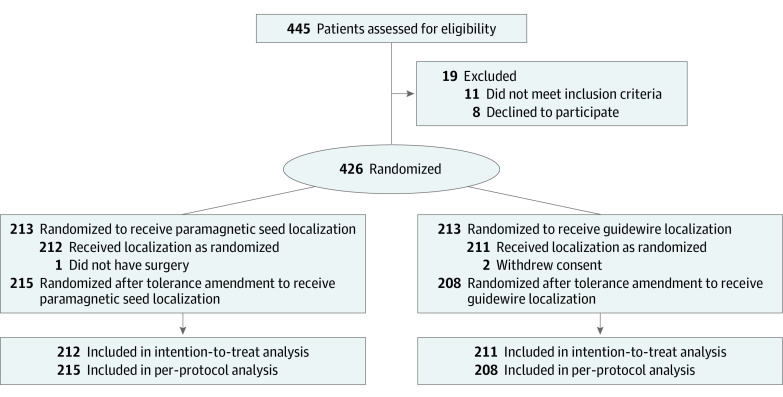
Of the 445 assessed patients, 430 were deemed eligible. After consent withdrawal from 4 patients, 426 women (median [IQR] age, 65 [56-71] years; median [IQR] tumor size, 11 [8-15] mm) were randomly assigned to 2 well-balanced arms of 213 participants (Table 1). In the per-protocol analysis, the totally magnetic arm included 215 participants whereas the guidewire arm included 208 (Figure); however, the discordance was not significant (McNemar test: difference, −0.9%; 95% CI, −2.6% to 0.8%; P = .34).
Table 1. Patient Characteristics.
| Characteristic | Allocation arm | |
|---|---|---|
| Guidewire | Magnetic marker | |
| Recruiting site, No. (%) | ||
| Uppsala | 121 (57.1) | 115 (54.5) |
| Västerås | 53 (25.0) | 54 (25.6) |
| Gothenburg | 38 (17.9) | 42 (19.9) |
| Age, median (IQR), y | 67 (56-72) | 64 (56-70) |
| Body mass index, median (IQR)a | 26.1 (23.8-29.8) | 26.7 (24.1-29.8) |
| Screening detected lesion, No. (%) | ||
| No | 16 (7.6) | 18 (8.5) |
| Yes | 195 (92.4) | 193 (91.5) |
| Palpable lesion, No. (%) | ||
| No | 199 (94.3) | 196 (92.9) |
| Diffusely palpable | 12 (5.7) | 15 (7.1) |
| Preoperative MRI, No. (%) | ||
| No | 133 (75.1) | 115 (66.5) |
| Yes | 44 (24.9) | 58 (33.5) |
| Lateralization, No. (%) | ||
| Right breast | 104 (49.5) | 101 (47.9) |
| Left breast | 106 (50.5) | 110 (52.1) |
| Location, No. (%) | ||
| Upper outer quadrant | 119 (56.1) | 115 (54.8) |
| Upper inner quadrant | 33 (15.6) | 40 (19.0) |
| Lower inner quadrant | 22 (10.4) | 20 (9.5) |
| Lower outer quadrant | 29 (13.7) | 20 (9.5) |
| Central/retroareolar | 7 (3.3) | 15 (7.1) |
| Multifocal/multicentric | 2 (0.9) | 1 (0) |
| Lesion size, median (IQR), mm | 10 (8-15) | 11 (8-15) |
| Histology, No. (%) | ||
| IDC (NST) | 170 (80.2) | 174 (84.1) |
| ILC | 27 (12.7) | 16 (7.7) |
| DCIS | 3 (1.4) | 3 (1.4) |
| Otherb | 12 (5.7) | 14 (6.8) |
| Nuclear grade, No. (%) | ||
| Grade 1 | 52 (25.2) | 63 (31.5) |
| Grade 2 | 123 (59.7) | 105 (52.5) |
| Grade 3 | 31 (15.0) | 32 (16.0) |
| Intrinsic subtype, No. (%) | ||
| Luminal A | 138 (69.0) | 117 (59.7) |
| Luminal B, ERBB2 negative | 41 (20.5) | 62 (31.6) |
| Luminal B, ERBB2 enriched | 4 (2.0) | 6 (3.1) |
| Basal-like, ERBB2 enriched | 5 (2.5) | 3 (1.5) |
| Triple-negative breast cancer | 12 (6.0) | 8 (4.1) |
| Primary systemic therapy | ||
| Yes | 7 (3.3) | 7 (3.3) |
| No | 205 (96.7) | 204 (96.7) |
| Type of surgery | ||
| Simple WLE | 180 (84.9) | 169 (81.3) |
| OPBCS level I | 24 (11.3) | 26 (12.5) |
| OPBCS level II | 8 (3.8) | 13 (6.3) |
Abbreviations: DCIS, ductal cancer in situ; IDC (NST), invasive ductal cancer (nonspecific type); ILC, invasive lobular cancer; MRI, magnetic resonance imaging; OPBCS, oncoplastic breast-conserving surgery; WLE, wide local excision.
Calculated as weight in kilograms divided by height in meters squared.
Other refers to mucinous breast cancer, medullary breast cancer, tubular breast cancer.
Figure. MAGTOTAL Trial Consolidated Standards of Reporting Trials (CONSORT) Flow Diagram.
Re-excision Rates, Resection Ratios, and SLND Outcomes
The overall re-excision rate was 2.90% (95% CI, 1.60%-4.80%). No differences were found between the guidewire and the paramagnetic seed (intention-to-treat analysis, 6 of 211 [2.84%] vs 6 of 209 [2.87%]; difference, −0.03%; 95% CI, −3.20% to 3.20%; P = .99 and per-protocol analysis, 6 of 206 [2.91%] vs 6 of 214 [2.84%]; difference, 0.07%; 95% CI, −3.10% to 3.30%; P = .95). Only the recruiting site was associated with re-excision rate in the univariable analysis (Uppsala: 0.9%; 95% CI, 0.2-2.7; Västerås, 3.8%; 95% CI, 1.3-8.7; Gothenburg, 7.6%; 95% CI, 3.2-15.0; P = .004), with logistic regression suggesting similar outcomes (1 [Reference] for free margins Uppsala; Västerås: OR, 0.219; 95% CI, 0.039-1.215; P = .08; Gothenburg: OR, 0.104; 95% CI, 0.020-0.529; P = .006).
The median (IQR) overall resection ratio was 1.96 (1.15-3.44). The outcomes were equivalent between the guidewire and the paramagnetic seed (intention-to-treat analysis: median, 1.93; IQR, 1.18-3.43 vs median, 2.01; IQR, 1.11-3.47; P = .70; per-protocol analysis: median, 1.96; IQR, 1.22-3.48 vs median, 1.97; IQR, 1.11-3.46; P = .82). In univariable analyses, resection ratio was associated with body mass index, recruiting site, diffusely palpable lesion, preoperative MRI, and type of breast conservation. In multivariable analyses, only body mass index, type of breast conservation, and recruiting site were found to affect the resection ratio (Table 2). Sites interacted with re-excision rates and were a surrogate of experience with the magnetic technique and (possibly) different operating styles; further analyses conducted showed that in the center with the longest experience with the probe, resection ratios and re-excision rates were the lowest. In this setting, the resection ratio for the paramagnetic seed was 0.3 lower than the guidewire (1.26 vs 1.57), but this did not reach statistical significance (eTable 1 in Supplement 2).
Table 2. Univariate and Multivariable Analysis for the Resection Ratio.
| Site/variable | Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|
| Resection ratio (IQR) | P value | β coefficient (95% CI) | P value | |
| Per intention-to-treat analysis | ||||
| Magnetic marker | 2.01 (1.11-3.47) | .70a | NA | NA |
| Guidewire | 1.93 (1.18-3.43) | |||
| Per-protocol analysis | ||||
| Magnetic marker | 1.97 (1.11-3.46) | .82a | NA | NA |
| Guidewire | 1.96 (1.22-3.48) | |||
| Recruiting site | 1.269 (0.763-1.775) | <.001 | ||
| Uppsala | 1.45 (0.78-2.13) | <.001b | 1 [Reference] | NA |
| Västerås | 3.33 (2.13-5.39) | 2.478 (1.650-3.036) | <.001 | |
| Gothenburg | 2.87 (2.00-4.38) | 1.729 (0.805-2.653) | <.001 | |
| Body mass indexc | 0.307 (0.213-0.395)d | <.001d | 0.181 (0.101-0.260) | <.001 |
| Palpable lesion | ||||
| No | 2.00 (1.18-3.52) | .03a | −0.957 (−2.491-0.577) | .22 |
| Diffusely palpable lesion | 1.60 (0.90-2.23) | |||
| Preoperative MRI | ||||
| Yes | 2.55 (1.50-4.27) | <.001a | −0.156 (−1.115-0.802) | .75 |
| No | 1.61 (0.95-2.83) | |||
| Multifocal disease | ||||
| No | 1.98 (1.18-3.46) | .13a | NA | NA |
| Yes | 1.37 (0.56-3.15) | |||
| Histology | ||||
| IDC (NST) | 1.95 (1.15-3.54) | .53b | NA | NA |
| ILC | 2.00 (1.04-2.81) | |||
| DCIS | 2.25 (1.57-3.06) | |||
| Other | 1.79 (1.07-2.85) | |||
| Type of breast-conserving surgery | 1.188 (0.475-1.901) | <.001 | ||
| Simple WLE | 2.07 (1.26-3.60) | <.001b | 1 [Reference] | NA |
| OPBCS level I | 1.37 (0.70-1.85) | −0.029 (−1.105-1.047) | .96 | |
| OPBCS level II | 2.69 (1.05-5.57) | 4.916 (3.367-6.466) | <.001 | |
| Overall | 1.96 (1.15-3.44) | |||
Abbreviations: DCIS, Ductal cancer in situ; IDC (NST), invasive ductal cancer (nonspecific type); ILC, invasive lobular cancer; MRI, magnetic resonance imaging; reference category; NA, not applicable; OPBCS, oncoplastic breast-conserving surgery; WLE, wide local excision.
Mann-Whitney U test.
Kruskal-Wallis test.
Calculated as weight in kilograms divided by height in meters squared.
Spearman ρ (95% CI in parentheses).
Overall SLN detection was (98.6%; 95% CI, 97.1%-99.4%). SLN detection rates were similar between the experimental and the control arms (203 of 207 [98.1%] vs 204 of 206 [99.0%]; difference, −0.9%; 95% CI, −3.6% to 1.8%; P = .72). A median (IQR) of 2 (1-3) SLNs were retrieved in both arms (P = .68). The prevalence of metastasis was also comparable (32 of 212 [15.1%] vs 21 of 204 [10.3%]; difference, −4.8%; 95% CI, −11.7% to 2.1%; P = .19) and did not affect detection rates or nodal yield.
Procedural Outcomes and Patterns of Implementation
Median (IQR) time to specimen excision was significantly shorter for the paramagnetic marker (15 [10-22] minutes vs 18 [12-30] minutes; P = .01) as was the total operative time (69 [56- 86] minutes vs 75.5 [59-101] minutes; P = .03) (Table 3). These outcomes were associated with type of breast surgery on univariable analysis, too. Multivariable regression demonstrated that the use of a paramagnetic marker for lesion localization still resulted in shorter excision and operative times.
Table 3. Univariate and Multivariable Regression for Time To Specimen Excision and Operative Time.
| Marker/surgery type | Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|
| Median (IQR) | P value | β coefficient (95% CI) | P valuea | |
| Time to specimen excision, min | ||||
| Type of marker | 3.768 (1.623-5.917) | .001 | ||
| Magnetic marker | 15 (10-22) | .01b | 1 [Reference] | NA |
| Guidewire | 18 (12-30) | 3.763 (1.613-5.913) | .001 | |
| Type of breast-conserving surgery | 4.913 (2.895-6.931) | <.001 | ||
| Simple WLE | 16 (11-24.5) | .01c | 1 [Reference] | NA |
| OPBCS level I | 20 (14-30) | 5.079 (1.819-8.339) | .002 | |
| OPBCS level II | 30 (11.5-36) | 9.656 (4.831-14.479) | <.001 | |
| Total operative time, min | ||||
| Type of marker | 10.227 (4.634-15.820) | <.001 | ||
| Magnetic marker | 69 (56- 86) | .03b | 1 [Reference] | NA |
| Guidewire | 75.5 (59-101) | 10.442 (4.873-16.011) | <.001 | |
| Type of breast-conserving surgery | 23.121 (17.782-28.460) | <.001 | ||
| Simple WLE | 69 (55-86) | <.001c | 1 [Reference] | NA |
| OPBCS level I | 78.5 (66-103) | 15.505 (6.969-24.041) | <.001 | |
| OPBCS level II | 115 (102-143) | 54.236 (41.505-66.967) | <.001 | |
Abbreviations: NA, not applicable; OPBCS, oncoplastic breast-conserving surgery; WLE, wide local excision.
P value refers to the outcomes of the multivariable regression analysis (linear regression).
Mann-Whitney U test.
Kruskal-Wallis test.
The rate of failed localizations in the trial was 5.9% (95% CI, 3.9-8.6). There were significantly more failed localizations in the guidewire arm compared with the paramagnetic marker (21 of 208 [10.1%] vs 4 of 215 [1.9%]; difference, 8.2%; 95% CI, 3.3%-13.2%; P < .001). From the 4 failed seed localizations, 1 was due to failed deployment and a guidewire was used instead; 3 were intraoperative due to superficial lesions, with the seed dislocated during dissection; in all cases, the tumor was identified with the SPIO magnetic signal. In the guidewire arm (n = 21), 8 localizations failed preoperatively due to tumor location or dense parenchyma and were replaced with a seed, and the remaining 13 were intraoperative dislocations, where resection was guided by the magnetic signal and brown staining of the SPIO. Re-excision was more common in failed localizations (2 of 25 [8%] vs 10 of 395 [2.5%]), but the difference was not significant (5.5%; 95% CI, −5.3% to 16.2%; P = .11) and did not differ per localization technique. Postoperative SPIO-induced skin staining at the postoperative visit was 10.5% (95% CI, 7.7%-13.8%) and was associated only with nonradiology-guided, free-hand peritumoral injection (17 of 108 [15.7%] vs 27 of 313 [8.6%]; difference, 7.1%; 95% CI, 0.04%-15.6%; P = .04; OR, 1.979; 95% CI, 1.032-3.795; P = .04). The rate of postoperative complications was 8.6% (95% CI, 6.1%-11.7%) and did not differ between the paramagnetic marker and the guidewire in frequency (9.8% vs 7.3%; difference, 2.5%; 95% CI, −3.3% to 8.3%; P = .45) or type (eTable 2 in Supplement 2).
There was significant variability in how lesion localization and SPIO administration were implemented (Table 4). However, none of these interacted with re-excision rates, resection ratios, or SLN detection. The localization time was shorter in the totally magnetic arm (median [IQR], 4 [3-5] minutes) than the guidewire arm (median [IQR], 5 [5-6] minutes) across all centers (P < .001).
Table 4. Patterns of Lesion Localization and Superparamagnetic Iron Oxide (SPIO) Administration.
| Localization/administration | Guidewire | Magnetic marker | P value |
|---|---|---|---|
| Localization modality, No. (%) | |||
| Ultrasound | 194 (93.3) | 189 (92.2) | .71a |
| Stereotactic | 14 (6.7) | 16 (7.8) | |
| Days from localization to surgery, median (IQR) | 0 | 5 (1-8) | <.001b |
| Time for lesion localization, median (IQR), min | 5 (5-6) | 4 (3-5) | <.001b |
| SPIO administration, No. (%) | |||
| Surgeonc | 86 (40.6) | 22 (10.5) | <.001a |
| Radiologist | 126 (59.4) | 188 (89.5) | |
| SPIO volume, mL, No. (%) | |||
| 1.0 | 187 (89.0) | 195 (92.9) | .23a |
| 1.5 | 23 (11.0) | 15 (7.1) | |
| Days from SPIO injection to surgery, median (IQR) | 7 (0-15) | 6 (1-8) | .04b |
| Single localization procedure (breast and axilla), No. (%) | |||
| Yes | 74 (34.9) | 180 (85.3) | <.001a |
| No | 138 (65.0) | 31 (14.7) |
Fisher exact test.
Mann-Whitney U test.
Surgeon denotes free-hand SPIO injection around the tumor.
Ease of Implementation
All the disciplines involved graded their experience on a Likert scale of 0 to 10 with higher scores denoting higher satisfaction. Overall, 15 surgeons, 4 radiologists, and 6 surgical coordinators were involved. Satisfaction was higher with the paramagnetic marker across all disciplines, with the difference being more pronounced for surgeons and coordinators (eTable 3 in Supplement 2).
Discussion
In this pragmatic, multicenter RCT, a paramagnetic marker was equivalent to the guidewire in terms of re-excision rates and excess tissue removal regardless of physician experience or localization routines. These results corroborate findings from previous cohort studies16,23,29 and provide stronger evidence. Moreover, the implementation of a totally magnetic technique for lesion removal and SLND was favorable compared with the guidewire in terms of shorter operative times and easier logistics, as shown by the preferences of all health care practitioners that were involved.
One of the concerns expressed regarding the combination of a paramagnetic marker for lesion localization and a peritumoral SPIO injection was that the overlapping signal might lead to the excision of larger specimens.24 Clearly, the combination is successful, regardless of SPIO injection location (subareolar or intraparenchymal in another quadrant of the breast), as smaller studies that tried to address this concern have suggested.24,30 Reassuringly, resection ratios in this RCT were similar between the trial arms, regardless of previous physician experience or practice patterns, suggesting that adaptation is safe. Moreover, in the center with the highest experience, the resection ratio in the totally magnetic arm was 0.3 lower (1.26 vs 1.57) and one of the lowest reported in the literature with only 0.9% re-excisions. Although this did not reach statistical significance, it is indicative of how familiarization with the technique yields potential for precision surgery and resection of smaller specimens. It seems that the totally magnetic technique for nonpalpable tumor localization used in the MAGTOTAL trial allows for the creation of a magnetic halo around the lesion, with the seed placed in the anterior aspect of the tumor, whereas the brown staining from SPIO in the surrounding tissue enables additional intraoperative visual navigation. This technique had lower failed localization rates than the guidewire, a finding similar to previous nonrandomized comparisons.16 Furthermore, injecting SPIO close to the tumor, especially under ultrasonographic guidance, results in reduced skin staining because the bulk of SPIO is removed. This may contribute to minimizing postoperative MRI artifacts, which has been a concern with SPIO-guided SLND.21,22 Currently, this hypothesis is being investigated in a prospective study from our group.31
Previous studies have investigated solely magnetic lesion localization and others solely magnetic SLN detection; the outcomes were comparable with the guidewire and, respectively, RI with or without BD.16,20 Paramagnetic markers and SPIO both have the benefit of decoupling the respective procedure from the day of surgery17,32,33; however, if not combined, this benefit is not being fully utilized. In this RCT, the combination was successful and was positively met by all health care professionals involved in planning and performing breast cancer surgery. The present RCT showed that the totally magnetic technique for nonpalpable tumor localization is currently the only wire- and RI-free technique, to the authors’ knowledge, where both lesion localization and SLN detection can be performed with the same probe, suggesting that the technique can be implemented in any setting.
Strengths and Limitations
Multiple, nonrandomized comparisons of the paramagnetic seed to the guidewire that had suggested similar outcomes served in providing baseline comparative evaluation. Therefore, an RCT was necessary for a definitive comparison of main efficacy and safety aspects, as suggested by the Idea, Development, Exploration, Assessment, and Long-term Follow-Up (IDEAL) Framework.34 The trial did not investigate superiority, but equivalence, as the rationale that a device per se can improve outcomes had not been demonstrated in similar trials11; however, because the investigated technique had other presumed benefits, an RCT was necessary, as relevant literature suggests.35 The pragmatic design ensures the external validity and that the intervention can be implemented with ease and flexibility and without expertise or previous familiarization.
On the other hand, the trial has several limitations. Differences in surgical style are hard to account for, which may be the reason for differences among sites, but, reassuringly, not between trial arms. Moreover, the inherent inability to mask the intervention may account for performance bias and the Pygmalion effect, but we chose end points that would minimize this as we investigated both re-excision and excess excision of healthy tissue at the same time.36 Finally, cost efficacy analyses are still pending, but the shorter localization and operating time, along with the ease of preoperative planning, may compensate for the higher cost of the device.
Conclusions
In this RCT, a paramagnetic marker was equivalent to the guidewire in re-excisions and excised specimen volumes, with advantages of shorter operative time, safer localization, and preferable logistics. Additionally, familiarization with the technique may offer the potential for more precise surgery. Moreover, a totally magnetic technique for lesion localization and SLND relieves the health care system from the restrictions posed by guidewire localization or radioisotope-based methods, making it an attractive alternative for numerous and diverse clinical settings.
Trial Protocol.
eTable 1. Resection Ratio Per Site and Type of Surgery
eTable 2. Type of Complication Per Received Localization Device
eTable 3. Health Care Practitioners’ Experience With Each Marker
Data Sharing Statement.
References
- 1.Cady B, Stone MD, Schuler JG, Thakur R, Wanner MA, Lavin PT. The new era in breast cancer—invasion, size, and nodal involvement dramatically decreasing as a result of mammographic screening. Arch Surg. 1996;131(3):301-308. doi: 10.1001/archsurg.1996.01430150079015 [DOI] [PubMed] [Google Scholar]
- 2.Frank HA, Hall FM, Steer ML. Preoperative localization of nonpalpable breast lesions demonstrated by mammography. N Engl J Med. 1976;295(5):259-260. doi: 10.1056/NEJM197607292950506 [DOI] [PubMed] [Google Scholar]
- 3.Hall FM, Kopans DB, Sadowsky NL, Homer MJ. Development of wire localization for occult breast lesions: Boston remembrances. Radiology. 2013;268(3):622-627. doi: 10.1148/radiol.13121943 [DOI] [PubMed] [Google Scholar]
- 4.Joret MO, El-Haddawi F. Intraperitoneal migration of a hookwire following wide local excision of a breast lesion presenting as a spontaneous pneumothorax. BMJ Case Rep. 2021;14(8):e244086. doi: 10.1136/bcr-2021-244086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seifi A, Axelrod H, Nascimento T, et al. Migration of guidewire after surgical breast biopsy: an unusual case report. Cardiovasc Intervent Radiol. 2009;32(5):1087-1090. doi: 10.1007/s00270-009-9620-9 [DOI] [PubMed] [Google Scholar]
- 6.Homer MJ. Transection of the localization hooked wire during breast biopsy. AJR Am J Roentgenol. 1983;141(5):929-930. doi: 10.2214/ajr.141.5.929 [DOI] [PubMed] [Google Scholar]
- 7.Martaindale S, Scoggins M, Bassett RL Jr, Whitman G. Retained localization wire fragments in the breast: long-term follow-up. Curr Probl Diagn Radiol. 2022;51(3):313-316. doi: 10.1067/j.cpradiol.2021.03.015 [DOI] [PubMed] [Google Scholar]
- 8.Chan BK, Wiseberg-Firtell JA, Jois RH, Jensen K, Audisio RA. Localization techniques for guided surgical excision of non-palpable breast lesions. Cochrane Database Syst Rev. 2015;2015(12):CD009206. doi: 10.1002/14651858.CD009206.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray RJ, Salud C, Nguyen K, et al. Randomized prospective evaluation of a novel technique for biopsy or lumpectomy of nonpalpable breast lesions: radioactive seed versus wire localization. Ann Surg Oncol. 2001;8(9):711-715. doi: 10.1007/s10434-001-0711-3 [DOI] [PubMed] [Google Scholar]
- 10.Alderliesten T, Loo CE, Pengel KE, Rutgers EJT, Gilhuijs KGA, Vrancken Peeters MJTFD. Radioactive seed localization of breast lesions: an adequate localization method without seed migration. Breast J. 2011;17(6):594-601. doi: 10.1111/j.1524-4741.2011.01155.x [DOI] [PubMed] [Google Scholar]
- 11.Langhans L, Tvedskov TF, Klausen TL, et al. Radioactive seed localization or wire-guided localization of nonpalpable invasive and in situ breast cancer: a randomized, multicenter, open-label trial. Ann Surg. 2017;266(1):29-35. doi: 10.1097/SLA.0000000000002101 [DOI] [PubMed] [Google Scholar]
- 12.Wazir U, Kasem I, Michell MJ, et al. Reflector-guided localisation of non-palpable breast lesions: a prospective evaluation of the SAVI SCOUT system. Cancers (Basel). 2021;13(10):2409. doi: 10.3390/cancers13102409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornella KN, Palafox BA, Razavi MK, Loh CT, Markle KM, Openshaw LE. SAVI SCOUT as a novel localization and surgical navigation system for more accurate localization and resection of pulmonary nodules. Surg Innov. 2019;26(4):469-472. doi: 10.1177/1553350619843757 [DOI] [PubMed] [Google Scholar]
- 14.Dauphine C, Reicher JJ, Reicher MA, Gondusky C, Khalkhali I, Kim M. A prospective clinical study to evaluate the safety and performance of wireless localization of nonpalpable breast lesions using radiofrequency identification technology. AJR Am J Roentgenol. 2015;204(6):W720-3. doi: 10.2214/AJR.14.13201 [DOI] [PubMed] [Google Scholar]
- 15.McGugin C, Spivey T, Coopey S, et al. Radiofrequency identification tag localization is comparable to wire localization for nonpalpable breast lesions. Breast Cancer Res Treat. 2019;177(3):735-739. doi: 10.1007/s10549-019-05355-0 [DOI] [PubMed] [Google Scholar]
- 16.Dave RV, Barrett E, Morgan J, et al. ; iBRA-NET Localisation Study collaborative . Wire- and magnetic-seed-guided localization of impalpable breast lesions: iBRA-NET localisation study. Br J Surg. 2022;109(3):274-282. doi: 10.1093/bjs/znab443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey JR, Lim Y, Murphy J, et al. Safety and feasibility of breast lesion localization using magnetic seeds (Magseed): a multicenter, open-label cohort study. Breast Cancer Res Treat. 2018;169(3):531-536. doi: 10.1007/s10549-018-4709-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thill M, Kurylcio A, Welter R, et al. The central-European SentiMag study: sentinel lymph node biopsy with superparamagnetic iron oxide (SPIO) vs. radioisotope. Breast. 2014;23(2):175-179. doi: 10.1016/j.breast.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 19.Karakatsanis A, Christiansen PM, Fischer L, et al. The Nordic SentiMag trial: a comparison of super paramagnetic iron oxide (SPIO) nanoparticles vs Tc(99) and patent blue in the detection of sentinel node (SN) in patients with breast cancer and a meta-analysis of earlier studies. Breast Cancer Res Treat. 2016;157(2):281-294. doi: 10.1007/s10549-016-3809-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pantiora E, Tasoulis MK, Valachis A, et al. Evolution and refinement of magnetically guided sentinel lymph node detection in breast cancer: meta-analysis. Br J Surg. 2023;110(4):410-419. doi: 10.1093/bjs/znac426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christenhusz A, Pouw JJ, Simonis FFJ, et al. Breast MRI in patients after breast conserving surgery with sentinel node procedure using a superparamagnetic tracer. Eur Radiol Exp. 2022;6(1):3. doi: 10.1186/s41747-021-00257-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chapman MC, Lee AY, Hayward JH, Joe BN, Price ER. Superparamagnetic iron oxide sentinel node tracer injection: effects on breast MRI quality. J Breast Imaging. 2020;2(6):577-582. doi: 10.1093/jbi/wbaa083 [DOI] [PubMed] [Google Scholar]
- 23.Zacharioudakis K, Down S, Bholah Z, et al. Is the future magnetic—Magseed localization for nonpalpable breast cancer: a multicenter nonrandomized control study. Eur J Surg Oncol. 2019;45(11):2016-2021. doi: 10.1016/j.ejso.2019.06.035 [DOI] [PubMed] [Google Scholar]
- 24.Spiekerman van Weezelenburg MA, van Haaren ERM, Aldenhoven L, et al. An adapted protocol for magnetic localisation of nonpalpable breast cancer lesions and sentinel lymph nodes using a magnetic seed and superparamagnetic iron oxide tracer. J Surg Oncol. 2023;127(5):776-781. doi: 10.1002/jso.27197 [DOI] [PubMed] [Google Scholar]
- 25.Hersi AF, Eriksson S, Ramos J, Abdsaleh S, Wärnberg F, Karakatsanis A. A combined, totally magnetic technique with a magnetic marker for nonpalpable tumor localization and superparamagnetic iron oxide nanoparticles for sentinel lymph node detection in breast cancer surgery. Eur J Surg Oncol. 2019;45(4):544-549. doi: 10.1016/j.ejso.2018.10.064 [DOI] [PubMed] [Google Scholar]
- 26.Zwarenstein M, Treweek S, Gagnier JJ, et al. ; CONSORT group; Pragmatic Trials in Healthcare (Practihc) group . Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ. 2008;337:a2390. doi: 10.1136/bmj.a2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uschner D, Schindler D, Hilgers RD, Heussen N. RandomizeR: an R package for the assessment and implementation of randomization in clinical trials. J Stat Softw. 2018;85(8):1-22. doi: 10.18637/jss.v085.i0830505247 [DOI] [Google Scholar]
- 28.Krekel NMA, Zonderhuis BM, Stockmann HBAC, et al. A comparison of 3 methods for nonpalpable breast cancer excision. Eur J Surg Oncol. 2011;37(2):109-115. doi: 10.1016/j.ejso.2010.12.006 [DOI] [PubMed] [Google Scholar]
- 29.Micha AE, Sinnett V, Downey K, et al. Patient and clinician satisfaction and clinical outcomes of Magseed compared with wire-guided localisation for impalpable breast lesions. Breast Cancer. 2021;28(1):196-205. doi: 10.1007/s12282-020-01149-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pohlodek K, Sečanský P, Haluzová I, Mečiarová I. Localization of impalpable breast lesions and detection of sentinel lymph nodes through magnetic methods. Eur J Radiol. 2019;120:108699. doi: 10.1016/j.ejrad.2019.108699 [DOI] [PubMed] [Google Scholar]
- 31.Postoperative breast MRI in patients undergoing sentinel node biopsy using super paramagnetic iron oxide nanoparticles. ISRCTN identifier: ISRCTN85167182. Accessed April 14, 2023. 10.1186/ISRCTN85167182 [DOI]
- 32.Karakatsanis A, Daskalakis K, Stålberg P, et al. Superparamagnetic iron oxide nanoparticles as the sole method for sentinel node biopsy detection in patients with breast cancer. Br J Surg. 2017;104(12):1675-1685. doi: 10.1002/bjs.10606 [DOI] [PubMed] [Google Scholar]
- 33.Shams S, Lippold K, Blohmer JU, Röhle R, Kühn F, Karsten MM. A pilot study evaluating the effects of Magtrace for sentinel node biopsy in breast cancer patients regarding care process optimization, reimbursement, surgical time, and patient comfort compared with standard technetium99. Ann Surg Oncol. 2021;28(6):3232-3240. doi: 10.1245/s10434-020-09280-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCulloch P, Feinberg J, Philippou Y, et al. Progress in clinical research in surgery and IDEAL. Lancet. 2018;392(10141):88-94. doi: 10.1016/S0140-6736(18)30102-8 [DOI] [PubMed] [Google Scholar]
- 35.Páez A, Rovers M, Hutchison K, Rogers W, Vasey B, McCulloch P; IDEAL Collaboration . Beyond the RCT: when are randomised trials unnecessary for new therapeutic devices, and what should we do instead? Ann Surg. 2022;275(2):324-331. doi: 10.1097/SLA.0000000000005053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceelen W, Soreide K. Randomized controlled trials and alternative study designs in surgical oncology. Eur J Surg Oncol. 2023;49(8):1331-1340. doi: 10.1016/j.ejso.2023.03.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eTable 1. Resection Ratio Per Site and Type of Surgery
eTable 2. Type of Complication Per Received Localization Device
eTable 3. Health Care Practitioners’ Experience With Each Marker
Data Sharing Statement.