Key Points
Question
Is benzodiazepine use during pregnancy associated with miscarriage, taking into account both measured and unmeasured confounding factors?
Findings
In this case-time-control study, benzodiazepine use during pregnancy was associated with an increased risk of miscarriage, even after accounting for unmeasured confounders, including those related to genetics and the family environment.
Meaning
The observation of an increased risk of miscarriage associated with benzodiazepine use during pregnancy suggests that benzodiazepines should only be used after a thorough evaluation of the potential benefits and risks for both the mother and child.
This case-time-control study evaluates the association between benzodiazepine use during pregnancy and risk of miscarriage.
Abstract
Importance
Benzodiazepine use during pregnancy has raised significant concerns due to the potential harmful effects of this drug class on neonates. Studies on the association between benzodiazepine use and the risk of miscarriage are limited.
Objective
To quantify the risk of miscarriage associated with benzodiazepine use during pregnancy after controlling for unmeasured confounders and exposure time trends.
Design, Setting, and Participants
This was a nationwide, population-based case-time-control study using Taiwan’s National Birth Certificate Application database and the National Health Insurance database. Pregnancies resulting in miscarriage between 2004 and 2018 were included in the case group and were 1:1 matched with exposure time-trend control individuals using disease risk score, considering demographic characteristics and prepregnancy comorbidities. Data were analyzed from August 2022 to March 2023.
Exposures
Discordant exposures to benzodiazepines during risk period (1-28 days before miscarriage) and 2 reference periods (31-58 days and 181-208 days before the last menstrual period) were compared for each pregnancy.
Main Outcomes and Measures
Miscarriage was defined as any pregnancy loss occurring between the first prenatal care visit (usually 8 weeks) and the 19th completed week of pregnancy.
Results
This study comprised a total of 3 067 122 pregnancies among 1 957 601 women, 136 134 of which (4.4%) resulted in miscarriage. The mean (SD) age of the study population was 30.61 (5.91) years. The use of benzodiazepines during pregnancy was associated with an increased risk of miscarriage (odds ratio [OR], 1.69; 95% CI, 1.52-1.87), and consistent findings were observed across multiple sensitivity analyses considering different time windows and accounting for misclassification. In subgroup analyses, an increased risk of miscarriage was associated with each commonly used individual benzodiazepine, ranging from case-time-control ORs of 1.39 (95% CI, 1.17-1.66) for alprazolam to 2.52 (95% CI, 1.89-3.36) for fludiazepam.
Conclusions and Relevance
This nationwide case-time-control study revealed an increased risk of miscarriage associated with benzodiazepine use during pregnancy after accounting for measurable confounders, and results were unlikely to be due to unmeasured confounding. These findings underscore the necessity for health care professionals to meticulously balance the risk-benefit ratio when considering the use of benzodiazepines to treat psychiatric and sleep disorders during pregnancy.
Introduction
Psychiatric and sleep disorders are common during pregnancy.1 Benzodiazepines, possessing anxiolytic and hypnotic properties, are thus widely prescribed to pregnant women for the treatment of anxiety and insomnia.2 Approximately 1.7% of pregnant women are prescribed benzodiazepines during the first trimester, and their use has been on the rise in recent years.3
When used during pregnancy, benzodiazepines can readily cross the placental barrier and accumulate substantially in embryo and fetal tissues.4 Given their potential role in cell proliferation and differentiation processes,5 it is plausible that benzodiazepines may cause fetal developmental abnormalities, ultimately leading to miscarriage. Several studies have found that benzodiazepine use during pregnancy is associated with an increased risk of miscarriage.6,7,8 One recent nested case-control study showed that women exposed to benzodiazepines during pregnancy had an 85% higher risk of miscarriage, after considering measured underlying diseases and sociodemographic factors.9 However, confounding by characteristics, including the severity of anxiety and insomnia, comorbidities, economic status, lifestyle, and genetic factors may also account for the associations.10
Randomized clinical trials may not be the optimal approach for assessing the safety of benzodiazepine use during pregnancy, primarily because pregnant women are typically precluded from clinical trials. Observational studies are thus crucial to support the safe use of these drugs in pregnant women, although confounders need to be well addressed.11 Recent observational studies have found that the association between medication use during pregnancy and adverse outcomes may be a result of confounding by indication, as well as genetic and environmental confounders.12,13,14 Therefore, ameliorating residual confounding bias in observational studies is essential to better understand the association between prenatal drug exposure and the risk of miscarriage. As a result, the present study aims to quantify the risk of miscarriage associated with benzodiazepines use during pregnancy, adopting a case-time-control design to address both measured and unmeasured confounding factors.
Methods
Data Source
A nationwide, population-based study was conducted in Taiwan using 2 data sources: the National Health Insurance (NHI) database from 2002 to 2019 and the National Birth Certificate Application (BCA) database from 2004 to 2018 (eTable 1 in Supplement 1). The NHI database comprises anonymized health insurance claims for visits, procedures, and prescriptions for more than 99% of the population in Taiwan (approximately 23 million people).15 The BCA records all live births and stillbirths with gestational age of more than 20 weeks or birth weight more than 500 g.16 Data of pregnant women from the NHI database and the BCA database were linked on an individual level using encrypted patient identification numbers. The study was approved by the Institutional Review Board of the National Taiwan University Hospital (202101129RINC), and the requirement for informed patient consent was waived due to the use of deidentified patient data. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline was followed. Data were analyzed from August 2022 to March 2023.
Study Population
Our study included women with pregnancies from both the NHI and BCA databases (eFigure 1 in Supplement 1). Women whose pregnancies resulted in miscarriage were identified from the NHI database (eFigure 2 in Supplement 1). We included pregnant women who had had their first prenatal care visit and excluded women with records of births in the BCA database, as well as those without a diagnosis of miscarriage. The end of the pregnancy was defined as the date of the miscarriage, and the last menstrual period was calculated as the date of the first prenatal care visit minus 56 days. This calculation assumes that most pregnant women had their first prenatal care visit at 8 weeks’ gestation based on clinical insights from obstetricians.
Women whose pregnancies resulted in live births or stillbirths were identified from the BCA database. The end of the pregnancy was defined as the birth date of the newborn, and the last menstrual period was calculated as the birth date of the newborn minus the number of days of pregnancy. Pregnant women with suspected inaccurate data and gestational periods less than 20 weeks or more than 42 weeks were further excluded.
Case-Time-Control Design
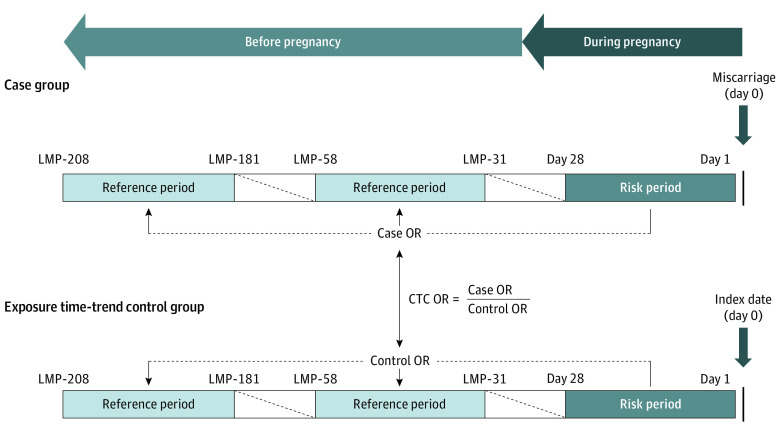
We used a case-time-control study design, which is a within-person comparison, to investigate the association between benzodiazepine use during pregnancy and the risk of miscarriage (Figure 1). The case-time-control design comprised 2 self-adjusted analyses: a case-crossover analysis and an exposure time-trend control crossover analysis.17,18 Through the case-crossover analysis, time-invariant factors, such as underlying disease severity and genetic factors, could be automatically controlled for by using case individuals as their own controls. However, a limitation of case-crossover design is that the estimated odds ratios for associations, obtained solely from the exposure of case individuals at different time points, may potentially reflect changes in drug use associated with the event occurrence—pregnancy in our study. To mitigate this bias, an exposure time-trend control crossover analysis was incorporated into the case-time-control design to adjust for background time trends.19 This design therefore adjusts for time-invariant confounders and exposure time-trend bias resulting from changing of prescription patterns over the pregnancy-related periods.20,21,22
Figure 1. Illustration of the Case-Time-Control (CTC) Design for Studying the Association Between Benzodiazepine Use During Pregnancy and Risk of Miscarriage.
LMP indicates last menstrual period, with number of days before LMP shown; OR indicates odds ratio.
The case individuals in our case-crossover analysis were women with pregnancies resulting in miscarriage. We then used risk-set sampling to identify exposure time-trend controls from the pregnant women.19 The control individuals were matched 1:1 to women with miscarriages based on their birth year and a disease risk score, within a caliper of 0.2 times the standard deviation of the logit of the score.23 The disease risk score was defined as a pregnant woman’s likelihood of experiencing a miscarriage conditional on age, psychiatric medical conditions, lifestyle factors, chronic comorbidities, medication use, and health care use measured within 180 days prior to pregnancy (eTable 2 in Supplement 1). The index date for women with miscarriages was defined as the date of miscarriage diagnosis, while for control individuals, the index date was determined by adding the number of days of pregnancy taken from the matched case to the last menstrual period of controls.
For both individuals with miscarriages and control individuals, we considered 28-day periods for exposure assessment, as recommendations have emphasized limiting the duration of benzodiazepine use to the short term (4-12 weeks).24,25 The risk period was defined as 1 to 28 days before the index date, and 2 reference periods were defined as 31 to 58 and 181 to 208 days before the last menstrual period, respectively.
Miscarriage
Miscarriage was defined as any pregnancy loss occurring between the first prenatal care visit (around the eighth week of gestation) and the 19th completed week of pregnancy, using International Classification of Diseases, Ninth Revision (ICD-9-CM) codes 631, 632, 634, 637, 640; Tenth Revision, Clinical Modification (ICD-10-CM) codes O02, O03, O20; or specific reimbursement codes for miscarriage procedures in Taiwan (eTable 3 in Supplement 1).9,26 Women who had a miscarriage before the first prenatal care visit were not included because they were potentially misclassified due to unrecognized early pregnancy losses. We also did not consider pregnancy losses occurring after 20 weeks of gestation since they were categorized as stillbirth deliveries.
Exposure to Benzodiazepine
The benzodiazepines included in the study are listed in eTable 4 in Supplement 1. Exposure was defined as women receiving at least 1 prescription of benzodiazepine during the risk or reference period. We further assessed exposure to short-acting (half-life ≤24 hours) or long-acting (half-life >24 hours) benzodiazepines based on their duration of action9,27 and exposure to the most commonly used individual medications (alprazolam, diazepam, lorazepam, oxazolam, and fludiazepam). A dose-dependent effect was estimated by the mean daily exposure of benzodiazepines within the risk or reference periods. The mean daily exposure was calculated using the defined daily dose, as defined by the World Health Organization Collaborating Center for Drug Statistics Methodology,28 and categorized as low dose (defined daily dose <1.0) and high dose (defined daily dose ≥1.0).
Statistical Analyses
We used a conditional logistic model to estimate the odds ratios (ORs) and 95% CIs of miscarriage. First, case-crossover ORs and exposure time-trend control crossover ORs were calculated separately. Second, the case-time-control ORs were calculated by dividing the case-crossover ORs by the control crossover ORs. Regression models fitted for case-time-control included an additional interaction term between exposure status in each period and an indicator that identifies an individual as either a case or a control. The regression coefficient for the interaction term provided an adjusted estimate of the OR between benzodiazepine use and miscarriage.17,19
To examine the robustness of our findings, we performed several sensitivity analyses. First, we assessed the impact of exposure duration on our findings by redefining the exposure assessment period as 7, 14, and 56 days. Second, to evaluate the potential impact of exposure misclassification, we extended the prescription period by 14 days. Third, we redefined the exposure as having filled at least 2 prescriptions to further minimize exposure misclassification. Fourth, to minimize outcome misclassification, we redefined miscarriage as at least 2 diagnosis codes on different dates. Fifth, we utilized commonly used diagnosis codes for miscarriage (ICD-9-CM codes 632 and 634; ICD-10-CM codes O02.1 and O03; or specific reimbursement codes for miscarriage procedures in Taiwan) to further minimize outcome misclassification. Sixth, to account for time-varying confounders, we adjusted the comedication use, such as antidepressants, opioid analgesics, anticonvulsant, Z-hypnotics, and other anxiolytics, in the model. Lastly, we conducted a negative control analysis by comparing 2 negative risk periods (defined as 31-58 and 91-118 days before the last menstrual period) with the reference period (181-208 days before the last menstrual period). If the main finding is subject to residual confounding, we can expect to observe a nonnull result from the negative control analysis. All analyses were performed using SAS version 9.4 (SAS Institute).
Results
Characteristics of the Identified Pregnancies
This study comprised a total of 3 067 122 pregnancies among 1 957 601 women, 136 134 of which (4.4%) resulted in miscarriage (eTable 5 in Supplement 1). The mean (SD) age of the study population was 30.61 (5.91) years. After matching with time-trend control individuals, we identified 134 864 pairs of pregnant women. The case and control groups were comparable in terms of age, psychiatric medical conditions, lifestyle factors, chronic comorbidities, medication use, and health care use, with a standardized difference less than 0.1 (Table 1). Additionally, the women with miscarriages demonstrated similar distributions of characteristics during the risk and 2 reference windows (eTables 6 and 7 in Supplement 1).
Table 1. Characteristics of Individuals With Miscarriages and Disease Risk Score–Matched Time-Trend Control Individuals.
| Characteristic | No. (%) | Standardized difference | |
|---|---|---|---|
| Individuals with miscarriages | Control individuals | ||
| Total, No. | 134 864 | 134 864 | NA |
| Age at pregnancy, mean (SD), y | 30.58 (5.90) | 30.56 (5.86) | 0.004 |
| Psychiatric medical conditions | |||
| Anxiety | 1228 (0.91) | 771 (0.57) | 0.040 |
| Insomnia | 1515 (1.12) | 858 (0.64) | 0.052 |
| Depression | 960 (0.71) | 741 (0.55) | 0.021 |
| Schizophrenia | 94 (0.07) | 81 (0.06) | 0.004 |
| Epilepsy | 144 (0.11) | 136 (0.10) | 0.002 |
| Bipolar disorder | 114 (0.08) | 96 (0.07) | 0.005 |
| Lifestyle factors | |||
| Obesity | 101 (0.07) | 84 (0.06) | 0.005 |
| Tobacco use | 90 (0.07) | 90 (0.07) | 0.000 |
| Alcohol use | 26 (0.02) | 22 (0.02) | 0.002 |
| Drug misuse | 18 (0.01) | 22 (0.02) | −0.002 |
| Chronic comorbidities | |||
| Hypertension | 634 (0.47) | 522 (0.39) | 0.013 |
| Hyperlipidemia | 450 (0.33) | 293 (0.22) | 0.022 |
| Diabetes | 564 (0.42) | 437 (0.32) | 0.016 |
| Chronic congestive heart failure | 23 (0.02) | 12 (0.01) | 0.007 |
| Chronic ischemic heart disease | 62 (0.05) | 58 (0.04) | 0.001 |
| Congenital heart disease | 72 (0.05) | 47 (0.03) | 0.009 |
| Cardiac valvular disease | 193 (0.14) | 169 (0.13) | 0.005 |
| Asthma | 594 (0.44) | 602 (0.45) | −0.003 |
| Hyperthyroidism | 732 (0.54) | 707 (0.52) | 0.002 |
| Chronic kidney disease | 128 (0.09) | 122 (0.09) | −0.001 |
| HIV | 7 (0.01) | 8 (0.01) | −0.001 |
| Sickle cell disease | 67 (0.05) | 39 (0.03) | 0.011 |
| Systemic lupus erythematosus | 220 (0.16) | 257 (0.19) | −0.007 |
| Medication use | |||
| Antidepressants | 2979 (2.21) | 2428 (1.80) | 0.029 |
| Opioid analgesics | 2567 (1.9) | 2535 (1.88) | 0.002 |
| Anticonvulsants | 792 (0.59) | 745 (0.55) | 0.005 |
| Z-hypnotics | 4182 (3.10) | 4148 (3.08) | 0.002 |
| Other anxiolytics | 4699 (3.48) | 4882 (3.62) | −0.007 |
| Antipsychotics | 1756 (1.30) | 1714 (1.27) | 0.003 |
| Antihypertensive drugs | 143 (0.11) | 146 (0.11) | −0.001 |
| Antidiabetic drugs | 1056 (0.78) | 1034 (0.77) | 0.002 |
| Lipid-modifying agents | 456 (0.34) | 444 (0.33) | 0.002 |
| Healthcare use | |||
| >5 Outpatient visits | 55 683 (41.29) | 55 180 (40.92) | 0.008 |
| >1 Inpatient visits | 5314 (3.94) | 5296 (3.93) | 0.001 |
Abbreviation: NA, not applicable.
Risk of Miscarriage
Of the pregnancies resulting in miscarriage, 1502 were exposed to benzodiazepines during the risk period only (1-28 days before miscarriage), and 2806 were exposed during the reference period only (181-208 days before the last menstrual period) (Table 2). For the time-trend control individuals, 753 and 2386 were exposed during the risk and reference periods, respectively. The case-time-control ORs revealed that exposure to benzodiazepines was associated with an increased risk of miscarriage (OR, 1.69; 95% CI, 1.52-1.87). Analysis of both long-acting and short-acting benzodiazepines showed an increased risk of miscarriage with case-time-control ORs of 1.67 (95% CI, 1.44-1.93) and 1.66 (95% CI, 1.47-1.87), respectively. Similar elevated risks were also observed in analyses using 31 to 58 days before the last menstrual period as the reference period (eTable 8 in Supplement 1).
Table 2. Associations Between Pregnancy Benzodiazepine Use and Risk of Miscarriage: Case-Time-Control (CTC) Design.
| Main analysis | Exposed during the risk period only, No.a | Exposed during the reference period only, No.b | OR (95% CI) |
|---|---|---|---|
| Overall benzodiazepine | |||
| CCO individuals with miscarriage | 1502 | 2806 | 0.54 (0.50-0.57) |
| CCO control individuals | 753 | 2386 | 0.32 (0.29-0.34) |
| CTC ratio | NA | NA | 1.69 (1.52-1.87) |
| Long-acting benzodiazepine | |||
| CCO individuals with miscarriage | 677 | 1358 | 0.50 (0.46-0.55) |
| CCO control individuals | 354 | 1202 | 0.30 (0.26-0.33) |
| CTC ratio | NA | NA | 1.67 (1.44-1.93) |
| Short-acting benzodiazepine | |||
| CCO individuals with miscarriage | 1083 | 1883 | 0.58 (0.54-0.62) |
| CCO control individuals | 555 | 1579 | 0.35 (0.32-0.39) |
| CTC ratio | NA | NA | 1.66 (1.47-1.87) |
Abbreviations: CCO, case-crossover; NA, not applicable; OR, odds ratio.
Risk period was defined as 1 to 28 days before miscarriage.
Reference period was defined as 181 to 208 days before the last menstrual period.
Subgroup Analyses
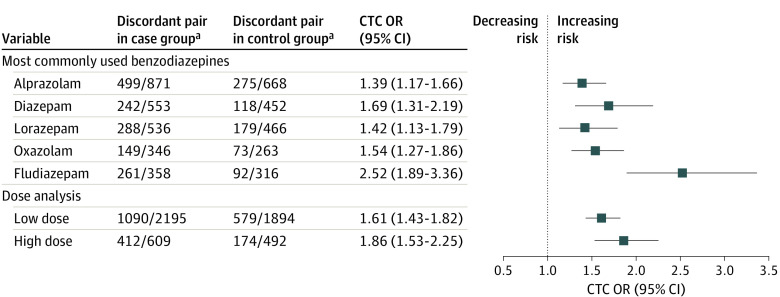
In the subgroup analyses, we identified an increased risk of miscarriage associated with each commonly used individual benzodiazepine, ranging from case-time-control ORs of 1.39 (95% CI, 1.17-1.66) for alprazolam to 2.52 (95% CI, 1.89-3.36) for fludiazepam (Figure 2; eTable 9 in Supplement 1). Additionally, a dose-response association was observed between benzodiazepine exposure and miscarriage, with the risk increasing from 1.61 (95% CI, 1.43-1.82) for low-dose exposure to 1.86 (95% CI, 1.53-2.25) for high-dose exposure.
Figure 2. Associations Between Pregnancy Benzodiazepine Use and Risk of Miscarriage: Subgroup Analyses.
CTC indicates case-time-control; OR, odds ratio.
aNumbers in the discordant pair refer to the number of pregnancies exposed to benzodiazepine in the risk period only and the number of pregnancies exposed to benzodiazepine in the reference period (181-208 days before the last menstrual period) only.
Sensitivity Analyses
Sensitivity analyses using risk and reference periods of 7 and 14 days, respectively, and redefining exposure as having filled at least 2 prescriptions yielded estimates that were generally consistent with those from the main analysis. When the exposure assessment period was redefined to 56 days (case-time-control OR, 1.28; 95% CI, 1.20-1.37) and the prescription period was extended by 14 days to address potential exposure misclassification (case-time-control OR, 1.33; 95% CI, 1.15-1.52), the risks of miscarriage were attenuated but still significantly increased. Moreover, consistent results were found in sensitivity analyses that redefined miscarriage using at least 2 diagnoses, adopted commonly used diagnosis codes for miscarriage, and accounted for potential time-varying confounders. In the negative control analyses, no increased risk was observed for miscarriage associated with benzodiazepine use (Table 3; eTable 10 in Supplement 1).
Table 3. Associations Between Benzodiazepine Use During Pregnancy and Risk of Miscarriage: Sensitivity Analyses.
| Sensitivity analyses | Reference period: 31-58 d before the last menstrual period | CTC, OR (95% CI) | Reference period: 181-208 d before the last menstrual period | CTC, OR (95% CI) | ||
|---|---|---|---|---|---|---|
| Discordant pair in case groupa | Discordant pair in control groupa | Discordant pair in case groupa | Discordant pair in control groupa | |||
| Time window of 7 d | 687/1426 | 309/1201 | 1.85 (1.59-2.14) | 751/1357 | 319/1197 | 2.04 (1.77-2.35) |
| Time window of 14 d | 946/1991 | 401/1777 | 2.09 (1.83-2.39) | 1058/1993 | 444/1677 | 1.96 (1.74-2.22) |
| Time window of 56 d | 2247/3903 | 1388/3421 | 1.41 (1.31-1.53) | 2592/4068 | 1627/3261 | 1.28 (1.20-1.37) |
| Dispensing ≥14 d | 560/1229 | 331/1065 | 1.48 (1.28-1.72) | 719/1357 | 465/1178 | 1.33 (1.15-1.52) |
| ≥2 Prescriptions dispensed | 525/1090 | 223/891 | 2.54 (2.11-3.07) | 418/1051 | 161/830 | 2.67 (2.16-3.29) |
| ≥2 Diagnoses of miscarriage | 756/1808 | 418/1646 | 1.68 (1.46-1.93) | 891/1839 | 512/1571 | 1.48 (1.31-1.68) |
| Redefine miscarriage | 728/1735 | 383/1665 | 1.83 (1.60-2.09) | 836/1797 | 463/1607 | 1.62 (1.42-1.85) |
| Adjust for time-varying factorsb | 1308/2778 | 623/2490 | 1.82 (1.63-2.04) | 1502/2806 | 753/2386 | 1.58 (1.41-1.78) |
| Negative control study | 2550/2383 | 2140/1906 | 0.96 (0.88-1.04)c | 2376/2241 | 1893/1785 | 1.00 (0.92-1.09)d |
Abbreviations: CTC, case-time-control; OR, odds ratio.
Numbers in the discordant pair refer to the number of pregnancies exposed to benzodiazepine in the risk period only and the number of pregnancies exposed to benzodiazepine in the reference period only.
Adjustment for comedication use (antidepressants, opioid analgesics, anticonvulsants, Z-hypnotics, and other anxiolytics).
The risk period was redefined as 31 to 58 days before the last menstrual period, and the reference period remained 181 to 208 days before the last menstrual period.
The risk period was redefined as 91 to 118 days before the last menstrual period, and the reference period remained 181 to 208 days before the last menstrual period.
Discussion
To the best of our knowledge, this is the first study using a self-controlled design that accounts for time-invariant confounders, such as genetic and sociodemographic factors, to investigate the association between benzodiazepine use during pregnancy and the risk of miscarriage. In this nationwide case-time-control study of approximately 3 million pregnancies, we found that benzodiazepine use during pregnancy was associated with an approximately 70% increased risk of miscarriage. Our results were generally consistent across diverse sensitivity analyses that considered exposure assessment periods, exposure and outcome misclassifications, and time-varying factors.
Our findings are consistent with a recent meta-analysis7 pooling data from 5 studies, which reported a significant association between benzodiazepine use during pregnancy and risk of miscarriage (OR,1.86; 95% CI, 1.43-2.42). Nevertheless, substantial heterogeneity observed across these studies suggests the presence of potential confounding variables in most prior studies. Additionally, limited power due to small sample size in previous studies often led to uncertainty about the risk estimates for miscarriage.7 By design, our case-time-control study eliminates the biasing effect of unmeasured confounding factors in situations where exposure varies over time.18 Moreover, the sample size of our population-based study, comprising 3 million pregnant women over the 15-year study period, allowed relatively precise estimates for miscarriage associated with benzodiazepine exposure during pregnancy, while addressing potential confounders.
A previous population-based study in the UK scrutinizing the effects of antenatal depression, anxiety, and benzodiazepine treatment on the risks of nonlive pregnancy found that pregnant women exposed to benzodiazepines during early pregnancy had a 60% higher risk of miscarriage compared to those with untreated depression or anxiety.6 While this approach may mitigate confounding by indication, it may not fully address disease severity as in our study. On the other hand, Sheehy et al9 took into consideration of mood, anxiety, and sleep disorders of pregnant women, and adjusted several proxies of illness severity in their study. They found a significant association between benzodiazepine use during early pregnancy and miscarriage, with an adjusted OR of 1.81. Moreover, the E-value of 3.1 with a lower limit of 2.6 in their study suggested that unmeasured confounding was unlikely to explain the findings. Nevertheless, the presence of unmeasured, inadequately measured, and unknown confounders may still impact the interpretation of their results.10
The dynamic characteristics of medication use, particularly during pregnancy,29 underscores the importance of considering the temporal aspect when examining medication effects. However, the case-crossover design may not be suitable when there is a time trend in exposure.22,30 In our study, we observed an inverse association when using a case-only design. The case-time-control design, therefore, mitigates bias stemming from exposure time trends by including a control group.18,20,21 In addition, it is worth noting that databases lacking precise last menstrual period data may hinder the ability to estimate the magnitude of the effect using the case-time-control design.31,32 The negative control design in our study, which redefined different risk periods before the last menstrual period, yielded null results, and further strengthened the accuracy of the approach we used to determine the last menstrual period.
While an increasing number of studies have shown that benzodiazepines used during pregnancy do not appear to be associated with an elevated risk of adverse pregnancy outcomes,27,33,34 concerns regarding teratogenicity and fetal developmental impairments persist. In our study, an increased risk of miscarriage associated with benzodiazepine use during early pregnancy was observed after accounting for measurable confounders, and the results were unlikely to be due to unmeasured confounding. In fact, aneuploidies account for nearly half of all pregnancy losses, and it is unlikely that benzodiazepines would induce such chromosomal abnormalities. Our study only included miscarriages from around 8 weeks of gestation until the beginning of week 20, and miscarriages due to aneuploidies are more likely to occur very early in pregnancy. Given this, anomalies in the central nervous system could be the most plausible mechanism for the observed increased risk of miscarriages in this study.
On the other hand, a recent study from Denmark,35 which reported the steady rates of first trimester miscarriages over the past 40 years, warrants further attention when interpreting our findings. We observed a relatively low percentage of miscarriage (4.4%) in our study compared to that reported in Denmark (consistently 10%).35 Given the notable increase in benzodiazepine use during this period, one might expect a discernible rise in early pregnancy losses if benzodiazepines significantly impact miscarriage risk. Yet, it is important to highlight that in Denmark, only a small proportion of pregnant women are prescribed the medications included in our study.36 Thus, a heightened risk of 70% in this small demographic might be overshadowed by the broader miscarriage statistics.
Limitations
With great efforts to bridge the knowledge gap, this study provides insights for future drug safety communications regarding benzodiazepine use during pregnancy. However, several limitations exist, mainly due to the nature of the birth certificate-based and claims-based databases. First, exposure classification relied on filled prescriptions, potentially leading to misclassification. To enhance reliability, we extended the prescription period by 14 days and redefined the exposure as having filled at least 2 prescriptions in sensitivity analyses and yielded consistent results. Second, pregnant individuals may sometimes report a voluntary termination of pregnancy as an involuntary one. It is also possible that some cases were inevitably missed, as not all miscarriages are reported or even recognized. However, we adopted a 3-step algorithm to capture miscarriage cases and conducted sensitivity analyses with redefined outcomes. The consistent findings affirm the validity of our approaches. Third, residual confounding or confounding by indication cannot be completely ruled out. While the pregnant women in the case group demonstrated similar distributions of benzodiazepines-associated indications during the risk and 2 reference windows (standardized difference <0.1), we may not have fully accounted for certain residual confounding factors not captured in the databases, such as underreporting anxiety symptoms or time-varying confounding by indication (eg, major life stressors, addiction, or benzodiazepine withdrawal). For example, withdrawal from benzodiazepines can lead to various physical and mental symptoms that might potentially affect fetal development. However, the E-value in our study suggested that unmeasured confounding was unlikely to explain the findings (eTable 11 in Supplement 1). Fourth, the low prevalence of benzodiazepine use during pregnancy in this study also makes it challenging to establish a specific exposure threshold. Fifth, although the case-time-control design allows for adjustment of the time trend of exposure, bias may emerge if there are differing exposure trends between case individuals and control individuals. To minimize the potential introduction of additional bias due to divergent time trends, we used a comprehensive disease-risk score to match individuals with miscarriages and control individuals. Still, further studies adopting different study designs or using different data sources are warranted to verify our findings.
Conclusions
In this study, benzodiazepine exposure during early pregnancy showed a significant association with an increased risk of miscarriage after accounting for measurable confounders, and results were unlikely to be due to unmeasured confounding. This association was observed across various analyses, including consideration of duration of action, specific individual agents, and dosage. These findings suggest that caution is warranted when using benzodiazepines during early pregnancy. The findings of this study also provide evidence to guide clinicians in making informed decisions regarding the treatment of psychiatric and sleep disorders in pregnant women. Prescribing benzodiazepines should only be considered following a comprehensive evaluation of the potential benefits and risks for both the mother and the child.
eFigure 1. Flow chart of study population selection
eFigure 2. Algorithms for miscarriage definition
eTable 1. Data sources
eTable 2. Definitions of covariates using for the disease risk score calculation
eTable 3. Study outcome: ICD codes and specific reimbursement codes
eTable 4. Study medication category and ATC codes
eTable 5. Characteristics of cases and controls before matched
eTable 6. Characteristics of cases between risk period and reference period (31-58 days before the LMP)
eTable 7. Characteristics of cases between risk period and reference period (181-208 days before the LMP)
eTable 8. Association between pregnancy benzodiazepine use and the risk of miscarriage: case-time-control design
eTable 9. Association between pregnancy benzodiazepine use and the risk of miscarriage: subgroup analyses
eTable 10. Association between pregnancy benzodiazepine use and the risk of miscarriage: sensitivity analyses
eTable 11. Calculating the E-value to assess the impact of unmeasured confounders
Data sharing statement
References
- 1.Gentile S. Anxiety and sleep disorders, psychopharmacology, and pregnancy. In: Galbally M, Snellen M, Lewis A, eds. Psychopharmacology and Pregnancy: Treatment Efficacy, Risks, and Guidelines. Springer; 2014:87-102. doi: 10.1007/978-3-642-54562-7_7 [DOI] [Google Scholar]
- 2.Bais B, Molenaar NM, Bijma HH, et al. Prevalence of benzodiazepines and benzodiazepine-related drugs exposure before, during and after pregnancy: A systematic review and meta-analysis. J Affect Disord. 2020;269:18-27. doi: 10.1016/j.jad.2020.03.014 [DOI] [PubMed] [Google Scholar]
- 3.Qato DM, Gandhi AB. Opioid and benzodiazepine dispensing and co-dispensing patterns among commercially insured pregnant women in the United States, 2007-2015. BMC Pregnancy Childbirth. 2021;21(1):350. doi: 10.1186/s12884-021-03787-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanto JH. Use of benzodiazepines during pregnancy, labour and lactation, with particular reference to pharmacokinetic considerations. Drugs. 1982;23(5):354-380. doi: 10.2165/00003495-198223050-00002 [DOI] [PubMed] [Google Scholar]
- 5.Lee DH, Kang SK, Lee RH, et al. Effects of peripheral benzodiazepine receptor ligands on proliferation and differentiation of human mesenchymal stem cells. J Cell Physiol. 2004;198(1):91-99. doi: 10.1002/jcp.10391 [DOI] [PubMed] [Google Scholar]
- 6.Ban L, Tata LJ, West J, Fiaschi L, Gibson JE. Live and non-live pregnancy outcomes among women with depression and anxiety: a population-based study. PLoS One. 2012;7(8):e43462. doi: 10.1371/journal.pone.0043462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grigoriadis S, Graves L, Peer M, et al. Pregnancy and delivery outcomes following benzodiazepine exposure: a systematic review and meta-analysis. Can J Psychiatry. 2020;65(12):821-834. doi: 10.1177/0706743720904860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H, Koh JW, Kim YA, et al. Pregnancy and neonatal outcomes after exposure to alprazolam in pregnancy. Front Pharmacol. 2022;13:854562. doi: 10.3389/fphar.2022.854562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheehy O, Zhao JP, Bérard A. Association between incident exposure to benzodiazepines in early pregnancy and risk of spontaneous abortion. JAMA Psychiatry. 2019;76(9):948-957. doi: 10.1001/jamapsychiatry.2019.0963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrade C. Gestational exposure to benzodiazepines, 1: the risk of spontaneous abortion examined through the prism of research design. J Clin Psychiatry. 2019;80(5):19f13076. doi: 10.4088/JCP.19f13076 [DOI] [PubMed] [Google Scholar]
- 11.Huybrechts KF, Bateman BT, Hernández-Díaz S. Use of real-world evidence from healthcare utilization data to evaluate drug safety during pregnancy. Pharmacoepidemiol Drug Saf. 2019;28(7):906-922. doi: 10.1002/pds.4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sujan AC, Rickert ME, Öberg AS, et al. Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder in offspring. JAMA. 2017;317(15):1553-1562. doi: 10.1001/jama.2017.3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Chan AYL, Coghill D, et al. Association between prenatal exposure to antipsychotics and attention-deficit/hyperactivity disorder, autism spectrum disorder, preterm birth, and small for gestational age. JAMA Intern Med. 2021;181(10):1332-1340. doi: 10.1001/jamainternmed.2021.4571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng LC, Lin CW, Lin YC, et al. Association between maternal benzodiazepine or Z-hypnotic use in early pregnancy and the risk of stillbirth, preterm birth, and small for gestational age: a nationwide, population-based cohort study in Taiwan. Lancet Psychiatry. 2023;10(7):499-508. doi: 10.1016/S2215-0366(23)00148-7 [DOI] [PubMed] [Google Scholar]
- 15.Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan National Health Insurance Research Database. JAMA Intern Med. 2015;175(9):1527-1529. doi: 10.1001/jamainternmed.2015.3540 [DOI] [PubMed] [Google Scholar]
- 16.Lin CM, Lee PC, Teng SW, Lu TH, Mao IF, Li CY. Validation of the Taiwan Birth Registry using obstetric records. J Formos Med Assoc. 2004;103(4):297-301. [PubMed] [Google Scholar]
- 17.Suissa S. The case-time-control design. Epidemiology. 1995;6(3):248-253. doi: 10.1097/00001648-199505000-00010 [DOI] [PubMed] [Google Scholar]
- 18.Suissa S. The case-time-control design: further assumptions and conditions. Epidemiology. 1998;9(4):441-445. doi: 10.1097/00001648-199807000-00016 [DOI] [PubMed] [Google Scholar]
- 19.Dong YH, Wang SV, Gagne JJ, Wu LC, Chang CH. Comparison of different case-crossover variants in handling exposure-time trend or persistent-user bias: using dipeptidyl peptidase-4 inhibitors and the risk of heart failure as an example. Value Health. 2020;23(2):217-226. doi: 10.1016/j.jval.2019.09.2746 [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Wu CS, Olsen J. Trimethoprim use before pregnancy and risk of congenital malformation: reanalyzed using a case-crossover design and a case-time-control design. Pharmacoepidemiol Drug Saf. 2014;23(10):1076-1083. doi: 10.1002/pds.3691 [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Pedersen LH, Wu CS, Petersen I, Sørensen HT, Olsen J. Antidepressant use during pregnancy and risk of congenital heart defects: a case-time-control study. Pharmacoepidemiol Drug Saf. 2019;28(9):1180-1193. doi: 10.1002/pds.4844 [DOI] [PubMed] [Google Scholar]
- 22.Hernández-Díaz S, Hernán MA, Meyer K, Werler MM, Mitchell AA. Case-crossover and case-time-control designs in birth defects epidemiology. Am J Epidemiol. 2003;158(4):385-391. doi: 10.1093/aje/kwg144 [DOI] [PubMed] [Google Scholar]
- 23.Connolly JG, Gagne JJ. Comparison of calipers for matching on the disease risk score. Am J Epidemiol. 2016;183(10):937-948. doi: 10.1093/aje/kwv302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675-700. doi: 10.1111/jsr.12594 [DOI] [PubMed] [Google Scholar]
- 25.Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14(Suppl 1):S1. doi: 10.1186/1471-244X-14-S1-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benson LS, Holt SK, Gore JL, et al. Early pregnancy loss management in the emergency department vs outpatient setting. JAMA Netw Open. 2023;6(3):e232639. doi: 10.1001/jamanetworkopen.2023.2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noh Y, Lee H, Choi A, et al. First-trimester exposure to benzodiazepines and risk of congenital malformations in offspring: a population-based cohort study in South Korea. PLoS Med. 2022;19(3):e1003945. doi: 10.1371/journal.pmed.1003945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC classification and DDD assignment, 2022. Accessed 8 April, 2023. https://www.whocc.no/atc_ddd_index_and_guidelines/guidelines/
- 29.Cohen JM, Selmer R, Furu K, Karlstad Ø. Interrupted time series analysis to assess changes in prescription filling around conception and implications for exposure misclassification. Pharmacoepidemiol Drug Saf. 2020;29(6):745-749. doi: 10.1002/pds.4974 [DOI] [PubMed] [Google Scholar]
- 30.Nicholas JM, Grieve AP, Gulliford MC. Within-person study designs had lower precision and greater susceptibility to bias because of trends in exposure than cohort and nested case-control designs. J Clin Epidemiol. 2012;65(4):384-393. doi: 10.1016/j.jclinepi.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 31.Shahn Z, Hernán MA, Robins JM. A formal causal interpretation of the case-crossover design. Biometrics. 2023;79(2):1330-1343. doi: 10.1111/biom.13749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernández-Díaz S, Huybrechts KF, Chiu YH, Yland JJ, Bateman BT, Hernán MA. Emulating a target trial of interventions initiated during pregnancy with healthcare databases: the example of COVID-19 vaccination. Epidemiology. 2023;34(2):238-246. doi: 10.1097/EDE.0000000000001562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grigoriadis S, Graves L, Peer M, et al. Benzodiazepine use during pregnancy alone or in combination with an antidepressant and congenital malformations: systematic review and meta-analysis. J Clin Psychiatry. 2019;80(4):18r12412. doi: 10.4088/JCP.18r12412 [DOI] [PubMed] [Google Scholar]
- 34.Chan AYL, Gao L, Howard LM, et al. Maternal benzodiazepines and Z-drugs use during pregnancy and adverse birth and neurodevelopmental outcomes in offspring: a population-based cohort study. Psychother Psychosom. 2023;92(2):113-123. doi: 10.1159/000529141 [DOI] [PubMed] [Google Scholar]
- 35.Lidegaard Ø, Mikkelsen AP, Egerup P, Kolte AM, Rasmussen SC, Nielsen HS. Pregnancy loss: a 40-year nationwide assessment. Acta Obstet Gynecol Scand. 2020;99(11):1492-1496. doi: 10.1111/aogs.13860 [DOI] [PubMed] [Google Scholar]
- 36.Bais B, Munk-Olsen T, Bergink V, Liu X. Prescription patterns of benzodiazepine and benzodiazepine-related drugs in the peripartum period: a population-based study. Psychiatry Res. 2020;288:112993. doi: 10.1016/j.psychres.2020.112993 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flow chart of study population selection
eFigure 2. Algorithms for miscarriage definition
eTable 1. Data sources
eTable 2. Definitions of covariates using for the disease risk score calculation
eTable 3. Study outcome: ICD codes and specific reimbursement codes
eTable 4. Study medication category and ATC codes
eTable 5. Characteristics of cases and controls before matched
eTable 6. Characteristics of cases between risk period and reference period (31-58 days before the LMP)
eTable 7. Characteristics of cases between risk period and reference period (181-208 days before the LMP)
eTable 8. Association between pregnancy benzodiazepine use and the risk of miscarriage: case-time-control design
eTable 9. Association between pregnancy benzodiazepine use and the risk of miscarriage: subgroup analyses
eTable 10. Association between pregnancy benzodiazepine use and the risk of miscarriage: sensitivity analyses
eTable 11. Calculating the E-value to assess the impact of unmeasured confounders
Data sharing statement