Abstract
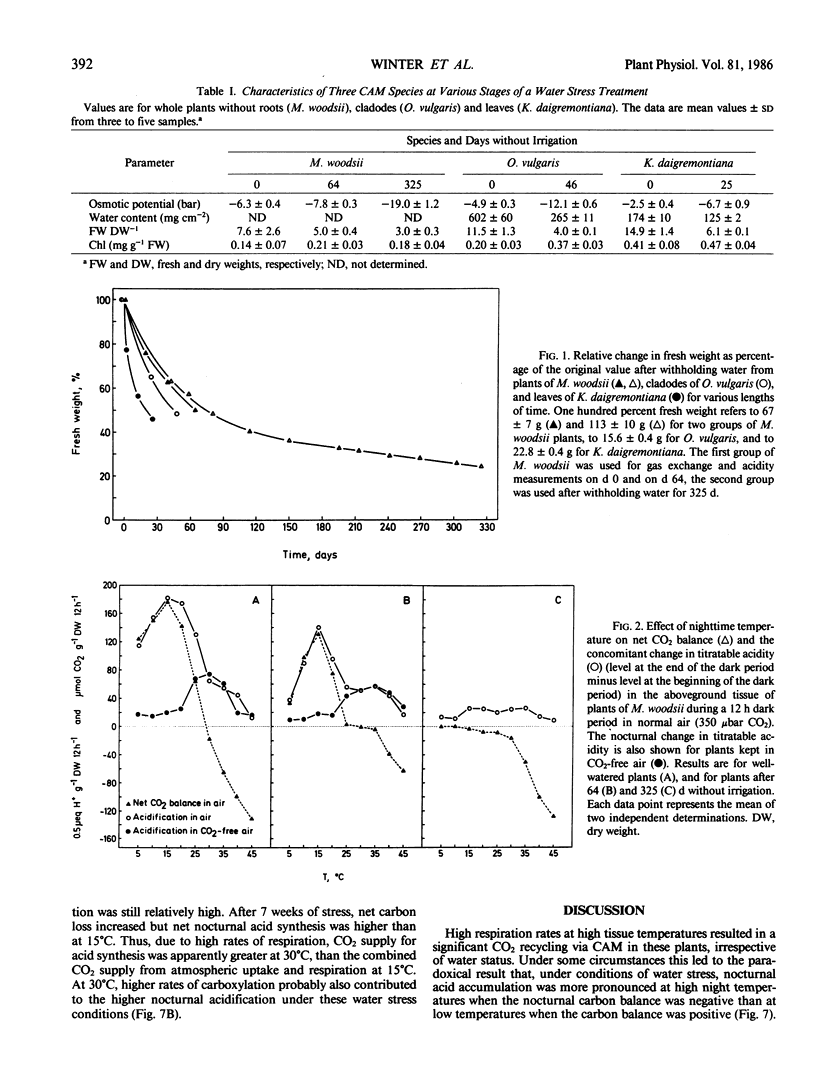
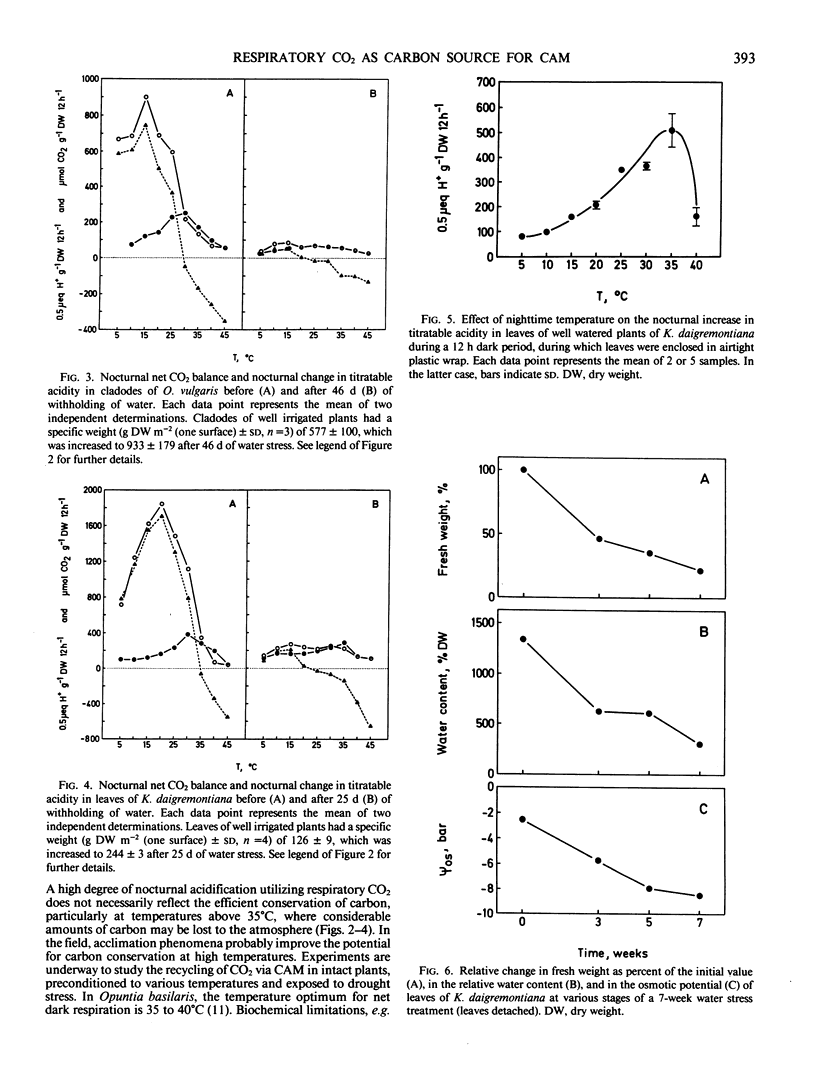
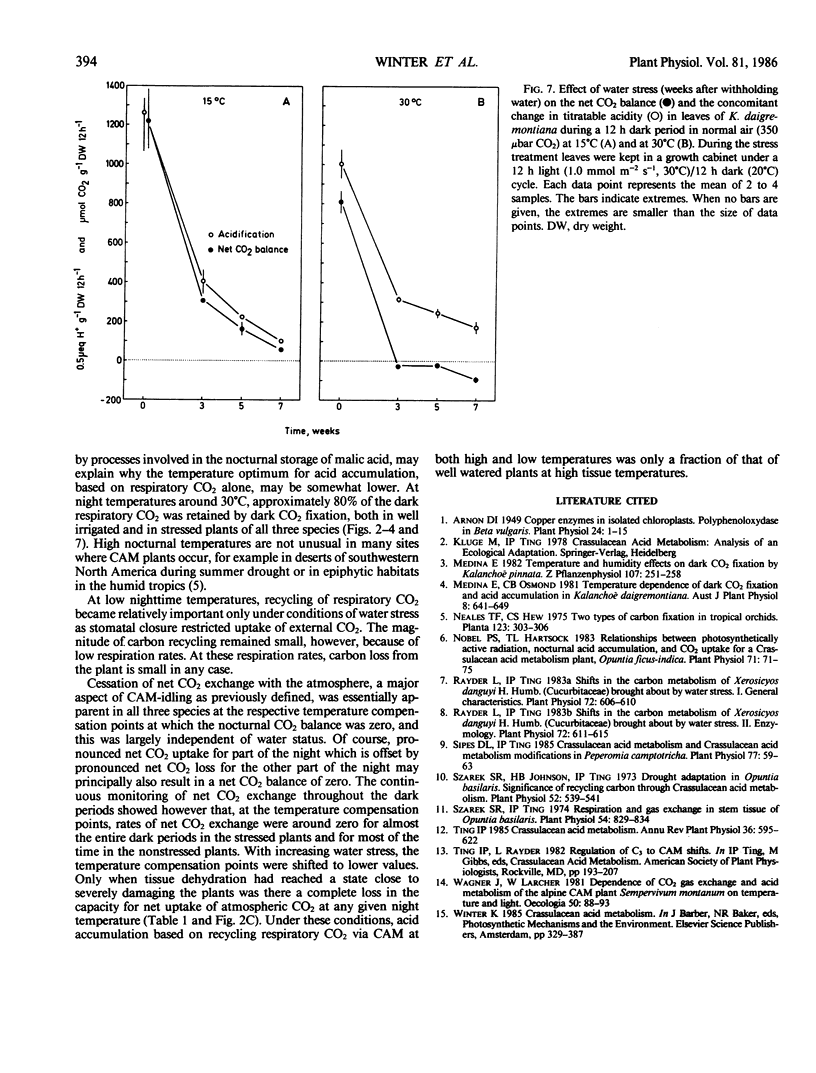
Temperature effects on nocturnal carbon gain and nocturnal acid accumulation were studied in three species of plants exhibiting Crassulacean acid metabolism: Mamillaria woodsii, Opuntia vulgaris, and Kalanchoë daigremontiana. Under conditions of high soil moisture, nocturnal CO2 gain and acid accumulation had temperature optima at 15 to 20°C. Between 5 and 15°C, uptake of atmospheric CO2 largely accounted for acid accumulation. At higher tissue temperatures, acid accumulation exceeded net carbon gain indicating that acid synthesis was partly due to recycling of respiratory CO2. When plants were kept in CO2-free air, acid accumulation based on respiratory CO2 was highest at 25 to 35°C. Net acid synthesis occurred up to 45°C, although the nocturnal carbon balance became largely negative above 25 to 35°C. Under conditions of water stress, net CO2 exchange and nocturnal acid accumulation were reduced. Acid accumulation was proportionally more decreased at low than at high temperatures. Acid accumulation was either similar over the whole temperature range (5-45°C) or showed an optimum at high temperatures, although net carbon balance became very negative with increasing tissue temperatures. Conservation of carbon by recycling respiratory CO2 was temperature dependent. At 30°C, about 80% of the dark respiratory CO2 was conserved by dark CO2 fixation, in both well irrigated and water stressed plants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina Banegas A., Robleda Prats E., García López M. Tuberculosis nasal. An Otorrinolaringol Ibero Am. 1982;9(4):251–258. [PubMed] [Google Scholar]
- Nobel P. S., Hartsock T. L. Relationships between Photosynthetically Active Radiation, Nocturnal Acid Accumulation, and CO(2) Uptake for a Crassulacean Acid Metabolism Plant, Opuntia ficus-indica. Plant Physiol. 1983 Jan;71(1):71–75. doi: 10.1104/pp.71.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayder L., Ting I. P. Shifts in the Carbon Metabolism of Xerosicyos danguyi H. Humb. (Cucurbitaceae) Brought About by Water Stress : I. General Characteristics. Plant Physiol. 1983 Jul;72(3):606–610. doi: 10.1104/pp.72.3.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayder L., Ting I. P. Shifts in the Carbon Metabolism of Xerosicyos danguyi H. Humb. (Cucurbitaceae) Brought About by Water Stress : II. Enzymology. Plant Physiol. 1983 Jul;72(3):611–615. doi: 10.1104/pp.72.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipes D. L., Ting I. P. Crassulacean Acid Metabolism and Crassulacean Acid Metabolism Modifications in Peperomia camptotricha. Plant Physiol. 1985 Jan;77(1):59–63. doi: 10.1104/pp.77.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarek S. R., Johnson H. B., Ting I. P. Drought Adaptation in Opuntia basilaris: Significance of Recycling Carbon through Crassulacean Acid Metabolism. Plant Physiol. 1973 Dec;52(6):539–541. doi: 10.1104/pp.52.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarek S. R., Ting I. P. Respiration and Gas Exchange in Stem Tissue of Opuntia basilaris. Plant Physiol. 1974 Dec;54(6):829–834. doi: 10.1104/pp.54.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]


