Abstract
Experimentally, the standard molar enthalpy of formation in the crystalline phase at 298.15 K, ΔfHm°(cr) for 7-methoxy-4-methylcoumarin (7M4MC) was calculated by traditional linear regression, which was obtained by combustion calorimetry. Similarly, the standard molar enthalpy of sublimation was determined through the standard molar enthalpy of fusion and by the standard molar enthalpy of vaporization, from differential scanning calorimetry and thermogravimetry, respectively; lately using these results, the standard molar enthalpy of formation in the gas phase was calculated at 298.15 K, ΔfHm°(g). In addition ML was used to predict the standard molar enthalpy of formation in the gas phase for the 7M4MC, constructing an experimental data set containing three kinds of functional groups: esters, coumarins, and aromatic compounds. The procedure was performed by using multiple linear regression algorithms and stochastic gradient descent with a R2 of 0.99. The obtained models were used to compare those predicted values versus experimental for coumarins, resulting in an average error rate of 9.0%. Likewise, four homodesmic reactions were proposed and predicted with the multiple linear regression algorithm of ML obtaining good results.
Introduction
Coumarins are heterocyclic compounds containing a lactone group. These compounds represent a wide range of natural, pharmaceutical, and phytochemical products. The interest in natural coumarins has significantly increased over time, leading to their discovery in plant species with different chemical structures and phases (crystalline and gas). In addition, many coumarins, such as 7-methoxy-4-methylcoumarin, have shown histamine release inhibition from mast cells and moderate adrenergic activity,1 as well as a focusing applications in lymphatic vasculature chronic disease.2−4 Albeit, coumarins and their derivatives exhibit antimicrobial,5,6 anti-inflammatory,7,8 antispasmodic, antiviral,9,10 antioxidant,11 and enzyme inhibitor properties.12,13 In addition, some coumarin-based products have demonstrated excellent results as antitumor or as photochemotherapeutic agents in psoriasis treatment.14−16
Despite the multiple potential applications described above, there are very few reports on their thermochemical properties. Among these properties, one of the most important is the standard molar enthalpy of formation, which provides a better explanation and support from the synthesis process.17,18 Thermal and calorimetric techniques are commonly used to determine experimentally this property19,20 by considering the differential scanning calorimetry (DSC), thermogravimetry, and combustion calorimetry, respectively. Furthermore, it is possible to predict this property with the use of computational techniques such as machine learning algorithms.21,22 This work presents the experimental results related to 7-methoxy-4-methyl coumarin (7M4MC) compounds, as seen in Figure 1, which was predicted using multiple linear regression (MLR) and stochastic gradient descent regression (SGD) models in the gas phase23,24 based on the Benson’s group additivity method.25
Figure 1.
7-Methoxy-4-methylcoumarin.
Experimental Section
Materials and Purification
The 7M4MC [CAS 2555-28-4] compound (supplied by Merck), with a 0.98 mass fraction purity, was determined by DSC, although the commercial label showed 0.99. Therefore, it was purified following a recrystallization process with ethyl acetate and dried under a high vacuum for 3 h, repeating this step twice. To determine the purity, melting temperature, and fusion enthalpy, a DSC in a TA Instrument Q2000 previously calibrated for temperature and energy with high-purity indium was used. The heating rate was 3.0 °C min–1 under a 20.0 cm3 min–1 dry nitrogen constant flux; furthermore, a temperature range from 333.15 to 453.15 K was considered. The correspondent thermograms are shown in figure A and figure B in the Supporting Information.
Crystalline phase 7M4MC heat capacities were obtained from 273.15 to 368.15 K using a TA Instrument DSC Q2000 that was previously calibrated with aluminum oxide at a heating rate of 10.0 K min–1 under a nitrogen flow of 50.0 cm3 min–1. For this reference compound, the calculated heat capacity was 0.7748 J g–1 K–1 at 298.15 K reaching 99.95%, this value is closer from those reported in the literature (0.7752 J g–1 K–1 at 298.15 K).26
Combustion Calorimetry
A static semimicro combustion bomb was used to determine the combustion energies. This instrument was calibrated using benzoic acid (NIST Standard Reference Material 39j) with a combustion mass energy of −(26434.0 ± 3.0) J g–1 (the uncertainty corresponds to expanded uncertainty), which was corrected using Coops et al. equation.27 The calorimetric equivalent of ϵ (calor) = (1281.2 ± 0.8) J K–1 (the uncertainty is twice the standard deviation of the mean) was calculated from six combustion experiments at 3.04 MPa pressure under a high purity gaseous oxygen (Air Liquide Corp., mass fraction of 0.99999) with 0.1 cm3 of deionized water.28
To maintain conditions similar to those of the reference material, the 7M4MC was oxidized considering the same parameters. The cotton-thread fuse (C1.000H1.742O0.921) used possess a combustion specific energy of −(16945.2 ± 4.2) J g–1 (the uncertainty is the standard deviation of the mean). Albeit the combustion energies in standard conditions were determined through Washburn corrections.27 The compounds physical properties are resumed in Table 1,29 where the elements’ atomic weights were those reported by IUPAC in 2021.30 To calculate the energy change associated with the pressure, the estimated value of (δu/δp)T = −0.2 J g–1 MPa–1 at 298.15 K was used, which is a typical value considered for most of the solid organic compounds.31
Table 1. Physical Properties at po = 0.1 MPa.
| compound | Ma(g mol–1) | ρ (g cm–3) | –(δu/δp)T (J g–1 MPa–1) | Cp(cr) (298.15 K) (J g–1 K–1) |
|---|---|---|---|---|
| 7M4MC | 190.198 | 1.195 ± 0.06b | 0.200c | 1.439 ± 0.023d |
| benzoic acid | 122.123 | 1.320c | 0.115c | 1.209c |
| cotton | 28.502 | 1.500c | 0.289c | 1.674c |
Based on the 2021 IUPAC recommendation.30
Calculated using Advanced Chemistry Development (ACD/Laboratories) Software v11.02.
Estimated value in the reference at T = 298.15 K.31
Experimental average value from two experiments using a DSC device. Its uncertainty corresponds to expanded uncertainty with a level of confidence of approximately 95%. Including the contributions from the calibration and u(T) = 0.1 K. The experiments were realized under average atmospheric pressure (78.8 kPa), u(p) = 1 kPa.
Thermogravimetry
The indirect method of thermal gravimetric analysis (TGA) was used to determine the vaporization enthalpy using the Langmuir equation.
| 1 |
where (dm/dt) is the rate of mass loss, A is the area which was subjected to the vaporization process, T is the temperature, p is the vapor pressure, R is the ideal gas constant, M is the molar mass of the compound, and γ is a vaporization constant.
Combining Clausius–Clapeyron’s to eq 1 yielded the expression that is applied to calculate the enthalpy of vaporization
| 2 |
where 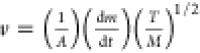 and B includes the integration
constant and the term
and B includes the integration
constant and the term  . Using eq 2, it was possible to obtain the vaporization enthalpies
by applying a linear adjustment to ln v vs 1/T. A TA Instruments Q500 device, previously calibrated for
mass and temperature, was used to register the term dm/dt with high precision. The thermogravimetric system
was tested with phenanthrene and pyrene secondary standards (J. T.
Baker). The standard molar enthalpy of vaporization results at 298.15
K were (77.9 ± 1.4) kJ mol–1 for phenanthrene
and (86.4 ± 1.4) kJ mol–1 for pyrene (Tables S1–S3 in the Supporting Information).
The calculated enthalpies are consistent with those reported in the
literature.32
. Using eq 2, it was possible to obtain the vaporization enthalpies
by applying a linear adjustment to ln v vs 1/T. A TA Instruments Q500 device, previously calibrated for
mass and temperature, was used to register the term dm/dt with high precision. The thermogravimetric system
was tested with phenanthrene and pyrene secondary standards (J. T.
Baker). The standard molar enthalpy of vaporization results at 298.15
K were (77.9 ± 1.4) kJ mol–1 for phenanthrene
and (86.4 ± 1.4) kJ mol–1 for pyrene (Tables S1–S3 in the Supporting Information).
The calculated enthalpies are consistent with those reported in the
literature.32
Computational Details
MLR Model
The MLR model is a versatile statistical model for evaluating a continuous target and predictors correlation.33
The predictors can be continuous, categorical, or derived fields so that nonlinear relationships are also supported. The model is considered linear because it consists of additive terms, where each term is a predictor which is multiplied by an estimated coefficient (βi) (see eq 3).
The constant term (intercept, β0) is also usually added to the model.34 Multiple regression models can be used to predict the value of the dependent variable or to assess the influence that the predictors have on it (the latter should be analyzed with caution so as not to misinterpret the cause-effect).35
| 3 |
The model relates a dependent variable (y) with n regressor variables (Xn) and finally, a random variable (β0) that collects all those factors that are not collectable and are associated with chance.36
It is important to bear in mind that the magnitude of each partial regression coefficient depends on the units in which the predictor variable is measured, so its magnitude is not associated with each predictor importance. To determine each variable impact over the model, the standardized partial coefficients are used.37
Stochastic Gradient Descent Regression
The SGD algorithm behaves like a straight-line formula, but it is based on a convex function.38 The starting point is just an arbitrary point, so the performance can be evaluated. From that consideration, the derivative (or slope) could be determined, the slope is associated with the parameter updates, i.e., weights and bias; at the starting point it will be steeper, but as new parameters are generated, the slope should gradually decrease until it reaches the curve’s lowest point, known as the convergence point.39 The SGD runs a training epoch for each example within the data set and updates each parameter of the training example, one at a time.40
Results and Discussion
Experimental Results
Table 2 shows four experimental results from the compound 7M4MC, which are the data from the purity, the melting point, the enthalpy of phase change, and the heat capacity at constant pressure, including the experimental uncertainties.
Table 2. Melting Temperature of 7M4MCa.
| purity | Tfus (K) | ΔcrlHm (Tfus) kJ mol–1 | Cp,m (cr, 298.15 K) J mol–1 K–1 |
|---|---|---|---|
| 0.9987 | 432.9 | 31.7 | |
| 0.9978 | 433.0 | 31.9 | |
| 0.9976 | 430.6 | 29.4 | |
| 0.9951 | 431.2 | 29.3 | |
| 0.9973 ± 0.0015 | 431.9 ± 1.2 | 30.6 ± 1.4 | 1.439 ± 0.023 |
The uncertainties correspond to the expanded uncertainty with a level of confidence of 95%, including uncertainty of calibration and u(T) = 0.1 K. The experiments were made under average atmospheric pressure (78.8 kPa), u(p) = 1 kPa.
The molar heat capacity was calculated from 273.15 to 388.15 K using the results obtained by DSC, these data are shown in Supporting Information in Table S4; for this calculus, the eq 4 is used, this was obtained from a polynomial regression applied to data in a graph of heat capacity vs temperature.
 |
4 |
On the other hand, Ngoc Toan41 determined that the melting temperature ranged from 432 to 435 K, which compared to our value presented a 0.37% error. It is important to mention that in the past decade in the literature, no value for 7M4MC enthalpy of fusion of the compound is reported.
Table 3 shows the 7M4MC combustion results. The six combustions’ complete data sets are shown in the Supporting Information in Table S5.
Table 3. Combustion Experiments for 7M4MC at 298.15 K and po = 0.1 MPa.
| 7M4MCa | |
|---|---|
| m (7M4MC)/g | 0.0408401 |
| m (cotton)/g | 0.0005911 |
| m (platinum)/g | 0.2311213 |
| ΔTc/K | 0.891702 |
| ϵ (calor) (−ΔTc)/kJ | –1.14245 |
| ϵ (cont) (−ΔTc)/kJ | –0.00111 |
| ΔUign/kJ | 0.00418 |
| ΔUIBP/kJ | –1.13938 |
| ΔUcorr/kJ | 0.00081 |
| (–mΔcuo)(cotton)/kJ | 0.01002 |
| (–mΔcuo) (7M4MC)/kJ | –1.12855 |
| Δcuo (7M4MC)/kJ g–1 | –27.6334 |
| average value ⟨−27.6912 ± 0.0162⟩/kJ g–1 | |
m is the mass corrected for buoyancy using densities listed in Table 1, ΔTc is the corrected temperature rise, ϵ (cont) is the energy equivalent of the contents of the bomb, ΔUign is the ignition energy, and ΔUIBP is the energy of the isothermal bomb process, which was calculated by ΔUIBP = [ϵ (calor)(−ΔTc) + ϵ (cont)(−ΔTc) + (ΔUign) (ΔUcorr)]. ΔUcorr is the correction to standard state and Δcuo (7M4MC) is the 7-methoxy-4-methylcoumarin mass energy of combustion. The uncertainty corresponds to the expanded uncertainty with a confidence level of 95%.
The average combustion energy, enthalpy, and uncertainty at 298.15 K and 0.1 MPa are shown in Table 4. To calculate the standard molar enthalpy of formation ΔfHm°(cr) from the molar enthalpy of combustion ΔcHm°(cr) the CO2(g) and H2O(l) molar enthalpy of formation values were −(393.51 ± 0.13) and −(285.83 ± 0.04) kJ mol–1 at 298.15 K, respectively.42
Table 4. Standard Molar Energy and Enthalpy of Combustion and Standard Molar Enthalpy of Formation in the Solid Phase at 298.15 K.
The uncertainties correspond to the expanded uncertainty with a confidence level of 95%, they include the calibration contributions from benzoic acid and energy of combustion of cotton thread.
The uncertainty corresponds to the expanded uncertainty with a confidence level of 95%, it includes the uncertainties of standard enthalpy of formation of H2O (l) and CO2 (g).
Table 5 shows the results of vaporization enthalpy for compound 7M4MC at Tm = 463.15 K (where Tm is the mean temperature), four series of experiments were performed, and an average of the obtained values is reported (Figures S1–S4 in the Supporting Information).
Table 5. Vaporization Enthalpies for 7M4MC.
| temperature T/K | mass m/mg | (dm/dt)·109 kg/s | ν·103 (kg K mol)1/2 m–2 s–1 | ln ν | 103/T K |
|---|---|---|---|---|---|
| 438.15 | 1.174 | 3.630 | 80.483 | –2.520 | 2.282 |
| 443.15 | 1.164 | 4.550 | 101.455 | –2.288 | 2.257 |
| 448.15 | 1.149 | 5.700 | 127.813 | –2.057 | 2.231 |
| 453.15 | 1.115 | 7.040 | 158.738 | –1.840 | 2.207 |
| 458.15 | 1.109 | 8.640 | 195.887 | –1.630 | 2.183 |
| 463.15 | 1.003 | 10.590 | 241.404 | –1.421 | 2.159 |
| 468.15 | 0.999 | 12.800 | 293.353 | –1.226 | 2.136 |
| 473.15 | 0.988 | 15.350 | 353.668 | –1.039 | 2.113 |
| 478.15 | 0.973 | 18.300 | 423.858 | –0.858 | 2.091 |
| 483.15 | 0.855 | 21.680 | 504.763 | –0.684 | 2.070 |
| 488.15 | 0.723 | 25.320 | 592.554 | –0.523 | 2.049 |
| series I | |||
| ln ν = 17.06–8570.44/T | r2 = 0.9994 | σm = 71.09, σy = 0.15 | ΔlgHm (463.15 K)/kJ mol–1 = (71.3 ± 0.6)a |
| series II | |||
| ln ν = 16.85–8461.06/T | r2 = 0.999 | σm = 120.77, σy = 0.26 | ΔlgHm (463.15 K)/kJ mol–1 = (70.3 ± 1.0)a |
| series III | |||
| ln ν = 16.96–8526.61/T | r2 = 0.9992 | σm = 110.89, σy = 0.24 | ΔlgHm (463.15 K)/kJ mol–1 = (70.9 ± 0.9)a |
| series IV | |||
| ln ν = 16.77–8455.23/T | r2 = 0.9997 | σm = 118.56, σy = 0.26 | ΔlgHm (463.15 K)/kJ mol–1 = (70.3 ± 1.0)a |
| weighted average: ⟨ΔlgHm (463.15 K) = (70.9 ± 0.8) kJ mol–1⟩ | |||
The uncertainty corresponds to the combined standard and includes the uncertainties of the slope, the rate of mass loss, and the temperature.
Table 6 contains the enthalpy of sublimation calculation at 298.15 K, in addition this table presents the results for the enthalpy of fusion and vaporization under experimental conditions. The pertinent adjustment to 298.15 K was determined by applying eqs 5–7.43,44
| 5 |
| 6 |
| 7 |
Table 6. Determination of Enthalpy of Sublimation at 298.15 Ka.
The enthalpy of sublimation was calculated by adding the enthalpy of vaporization and the enthalpy of fusion at 298.15 K. The enthalpy of sublimation for this compound has not been reported elsewhere yet.
Meanwhile, the standard molar enthalpy of formation in the gas phase was obtained from the standard molar enthalpy of formation in the crystalline phase plus the enthalpy of sublimation; as seen in Table 7.
Table 7. Standard Molar Enthalpies of Formation and Sublimation of 7M4MC at 298.15 Ka.
| ΔfHmo(cr) (kJ mol–1) | ΔcrgHm (298.15 K) (kJ mol–1) | ΔfHmo(g) (kJ mol–1) |
|---|---|---|
| –488.5 ± 6.4 | 104.9 ± 2.1 | –383.6 ± 6.7 |
The uncertainty corresponds to the expanded uncertainty with a level of confidence of 95%.
Theoretical Results
For assessing the precision from those values obtained experimentally, machine learning was used. To predict the 7M4MC enthalpy of formation in the gas phase, a data set was created based on the functional groups separation proposed by Benson;23 for these analysis, the ester family compounds were considered because this is the main functional group presented in coumarins.
From a literature review, a data set of 84 experimental values was obtained, and the data was separated into training and testing using the hold out model (70/30) and the seed 204, respectively; so as a result, the values obtained in this work can be reproducible. The metrics results are shown in Table 8, likewise Figure 2 presents the comparison between the experimental and predicted value for all compounds as well as the linear regression is included as a perfect fit.
Table 8. Algorithm Evaluation Metrics.
Mean absolute error.
Root mean squared error.
Figure 2.
Experimental and predicted value comparison from −ΔfHm° (g, 298.15 K) by MLR.
The evaluation metrics in the test set are low due to the fact that the data set does not consider the esters’ aromatic interaction; thus, not enough experimental values were found, and as a result an addition of 24 aromatic compounds was permitted to compensate for the precision. The result after that consideration was favorable, as is shown in Table 9, where the standard molar enthalpies of formation in the gas phase exclusively for the coumarins are reported; furthermore, a Δ parameter was added, which represents the error between the experimental and predicted values.
Table 9. Comparison between Literature and Predicted Values of −ΔfHm° (g, 298.15 K) in kJ mol–1 and Benson.
| compound | literature | predicteda | Δ | predictedb | Δ | Bensonc | Δ |
|---|---|---|---|---|---|---|---|
| coumarin | 176.8d | 176.8 | 0 | 179.4 | 2.6 | 173.7 | –3.1 |
| 3-hydroxycoumarin | 367.7e | 353.6 | –14.1 | 354.3 | –13.4 | 371.1 | 3.4 |
| 4-hydroxycoumarin | 351.4e | 349.5 | –1.9 | 350.7 | –0.7 | 371.1 | 19.7 |
| 5-hydroxycoumarin | 344.9f | 345.4 | 0.5 | 347.0 | 2.1 | 371.1 | 24.6 |
| 6-hydroxycoumarin | 339.8g | 341.3 | 1.5 | 343.4 | 3.6 | 371.1 | 31.3 |
| 7-hydroxycoumarin | 337.5f | 337.2 | –0.3 | 339.7 | 2.2 | 371.1 | 33.6 |
| 8-hydroxycoumarin | 349.2f | 333.0 | –16.2 | 336.0 | –13.2 | 371.1 | 21.9 |
| 5-methoxycoumarin | 329.7h | 327.9 | –1.8 | 329.2 | –0.5 | 327.1 | –2.6 |
| 6-methoxycoumarin | 321.5h | 323.8 | 2.3 | 325.5 | 4.0 | 327.1 | 5.6 |
| 7-methoxycoumarin | 321.6h | 319.7 | –1.9 | 321.9 | 0.3 | 327.1 | 5.5 |
| 8-methoxycoumarin | 314.2h | 315.6 | 1.4 | 318.2 | 4.0 | 327.1 | 12.9 |
| 7M4MC | 383.6i | 384.9 | –1.3 | 382.2 | 1.4 | 415.5 | 31.9 |
As observed in Table 9, the MLR and SGD values are quite close to the experimental value, so it means that the proposed models can be applied to predict the compound of interest’s enthalpies.
By analyzing the coumarin behavior and the two regression approach, we have observed that those theoretical tools are useful to predict the enthalpy of a desired compound because the difference between the two methods has a similar variation with respect to the experimental value.
On the other hand, using the Benson’s method results in a greater error due to the lack of updating of the data and by not considering the difference in between isomers, it falls into the same result for different kinds of molecules. Another theoretical method to obtain the enthalpy of formation in the gas phase is through the use of homodesmic reactions and, thus, is necessary to propose the 7M4MC reactions, as shown in Figure 3.
Figure 3.
Homodesmic reactions used in the 7M4MC enthalpy of formation determination.
The molecules proposed in reactions I, II, III, and IV were predicted by MLR. Besides for the ester compounds, a data set was constructed using 78 gas phase enthalpy of formation values in total; however, for the aromatic compounds, 53 values were used; in both cases, a hold-out (70/30) was maintained.
The seeds used were 39 and 508 for each type of compound, respectively (evaluation metrics are shown in Table S6 in the Supporting Information and Figures S5 and S6).
Table 10 shows the prediction results from those molecules presented in Figure 3. However, in Table 11, the homodesmic reaction results are presented, and the difference between the 7M4MC predicted value against the experimental value for each homodesmic reaction is given in brackets.
Table 10. Theoretical Results for the Molecules Used in Homodesmic Reactions.
| compound | ΔfHexp°(g, 298.15 K) | ΔfHpred°(g, 298.15 K) | Δ |
|---|---|---|---|
| coumarin | –176.8a | –176.8 | 0.0 |
| 1-methoxy-2-methylbenzene | –106.6 ± 1.6b | –109.5 | –2.9 |
| 1-methoxy-3-methylbenzene | –102.6 ± 5.0b | –100.6 | 2.0 |
| 1-methoxy-4-methylbenzene | –99.0 ± 2.0b | –98.4 | 0.6 |
| 7-methoxycoumarin | –321.6 ± 2.8c | –319.7 | 1.9 |
| benzene | 82.9 ± 0.9d | 83.5 | –0.6 |
| toluene | 50.1 ± 1.1d | 49.3 | 0.8 |
| 7M4MC | –383.6 ± 6.7e | –384.9 | –1.3 |
Table 11. Computational Estimates of the Standard Enthalpy of Formation in the Gas Phase at 298.15 K of the 7M4MC.
| R | –ΔfHpred°(g)/(kJ mol–1) | –exp | |
|---|---|---|---|
| 7M4MC | I | 381.3 (–2.1) | 383.6 ± 6.7 |
| II | 386.2 (2.6) | ||
| III | 384.8 (1.2) | ||
| IV | 385.4 (1.8) |
As observed from those results reported in Table 11, it shows that although all the proposed reactions values are close to the experimental value, the best reaction is III where the 1-methoxy-4-methylbenzene compound is presented. From this analysis, it can be suggested that the use of MLR to predict the enthalpy of formation of organic compounds is fast and reliable to the conventional software already used.50
Although one of the purposes of SGD is to improve the coefficients presented for MLR, we observed that similar results are obtained with both MLR and SGD, so the application of MLR is also a trustable option to be applied in these thermochemical property prediction.
To estimate the enthalpy of formation in the crystalline phase, a conventional regression was performed based on the coumarins reported experimentally in this phase because for esters and aromatics the reported condensed phase is the liquid phase. The regressors considered were the amount of C, H, and O atoms together and X3 and X4 as variables, which indicates where each of the coumarin radicals used binds, as seen in eq 8. The coefficient of determination (R2) was 0.9951. The resultant predictions are listed in Table 12.
| 8 |
where X1 represents the number of H atoms, X2 is the number of O atoms, X3 is the radical 1, and X4 is the radical 2. To identify the radical position, the numbering must begin from the carbonyl group toward the methoxy group, as shown in Figure 1.
Table 12. Comparison between Experimental and Predicted Values of −ΔfH°(cr, 298.15 K) Using the Regression in kJ mol–1.
| compounda | experimental | predicted | Δ |
|---|---|---|---|
| Cb | 259.9 | 259.9 | 0 |
| 7H4MCc | 540.8 | 538.5 | –2.3 |
| 6M4MCc | 488 | 490.3 | 2.3 |
| 6HCd | 466.2 | 471.4 | 5.2 |
| 7HCe | 471.1 | 473.0 | 1.9 |
| 3HCf | 459.6 | 466.5 | 6.9 |
| 4HCf | 479.9 | 468.2 | –11.7 |
| 7MCg | 428.9 | 426.6 | –2.3 |
| 7M4MCh | 488.5 | 492.0 | 3.5 |
Compound: coumarin (C); 7-hydroxy-4-methylcoumarin (7H4MC); 6-methoxy-4-methylcoumarin (6M4MC); 6-hydroxycoumarin (6HC); 7-hydroxycoumarin (7HC); 3-hydroxycoumarin (3HC); 4-hydroxycoumarin (4HC); and 7-methoxycoumarin (7MC).
Taken of ref (23).
Taken of ref (53).
Taken of ref (48).
Taken of ref (47).
Taken of ref (46).
Taken of ref (49).
Experimental value of this work.
As can be seen from eq 8, the carbon atoms amount within the compounds does not affect the enthalpy of formation in the crystalline phase estimation, the resultant value for compound 7M4MC was −(492.0 ± 4.1) kJ mol–1 (the uncertainty represents the average absolute error of the coumarins presented in Table 12), this value has a difference of 3.5 kJ mol–1 with respect to the experimentally obtained.
The predicted values of coumarins using this regression are quite close to those reported in the literature, so it is a good option to perform this type of analysis when few data are available, and it is necessary to compare with an experimental value.
Finally, an additional advantage for MLR is that it is possible to obtain regression coefficients; these coefficients represent a change and update to the conventional ones shown by Benson, as it is shown in Table 13.
Table 13. Update of Benson Functional Groups of −ΔfHm° (g, 298.15 K) for Esters and Coumarins in kJ mol–1.
| group | value | group | value |
|---|---|---|---|
| CO–(CD)(O) | 11.32 | O–(CB)(CO) | 28.56 |
| CO–(C)(O) | 25.79 | CD–(O)(H) | 0 |
| CO–(O)(CB) | 24.17 | C–(H)2(CO)2 | 127.97 |
| O–(H)(CO) | 0 | C–(H)3(CO) | 75.37 |
| O–(C)(CB) | –12.55 | C–(H)2(CO)(CD) | –3.29 |
| O–(H)(CB) | 86.57 | C–(H)2(CO)(CT) | 0 |
| CD–(H)(CO) | 3.71 | C–(O)(C)3 | –54.98 |
| CD–(C)(CO) | 7.61 | C–(H)(O)(C)2 | –8.64 |
| CB–(CO)(CB)2 | 36.31 | C–(H)2(O)(C) | 32.42 |
| CB–(O)(CB)2 | 102.58 | O–(CO)(O) | 0 |
| C–(CO)(C)3 | –202.02 | CD–(H)(C) | 6.12 |
| C–(H)(CO)(C)2 | –23.98 | CD–(H)(CD) | 0 |
| C–(H)2(CO)(C) | 14.58 | C–(H)2(C)(CD) | 5.70 |
| C–(H)3(O) | 82.38 | CT–(C) | 0 |
| C–(H)3(CD) | 11.32 | CB–(CB)3 | 22.86 |
| C–(H)3(C) | 82.40 | CB–(H)(CO)(CB) | –12.55 |
| C–(H)3(CB) | 41.51 | CB–(CO)2(CB) | 0 |
| C–(H)2(C)2 | 20.33 | rsc | –17.59 |
| C–(H)(C)3 | –9.91 | radical 1 | –4.11 |
| CD–(H)2 | 16.60 | radical 2 | –1.86 |
| CB–(H)(CB)2 | –11.82 | radical 3 | 0.68 |
| CB–(C)(CB)2 | –6.02 | correction O- | –10.37 |
| C–(H)2(C)(CB) | –20.83 | correction M- | –4.82 |
| CH3(tert) | 2.46 | correction P- | –4.09 |
| CH3(qua) | 0 | group aromatic | –57.16 |
| CO–(C)(CO) | 81.83 | group coumarin | 22.86 |
| CO–(C)(CB) | 35.00 | group ester | 34.30 |
| O–(CD)(CO) | 0 | O–(C)(CO) | 158.54 |
| H0 = 34.86a | |||
Represents the β0 value.
Conclusions
The enthalpy of fusion was determined by DSC and the enthalpy of vaporization was obtained by thermogravimetric analysis. The experimental standard molar enthalpy of formation in the gas phase of 7M4MC as a result from the standard molar enthalpy of formation in the solid phase and the standard molar enthalpy of sublimation resulting in −383.6 kJ mol–1, this value represents an excellent agreement concerning the value predicted from machine learning algorithms, which have a difference of 1.3 kJ mol–1 with respect to MLR and 1.4 kJ mol–1 with respect to the SGD regression. Based on that obtained from the experimental section, the enthalpy of formation in the crystalline phase was predicted using a fitting equation that was able to distinguish between the structural isomerism on different compounds and although it was only applied to a small data set, it was possible to demonstrate a prediction path when limited experimental values are available. Finally by using the fitting equation, a difference of 3.5 kJ mol–1 was obtained, and with the homodesmic reactions it was possible to propose an alternative method capable of predicting the enthalpy of formation in the gas phase; thus, the optimal reaction had a difference with an experimental value of 1.2 kJ mol–1 and the biggest difference of 2.6 kJ mol–1 with respect to the experimental value.
Acknowledgments
M.A.G.C. thanks Conacyt (Mexico) for his scholarship (registration number 200481) and thank VIEP-BUAP for financial support through project number 100420555-VIEP2021 “Mecanosíntesis y estudio termoquímico de la o-diimida diácida derivada del anhídrido ftálico.” The authors thankfully acknowledge technical expertise and support provided by the Departamento de Química, Centro de Investigación y de Estudios Avanzados del IPN.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c06756.
Data set consisting of values obtained experimentally, MLR and SGD, 39 for the set with ethers and 508 for the aromatic compounds (ZIP)
The authors declare no competing financial interest.
Supplementary Material
References
- Li D.; Wu L. Coumarins from the roots of Angelica dahurica cause anti-allergic inflammation. Exp. Ther. Med. 2017, 14, 874–880. 10.3892/etm.2017.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinola N.; Piller N. Pharmacogenomics: Its Role in Re-establishing Coumarin as Treatment for Lymphedema. Lymphatic Res. Biol. 2005, 3, 81–86. 10.1089/lrb.2005.3.81. [DOI] [PubMed] [Google Scholar]
- Kapoor S. The anti-neoplastic effects of coumarin: an emerging concept. Cytotech 2013, 65, 787–788. 10.1007/s10616-013-9538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacchio A.; Prencipe R.; Bertone M.; De Benedictis L.; Taglieri L.; D’Elia E.; Centoletti C.; Di Carlo G. Effectiveness and safety of a product containing diosmin, coumarin, and arbutin (Linfadren) in addition to complex decongestive therapy on management of breast cancer-related lymphedema. Support. Care Cancer 2019, 27, 1471–1480. 10.1007/s00520-018-4514-5. [DOI] [PubMed] [Google Scholar]
- Reen F. J.; Gutiérrez-Barranquero J. A.; Parages M. L.; ÓGara F. Coumarin: a novel player in microbial quorum sensing and biofilm formation inhibition. Appl. Microbiol. Bioeng. 2018, 102, 2063–2073. 10.1007/s00253-018-8787-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. C.; Hu C. F.; Zhang P. L.; Li S.; Hu C. S.; Geng R. X.; Zhou C. H. Coumarin thiazoles as unique structural skeleton of potential antimicrobial agents. Bio. Chem. 2022, 124, 105855. 10.1016/j.bioorg.2022.105855. [DOI] [PubMed] [Google Scholar]
- Chen L. Z.; Sun W. W.; Bo L.; Wang J. Q.; Xiu C.; Tang W. J.; Shi J. B.; Zhou H. P.; Liu X. H. New arylpyrazoline-coumarins: Synthesis and anti-inflammatory activity. Eur. J. Med. Chem. 2017, 138, 170–181. 10.1016/j.ejmech.2017.06.044. [DOI] [PubMed] [Google Scholar]
- Di Stasi L. C. Coumarin Derivatives in Inflammatory Bowel Disease. Molecules 2021, 26, 422. 10.3390/molecules26020422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y. F.; Liu L.; Feng C. Z.; Hu Y.; Chen C.; Wang G. X.; Zhu B. Synthesis and antiviral activity of a new coumarin derivative against spring viraemia of carp virus. Fish Shellfish Immunol. 2018, 81, 57–66. 10.1016/j.fsi.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Mishra S.; Pandey A.; Manvati S. Coumarin: An emerging antiviral agent. Heliyon 2020, 6, e03217 10.1016/j.heliyon.2020.e03217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Majedy Y.; Al-Amiery A.; Kadhum A. A.; BakarMohamad A. Antioxidant Activity of Coumarins. Syst. Rev. Pharm. 2016, 8, 24–30. 10.5530/srp.2017.1.6. [DOI] [Google Scholar]
- Chilin A.; Battistutta R.; Bortolato A.; Cozza G.; Zanatta S.; Poletto G.; Mazzorana M.; Zagotto G.; Uriarte E.; Guiotto A.; Pinna L.; Meggio F.; Moro S. Coumarin as Attractive Casein Kinase 2 (CK2) Inhibitor Scaffold: An Integrate Approach To Elucidate the Putative Binding Motif and Explain Structure–Activity Relationships. J. Med. Chem. 2008, 51, 752–759. 10.1021/jm070909t. [DOI] [PubMed] [Google Scholar]
- Rubab L.; Afroz S.; Ahmad S.; Hussain S.; Nawaz I.; Irfan A.; Batool F.; Kotwica-Mojzych K.; Mojzych M. An Update on Synthesis of Coumarin Sulfonamides as Enzyme Inhibitors and Anticancer Agents. Molecules 2022, 27, 1604. 10.3390/molecules27051604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim Y. A.; Ouf N. B. Anti-inflammatory new coumarin from the Ammi majus L. Org. Med. Chem. Lett. 2012, 2, 1. 10.1186/2191-2858-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M. I.; Syed Q. A.; Khattak M. N. K.; Hafez B.; Reigosa M. J.; El-Keblawy A. Natural product coumarins: biological and pharmacological perspectives. Biologia 2019, 74, 863–888. 10.2478/s11756-019-00242-x. [DOI] [Google Scholar]
- Ismael R. N.; Mustafa Y. F.; Al-Qazaz H. K. Coumarin-based products: Their biodiversity and pharmacology. Iraqi J. Pharm. 2022, 18, 162–179. 10.33899/iphr.2022.170405. [DOI] [Google Scholar]
- Vitorino J.; Bernardes C. E. S.; Minas da Piedade M. E. A general strategy for the experimental study of the thermochemistry of protic ionic liquids: enthalpy of formation and vaporisation of 1-methylimidazolium ethanoate. Phys. Chem. Chem. Phys. 2012, 14, 4440–4446. 10.1039/c2cp40213k. [DOI] [PubMed] [Google Scholar]
- Ogorodova L.; Vigasina M.; Mel’chakova L.; Rusakov V.; Kosova D.; Ksenofontov D.; Bryzgalov I. Enthalpy of formation of natural hydrous iron phosphate: Vivianite. J. Chem. Thermodyn. 2017, 110, 193–200. 10.1016/j.jct.2017.02.020. [DOI] [Google Scholar]
- García-Castro M. A.; Amador P.; Hernández-Pérez J. M.; Medina-Favela A. E.; Flores H. Experimental and computational thermochemistry of 3- and 4-nitrophthalic anhydride. J. Phys. Chem. A 2014, 118, 3820–3826. 10.1021/jp5003929. [DOI] [PubMed] [Google Scholar]
- López-Ortega M.; García-Castro M. A.; Galicia-Aguilar J. A.; Aranda-García R.; Ramos-Hernández H.; Díaz-Sánchez F. Standard enthalpies of formation of 3-hydroxyphthalic anhydride. Rev. Mex. Fis. 2022, 68, 061701. 10.31349/RevMexFis.68.061701. [DOI] [Google Scholar]
- Gharagheizi F.; Gohar M. R. S.; Vayeghan M. G. A quantitative structure–property relationship for determination of enthalpy of fusion of pure compounds. J. Therm. Anal. Calorim. 2012, 109, 501–506. 10.1007/s10973-011-1727-y. [DOI] [Google Scholar]
- Ramakrishnan R.; Dral P. O.; Rupp M.; von Lilienfeld O. A. Big data meets quantum chemistry approximations: The Δ-Machine Learning Approach. J. Chem. Theory Comput. 2015, 11, 2087–2096. 10.1021/acs.jctc.5b00099. [DOI] [PubMed] [Google Scholar]
- Pesme S.; Flammarion N.. Online robust regression via SGD on the l1 loss. Advances in Neural Information Processing Systems, 2020; Vol. 33, pp 2540–2552. [Google Scholar]
- Zou D.; Wu J.; Braverman V.; Gu Q.; Foster D. P.; Kakade S.. The benefits of implicit regularization from SGD in least squares problems. 35th Conference on Neural Information Processing Systems, 2021; Vol. 34, pp 5456–5468.
- Cohen N. Revised group additivity values for enthalpies of formation (at 298 K) of carbon-hydrogen and carbon-hydrogen-oxygen compounds. J. Phys. Chem. Ref. Data 1996, 25, 1411–1481. 10.1063/1.555988. [DOI] [Google Scholar]
- Archer D. G. Thermodynamic properties of synthetic sapphire (α-Al2O3), standard reference material 720 and the effect of temperature-scale differences on thermodynamic properties. J. Phys. Chem. Ref. Data 1993, 22, 1441–1453. 10.1063/1.555931. [DOI] [Google Scholar]
- Coops J.; Jessup R. S.; Van Nes K.; Rossini F. D.. Experimental Thermochemistry; Rossini F. D., Ed.; Interscience: New York, 1956. [Google Scholar]
- Salas-López K.; García-Castro M. A.; Amador P.; Herrera-González A. M.; Galicia-Aguilar A.; Amador F. A.; Hernandez-Pascasio F.; Flores H. Standard enthalpies of formation of N,N-(1,3-phenylene)bis(phthalimide) and N,N-(1,3-phenylene)bis(phthalimide-5-carboxilic acid). Thermochim. Acta 2021, 697, 178861–178869. 10.1016/j.tca.2021.178861. [DOI] [Google Scholar]
- Wagman D.; Evans W.; Parker V.; Schumm R.; Halow I.; Bailey S.; Churney K.; Nutall R.. The NBS tables of chemical thermodynamic properties. J. Phys. Chem. Ref. Data 1982, 11. [Google Scholar]
- Prohaska T.; Irrgeher J.; Benefield J.; Böhlke J. K.; Chesson L. A.; Coplen T. B.; Ding T.; Dunn P. J. H.; Gröning M.; Holden N. E.; Meijer H. A. J.; Moossen H.; Possolo A.; Takahashi Y.; Vogl J.; Walczyk T.; Wang J.; Wieser M. E.; Yoneda S.; Zhu X. K.; Meija J. Standard atomic weights of the elements 2021 (IUPAC Technical Report). Pure Appl. Chem. 2022, 94 (5), 573–600. 10.1515/pac-2019-0603. [DOI] [Google Scholar]
- Good W. D.; Smith N. K. Enthalpies of combustion of toluene, benzene, cyclohexane, cyclohexene, methylcyclopentane, 1-methylcyclopentene, and n-hexane. J. Chem. Eng. Data 1969, 14, 102–106. 10.1021/je60040a036. [DOI] [Google Scholar]
- Sabbah France Chairman R.; Xu-wu China A.; Chickos Usa J.; Leitão Portugal M.; Roux Spain M.; Torres México L. Reference materials for calorimetry and differential thermal analysis. Thermochim. Acta 1999, 331, 93–204. 10.1016/s0040-6031(99)00009-x. [DOI] [Google Scholar]
- Altman D. G.; Bland J. M. How to obtain the P value from a confidence interval. BMJ 2011, 343, d2304. 10.1136/bmj.d2304. [DOI] [PubMed] [Google Scholar]
- Daoud J. I. Multicollinearity and Regression Analysis. J. Phys.: Conf. Ser. 2017, 949, 012009. 10.1088/1742-6596/949/1/012009. [DOI] [Google Scholar]
- Jensen D. R.; Ramirez D. E. Variance Inflation in Regression. Adv. Decis. Sci. 2013, 2013, 671204. 10.1155/2013/671204. [DOI] [Google Scholar]
- Wold S.; Sjöström M.; Eriksson L. PLS-regression: a basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. 10.1016/s0169-7439(01)00155-1. [DOI] [Google Scholar]
- Bartlett P. L.; Long P. M.; Lugosi G.; Tsigler A. Benign overfitting in linear regression. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 30063–30070. 10.1073/pnas.1907378117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower R. M.; Loizou N.; Qian X.; Sailanbayev A.; Shulgin E.; Richtarik P.. SGD: General Analysis and Improved Rates. Proceedings of the 36th International Conference on Machine Learning; Proceedings of Machine Learning Research: Long Beach, California, 2019.
- Zou D.; Wu J.; Braverman V.; Gu Q.; Kakade S. M.. Benign Overfitting of Constant-Stepsize SGD for Linear Regression. Proceedings of the 34th International Conference on Learning Theory; Proceedings of Machine Learning Research, 2021; pp 4633–4635.
- Wu J.; Zou D.; Braverman V.; Gu Q.; Kakade S. M.. Last Iterate Risk Bounds of SGD with Decaying Stepsize for Overparameterized Linear Regression. Proceedings of the 39th International Conference on Machine Learning; Proceedings of Machine Learning Research: Baltimore, Maryland, USA, 2022.
- Ngoc Toan V.; Dinh Thanh N. Synthesis of 6- and 7-alkoxy-4-methylcoumarins from corresponding hydroxy coumarins and their conversion into 6- and 7-alkoxy-4-formylcoumarin derivatives. Synth. Commun. 2020, 50 (23), 3603–3615. 10.1080/00397911.2020.1807571. [DOI] [Google Scholar]
- CODATA Recommended Key Values for Thermodynamics. J. Chem. Thermodyn. 1978, 10, 903. [Google Scholar]
- Acree W.; Chickos J. S. Phase transition enthalpy measurements of organic compounds. An update of sublimation, vaporization, and fusion enthalpies from 2016 to 2021. J. Phys. Chem. Ref. Data 2022, 51, 043101. 10.1063/5.0081916. [DOI] [Google Scholar]
- Chickos J. S.; Hosseini S.; Hesse D. G.; Liebman J. F. Heat capacity corrections to a standard state: a comparison of new and some literature methods for organic liquids and solids. Struct. Chem. 1993, 4, 271–278. 10.1007/bf00673701. [DOI] [Google Scholar]
- Watik L. E.; Sabbah R. Etude thermodynamique de la coumarine et de l’anthrone. Bull. Soc. Chim. Fr. 1991, 128, 344–349. [Google Scholar]
- Sousa C. C. S.; Morais V. M. F.; Matos M. A. R. Energetics of the isomers: 3- and 4-hydroxycoumarin. J. Chem. Thermodyn. 2010, 42, 1372–1378. 10.1016/j.jct.2010.06.003. [DOI] [Google Scholar]
- Sousa C. C. S.; Matos M. A. R.; Morais V. M. F. Calorimetric and computational study of 7-hydroxycoumarin. J. Chem. Thermodyn. 2011, 43, 1435–1440. 10.1016/j.jct.2011.04.015. [DOI] [Google Scholar]
- Silva A. L. R.; C M O Lima A.; Agostinha R Matos M.; Morais V. M. F. Thermochemical study of the antioxidant activity of some hydroxycoumarins. J. Chem. Thermodyn. 2023, 180, 107006. 10.1016/j.jct.2023.107006. [DOI] [Google Scholar]
- Morais V. M. F.; Sousa C. C. S.; Matos M. A. R. Experimental and computational study of the energetics of methoxycoumarins. J. Mol. Struct.: THEOCHEM 2010, 946, 13–19. 10.1016/j.theochem.2009.11.036. [DOI] [Google Scholar]
- Domalski E. S.; Hearing E. D. Estimation of the Thermodynamic Properties of C-H-N-O-S-Halogen Compounds at 298.15 K. J. Phys. Chem. 1993, 22, 805–1159. 10.1063/1.555927. [DOI] [Google Scholar]
- Emel’yanenko V. N.; Zaitseva K. V.; Agapito F.; Martinho Simões J. A.; Verevkin S. P. Benchmark thermodynamic properties of methylanisoles: Experimental and theoretical study. J. Chem. Thermodynamics 2015, 85, 155–162. 10.1016/j.jct.2015.02.001. [DOI] [Google Scholar]
- Roux M. V.; Temprado M.; Chickos J. S.; Nagano Y. Critically Evaluated Thermochemical Properties of Polycyclic Aromatic Hydrocarbons. J. Phys. Chem. Ref. Data 2008, 37, 1855–1996. 10.1063/1.2955570. [DOI] [Google Scholar]
- Amador P.; Pineda B.; López A.; Flores H. Standard molar enthalpies of formation in the crystalline phase of 7-hydroxy-4-methylcoumarin, 7 ethoxy-4-methylcoumarin, and 6-methoxy-4-methylcoumarin. J. Chem. Thermodynamics 2011, 43, 1414–1416. 10.1016/j.jct.2011.04.013. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






