Abstract
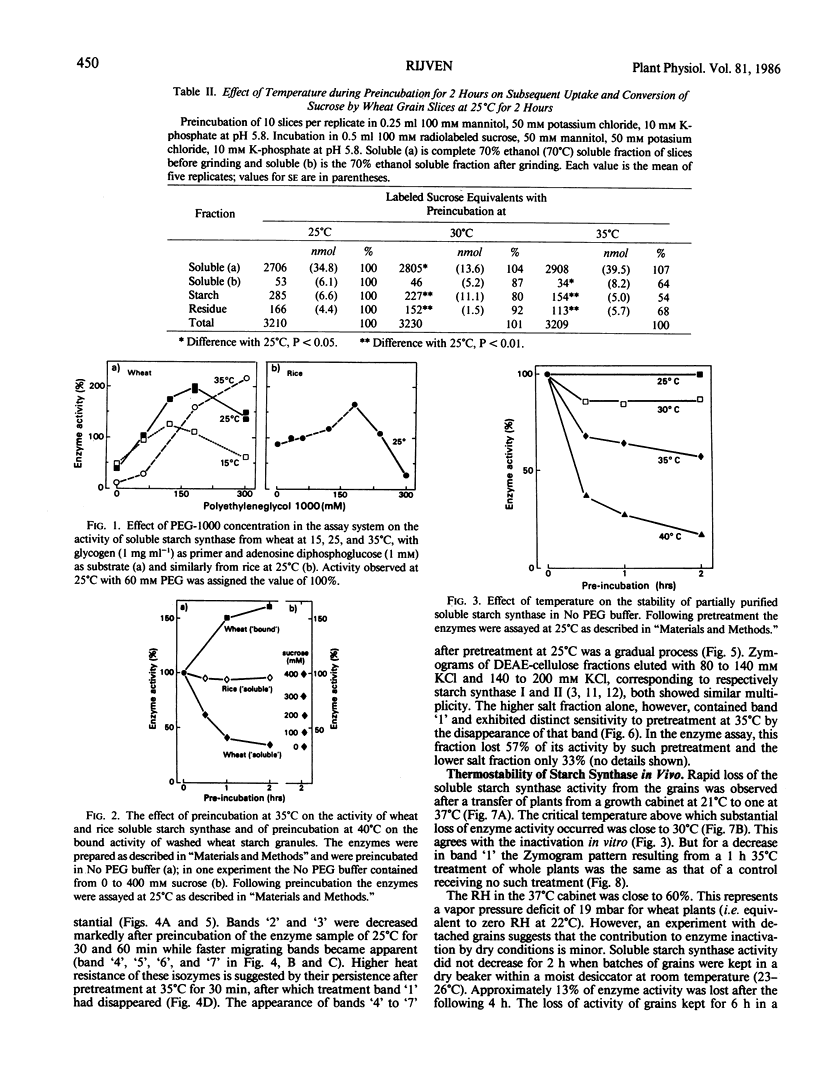
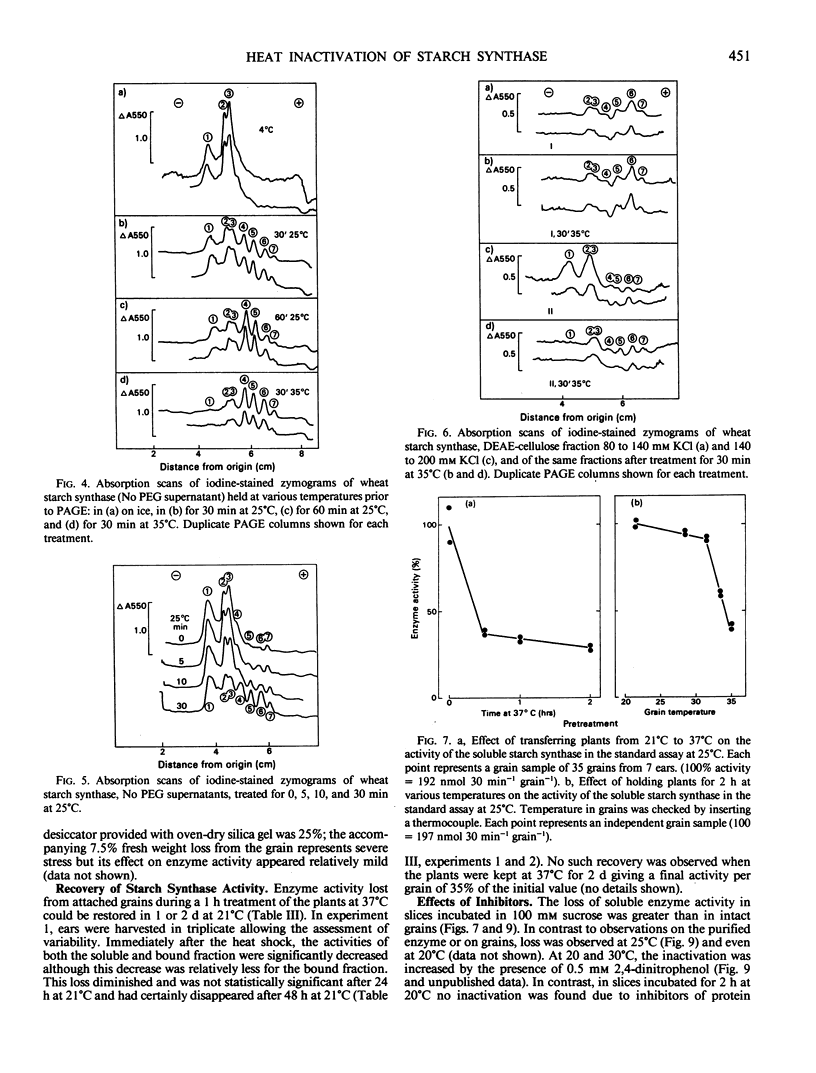
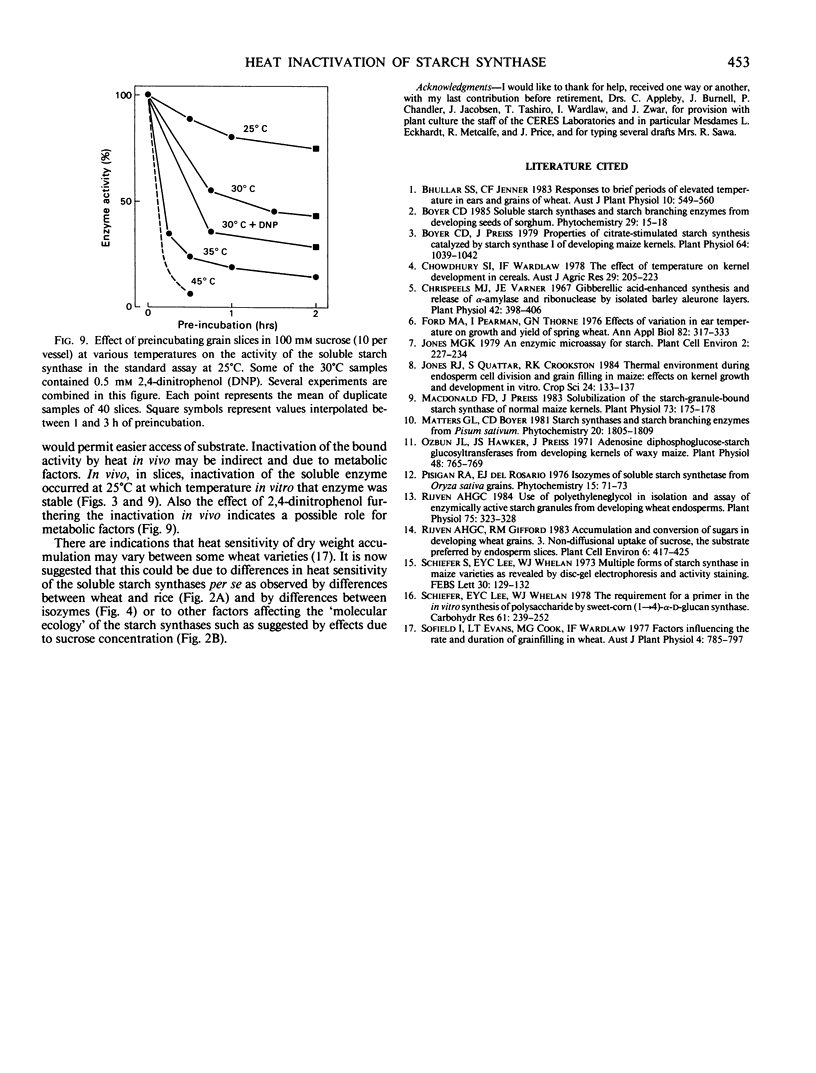
The effect of temperature on accumulation of starch was studied in grain slices of wheat (Triticum aestivum cv SUN9E), taken 15 days after anthesis. As compared with pretreatment of such slices at 25°C, pretreatment at 30 or 35°C reduced the subsequent conversion of sucrose to starch. In contrast to rice (Oryza sativa cv Calrose), pretreatment of wheat soluble starch synthase in vitro at 30°C or higher temperatures reduced its activity. In zymograms using nondenaturing polyacrylamide gel electrophoresis followed by activity staining, the slowest migrating band represented the most temperature sensitive isozyme. Although preincubation of a soluble enzyme sample in vitro at 25°C did not result in loss of starch synthase activity, it did result in a gradual shift of zymogram banding pattern toward faster migrating species. Pretreatment of isolated starch granules at 40°C increased their bound starch synthase activity. Both soluble and bound enzymes in the grains of whole wheat plants lost activity when the plants were held above 30°C for 30 minutes or longer. Both activities lost from the grains after a 1 hour treatment at 37°C were restored in 1 to 2 days by a return to 21°C. In slices, inactivation of the soluble starch synthase was increased by incubation with 2,4-dinitrophenol. It is tentatively suggested that in vivo heat inactivation of soluble starch synthase may be a direct effect of heat on the enzyme protein and that of bound enzyme an indirect effect involving metabolic factors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer C. D., Preiss J. Properties of Citrate-stimulated Starch Synthesis Catalyzed by Starch Synthase I of Developing Maize Kernels. Plant Physiol. 1979 Dec;64(6):1039–1042. doi: 10.1104/pp.64.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Varner J. E. Gibberellic Acid-enhanced synthesis and release of alpha-amylase and ribonuclease by isolated barley and aleurone layers. Plant Physiol. 1967 Mar;42(3):398–406. doi: 10.1104/pp.42.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald F. D., Preiss J. Solubilization of the starch-granule-bound starch synthase of normal maize kernels. Plant Physiol. 1983 Sep;73(1):175–178. doi: 10.1104/pp.73.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbun J. L., Hawker J. S., Preiss J. Adenosine diphosphoglucose-starch glucosyltransferases from developing kernels of waxy maize. Plant Physiol. 1971 Dec;48(6):765–769. doi: 10.1104/pp.48.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijven A. H. Use of polyethylene glycol in isolation and assay of stable, enzymically active starch granules from developing wheat endosperms. Plant Physiol. 1984 Jun;75(2):323–328. doi: 10.1104/pp.75.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer S., Lee E. Y.C., Whelan W. J. Multiple forms of starch synthetase in maize varieties as revealed by disc-gel electrophoresis and activity staining. FEBS Lett. 1973 Feb 15;30(1):129–132. doi: 10.1016/0014-5793(73)80634-9. [DOI] [PubMed] [Google Scholar]


