Abstract
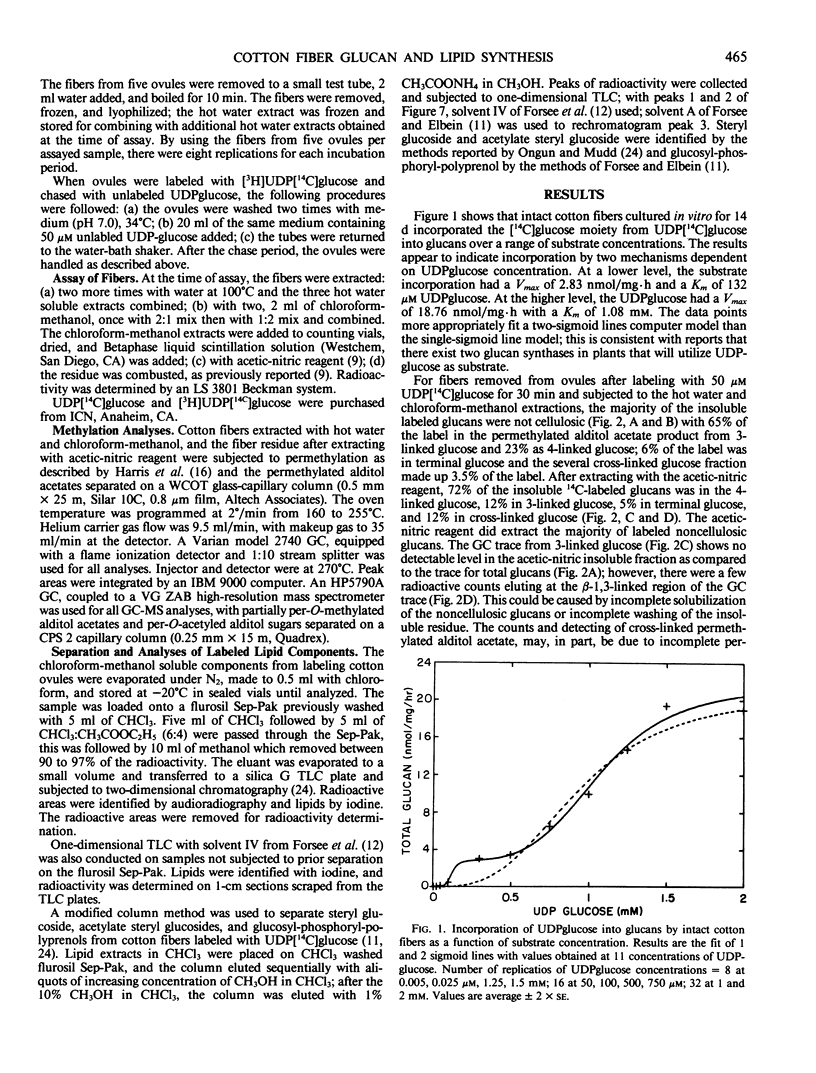
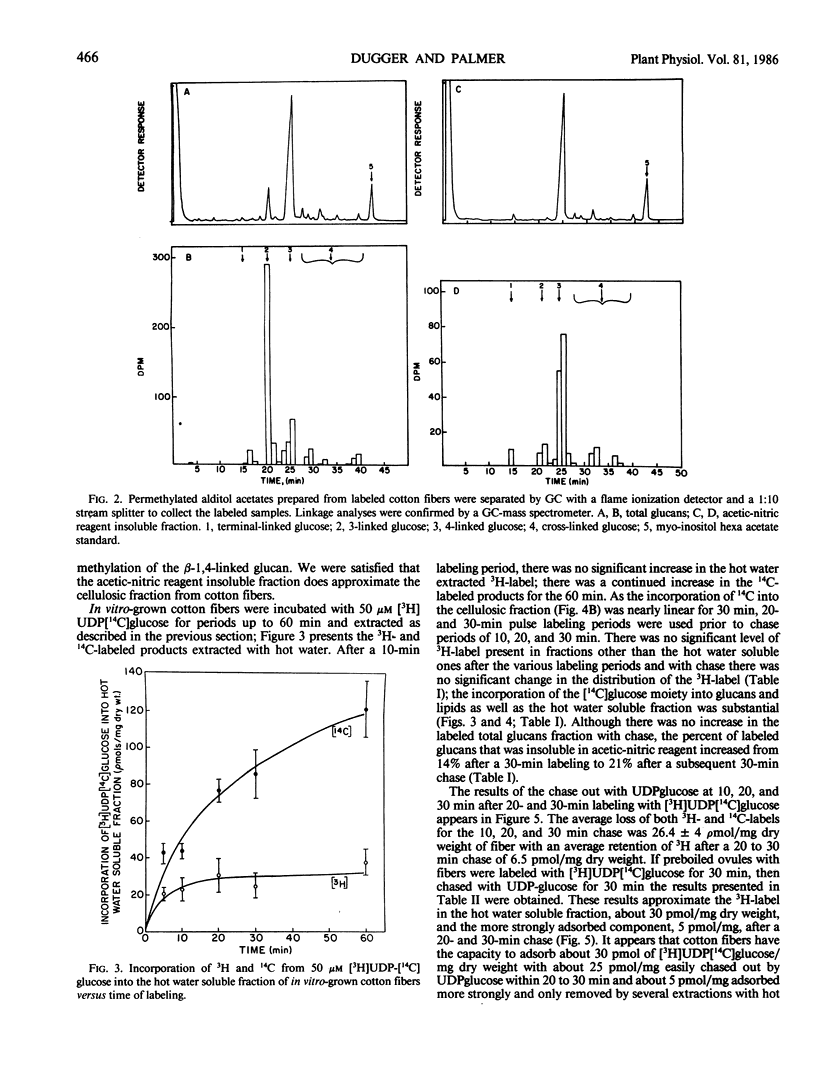
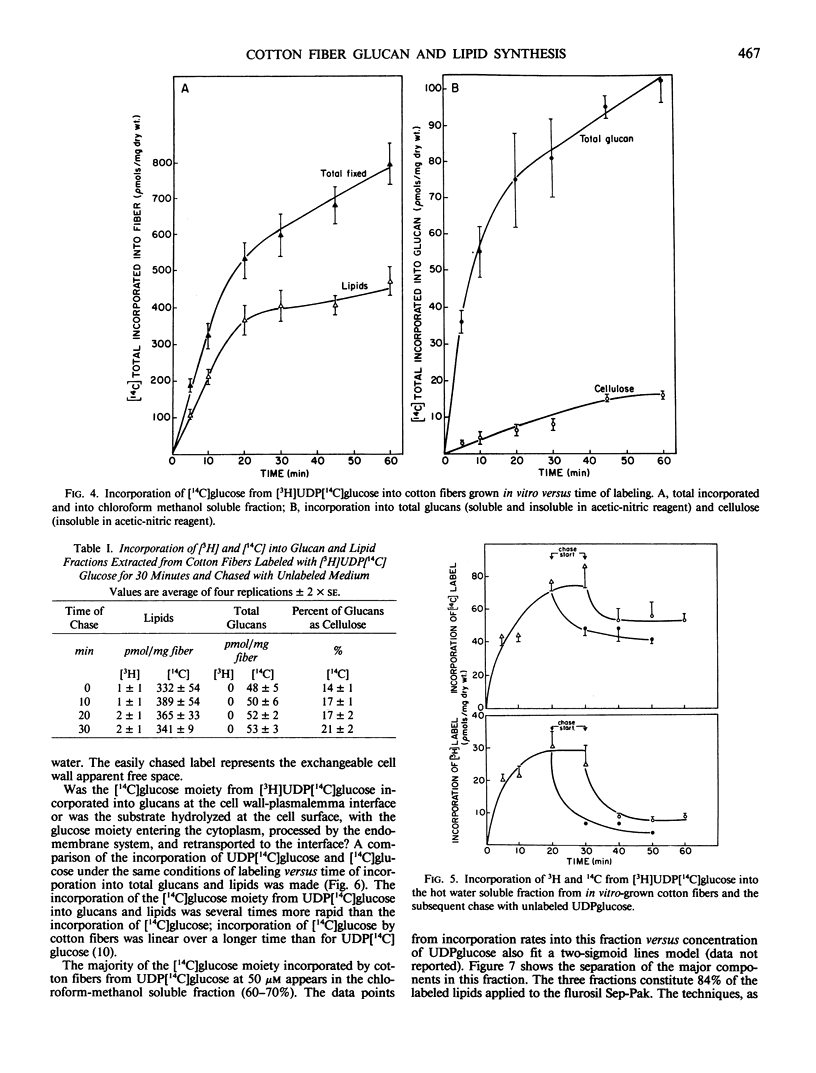
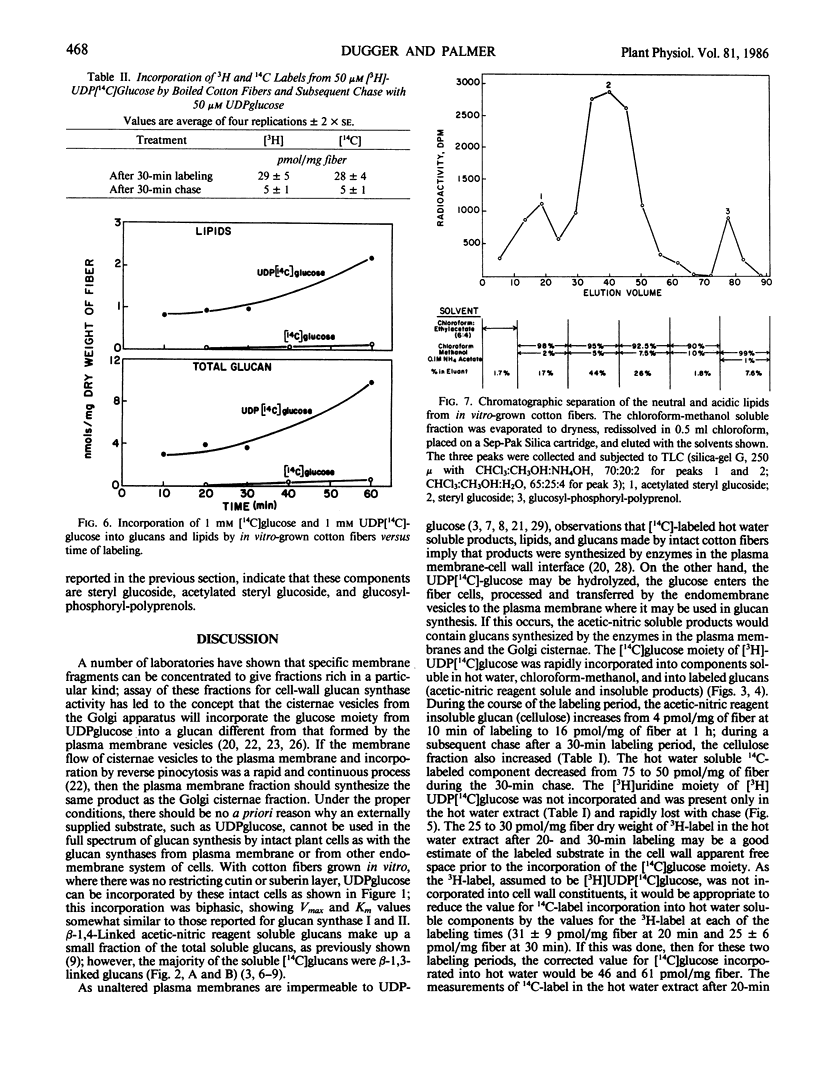
The [14C] moiety from [3H]UDP[14C]glucose was incorporated by intact cotton fibers into hot water soluble, acetic-nitric reagent soluble and insoluble components, and chloroform-methanol soluble lipids; the [3H] UDP moiety was not incorporated. The 3H-label can be exchanged rapidly with unlabeled substrate in a chase experiment. The cell wall apparent free space of cotton fibers was in the order of 30 picomoles per milligram of dry fibers; 25 picomoles per milligram easily exchanged and about 5 picomoles per milligram more tightly adsorbed. At 50 micromolar UDPglucose, 70% of the [14C]glucose was found in the lipid fraction after both a short labeling period and chase. The percent of [14C]glucose incorporated into total glucan increased slightly with chase, but the fraction of total glucans incorporated into insoluble acetic-nitric reagent (cellulose) did increase within a 30-minute chase period. The data supports the concept that glucan synthesis, including cellulose, as well as the synthesis of steryl glucosides, acetylated steryl glucosides, and glucosyl-phosphoryl-polyprenol from externally supplied UDPglucose occurs at the plasma membrane-cell wall interface. The synthase enzymes for such synthesis must be part of this interfacial membrane system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaum E. H., Dugger W. M., Beasley B. C. Interaction of boron with components of nucleic Acid metabolism in cotton ovules cultured in vitro. Plant Physiol. 1977 Jun;59(6):1034–1038. doi: 10.1104/pp.59.6.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogomolni R. A., Stubbs L., Lanyi J. K. Illumination-dependent changes in the intrinsic fluorescence of bacteriorhodopsin. Biochemistry. 1978 Mar 21;17(6):1037–1041. doi: 10.1021/bi00599a015. [DOI] [PubMed] [Google Scholar]
- Carpita N. C., Delmer D. P. Concentration and metabolic turnover of UDP-glucose in developing cotton fibers. J Biol Chem. 1981 Jan 10;256(1):308–315. [PubMed] [Google Scholar]
- Carpita N. C., Delmer D. P. Protection of cellulose synthesis in detached cotton fibers by polyethylene glycol. Plant Physiol. 1980 Nov;66(5):911–916. doi: 10.1104/pp.66.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P. Biosynthesis of cellulose. Adv Carbohydr Chem Biochem. 1983;41:105–153. doi: 10.1016/s0065-2318(08)60057-8. [DOI] [PubMed] [Google Scholar]
- Delmer D. P., Heiniger U., Kulow C. UDP-glucose: Glucan Synthetase in Developing Cotton Fibers: I. Kinetic and Physiological Properties. Plant Physiol. 1977 Apr;59(4):713–718. doi: 10.1104/pp.59.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugger W. M., Palmer R. L. Effect of Boron on the Incorporation of Glucose from UDP-Glucose into Cotton Fibers Grown in Vitro. Plant Physiol. 1980 Feb;65(2):266–273. doi: 10.1104/pp.65.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsee W. T., Elbein A. D. Biosynthesis of mannosyl- and glucosyl-phosphoryl-polyprenols in cotton fibers. J Biol Chem. 1973 Apr 25;248(8):2858–2867. [PubMed] [Google Scholar]
- Forsee W. T., Valkovich G., Elbein A. D. Acylation of steryl glucosides by phospholipids. Solubilization and properties of the acyl transferase. Arch Biochem Biophys. 1976 Feb;172(2):410–418. doi: 10.1016/0003-9861(76)90092-8. [DOI] [PubMed] [Google Scholar]
- Harris P. J., Henry R. J., Blakeney A. B., Stone B. A. An improved procedure for the methylation analysis of oligosaccharides and polysaccharides. Carbohydr Res. 1984 Apr 2;127(1):59–73. doi: 10.1016/0008-6215(84)85106-x. [DOI] [PubMed] [Google Scholar]
- Hopp H. E., Romero P. A., Daleo G. R., Pont Lezica R. Synthesis of cellulose precursors. The involvement of lipid-linked sugars. Eur J Biochem. 1978 Mar 15;84(2):561–571. doi: 10.1111/j.1432-1033.1978.tb12199.x. [DOI] [PubMed] [Google Scholar]
- Ongun A., Mudd J. B. The biosynthesis of steryl glucosides in plants. Plant Physiol. 1970 Mar;45(3):255–262. doi: 10.1104/pp.45.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore G., Maclachlan G. A. The site of cellulose synthesis. Hormone treatment alters the intracellular location of alkali-insoluble beta-1,4-glucan (cellulose) synthetase activities. J Cell Biol. 1975 Mar;64(3):557–571. doi: 10.1083/jcb.64.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe D. M., Heath M. C. Callose induction in cowpea by uridine diphosphate glucose and calcium phosphate-boric Acid treatments. Plant Physiol. 1982 Feb;69(2):366–370. doi: 10.1104/pp.69.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegraff D. M. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969 Dec;32(3):420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]


