Abstract
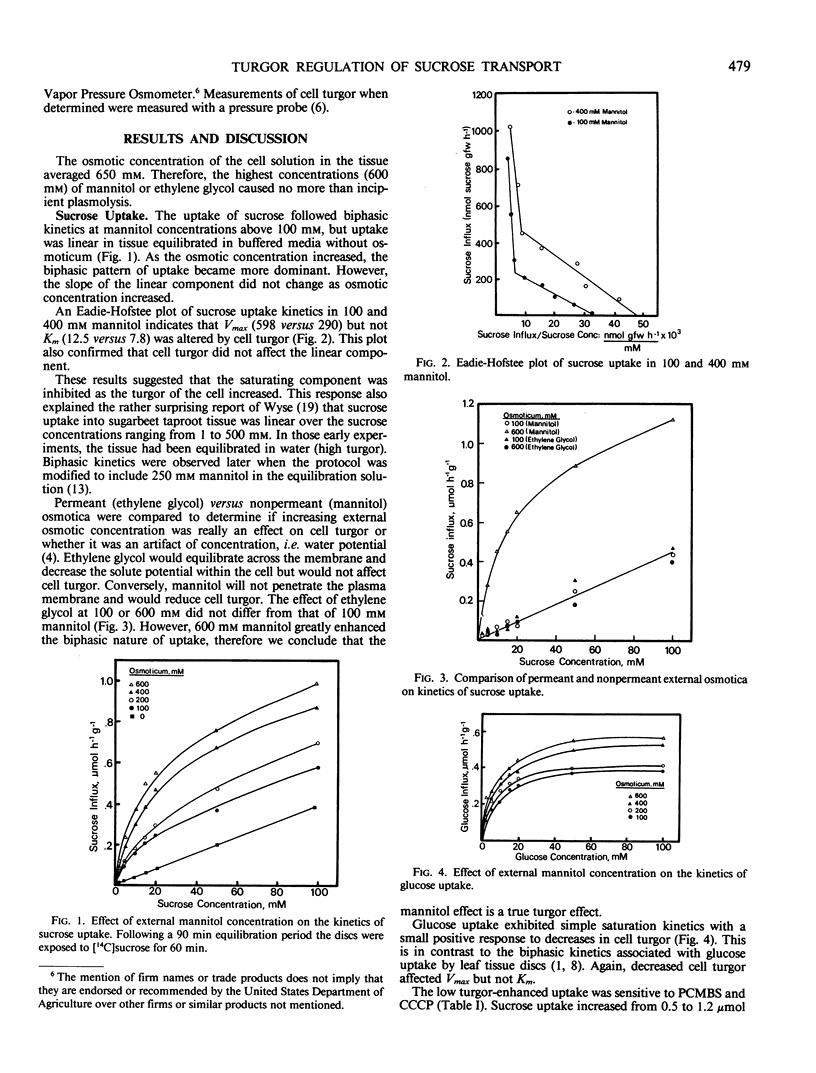
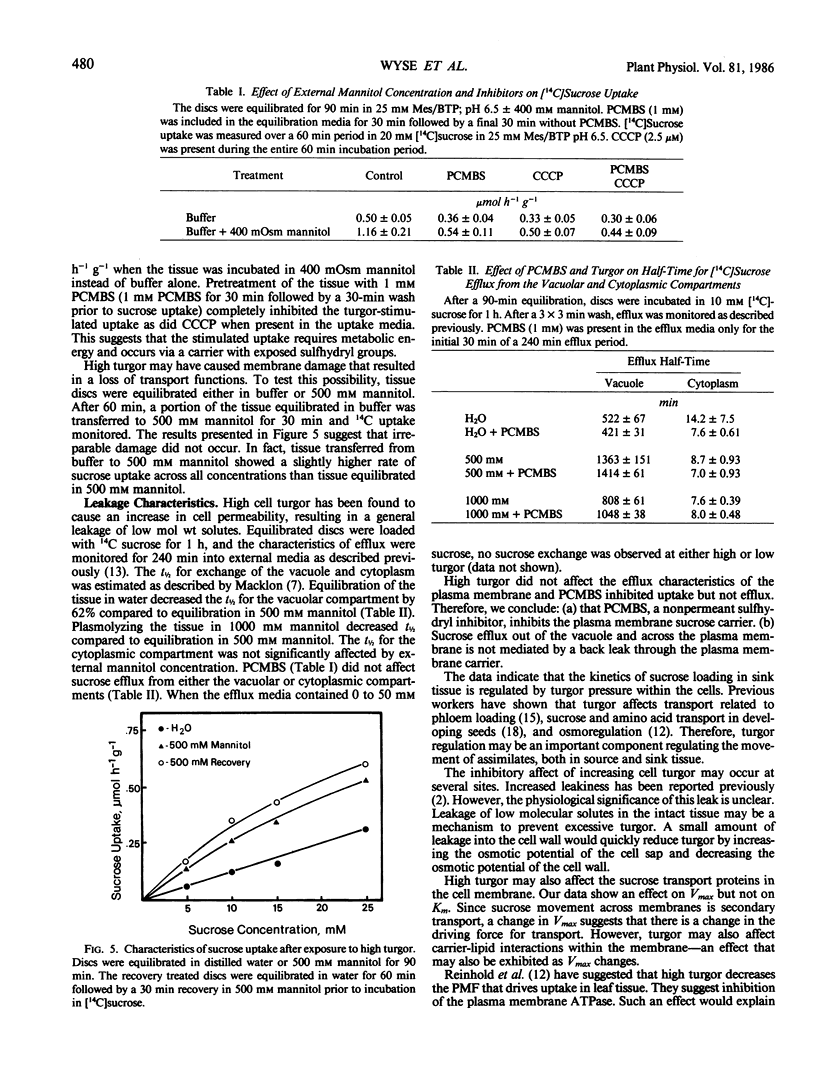
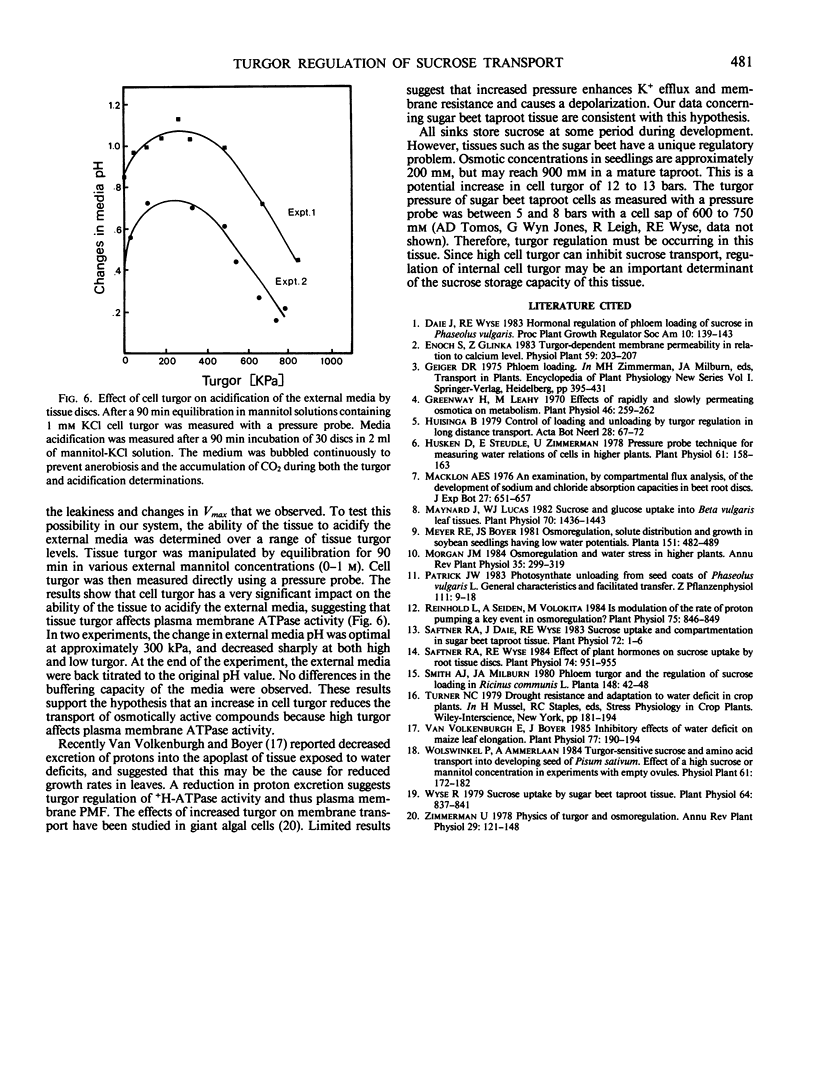
Sink tissues that store osmotically active compounds must osmoregulate to prevent excessively high turgor. The ability to regulate turgor may be related to membrane transport of solutes and thus sink strength. To study this possibility, the kinetics of sugar uptake were determined in sugar beet (Beta vulgaris L.) taproot tissue discs over a range of cell turgors. Sucrose uptake followed biphasic kinetics with a high affinity saturating component below 20 millimolar and a low affinity linear component at higher concentrations. Glucose uptake exhibited only simple saturation type kinetics. The high affinity saturating component of sucrose and glucose uptake was inhibited by increasing cell turgor (decreasing external mannitol concentrations). The inhibition was evident as a decrease in Vmax but no effect on Km. Sucrose uptake by tissue equilibrated in dilute buffer exhibited no saturating component. Ethylene glycol, a permeant osmoticum, had no effect on uptake kinetics, suggesting that the effect was due to changes in cell turgor and not due to decreased water potential per se. p-(Chloromercuri)benzene sulfonic acid (PCMBS) inhibited sucrose uptake at low but not high cell turgor. High cell turgor caused the tissue to become generally leaky to potassium, sucrose, amino acids, and reducing sugars. PCMBS had no effect on sucrose leakage, an indication that the turgor-induced leakage of sucrose was not via back flow through the carrier. The ability of the tissue to acidify the external media was turgor dependent with an optimum at 300 kilopascals. Acidification was sharply reduced at cell turgors above or below the optimum. The results suggest that the secondary transport of sucrose is reduced at high turgor as a result of inhibition of the plasma membrane ATPase. This inhibition of ATPase activity would explain the reduced Vmax and leakiness to low molecular weight solutes. Cell turgor is an important regulator of sucrose uptake in this tissue and thus may be an important determinant of sink strength in tissues that store sucrose.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Greenway H., Leahy M. Effects of rapidly and slowly permeating osmotica on metabolism. Plant Physiol. 1970 Aug;46(2):259–262. doi: 10.1104/pp.46.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüsken D., Steudle E., Zimmermann U. Pressure probe technique for measuring water relations of cells in higher plants. Plant Physiol. 1978 Feb;61(2):158–163. doi: 10.1104/pp.61.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard J. W., Lucas W. J. Sucrose and Glucose Uptake into Beta vulgaris Leaf Tissues : A Case for General (Apoplastic) Retrieval Systems. Plant Physiol. 1982 Nov;70(5):1436–1443. doi: 10.1104/pp.70.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold L., Seiden A., Volokita M. Is modulation of the rate of proton pumping a key event in osmoregulation? Plant Physiol. 1984 Jul;75(3):846–849. doi: 10.1104/pp.75.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saftner R. A., Wyse R. E. Effect of plant hormones on sucrose uptake by sugar beet root tissue discs. Plant Physiol. 1984 Apr;74(4):951–955. doi: 10.1104/pp.74.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Volkenburgh E., Boyer J. S. Inhibitory effects of water deficit on maize leaf elongation. Plant Physiol. 1985 Jan;77(1):190–194. doi: 10.1104/pp.77.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse R. Sucrose uptake by sugar beet tap root tissue. Plant Physiol. 1979 Nov;64(5):837–841. doi: 10.1104/pp.64.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]


