Abstract
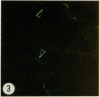
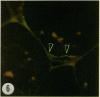
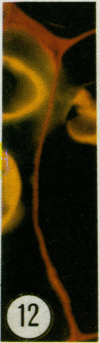
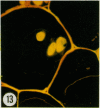
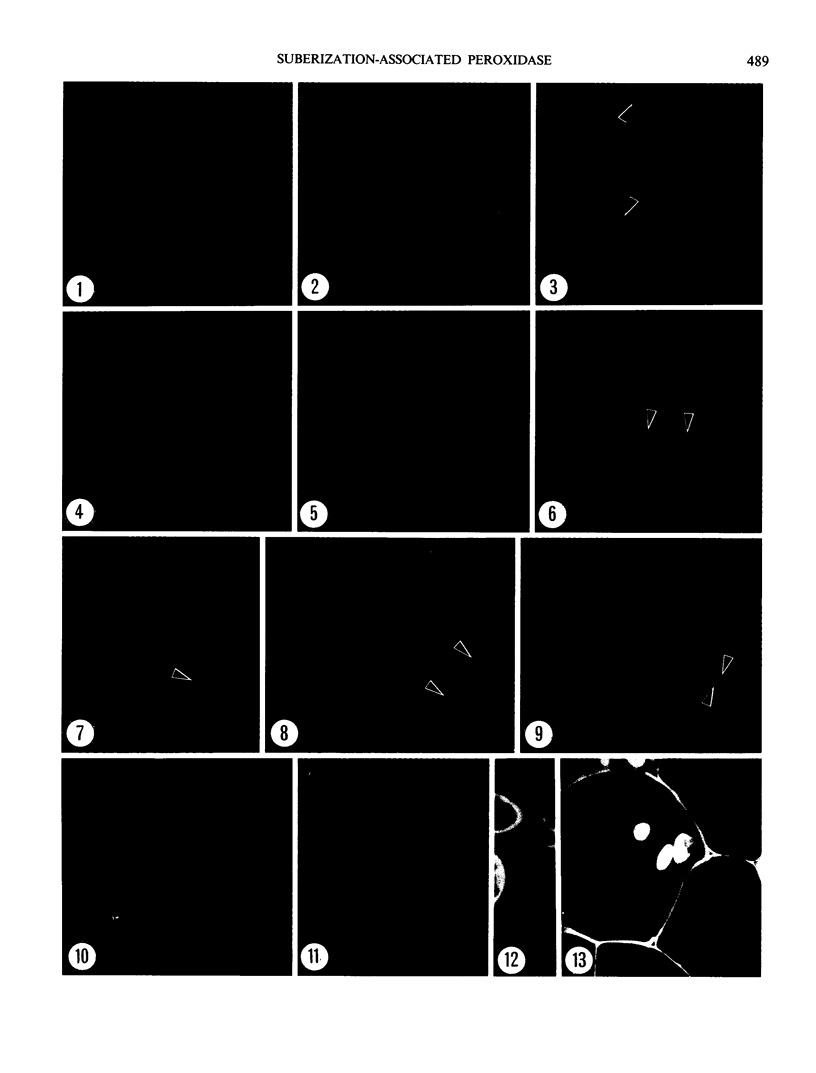
Thin sections of wound-healing potato tuber tissue were stained with rabbit antibody prepared against a suberization-associated anionic peroxidase and then stained with a goat anti-rabbit antibody-fluorescein conjugate. When these sections were examined with an epiilluminating fluorescence microscope, bright green fluorescent linear deposits were observed on the inner side of cell walls in the periderm layer. Initial deposits which were often not contiguous throughout the wall were first observed in some cells after 3 days of wound-healing and subsequently these layers became more pronounced so that all 6 day old periderm cells had green fluorescent layers on their inner walls. This fluorescence was not present in the walls of parenchyma cells or in the walls of periderm cells treated with preimmune serum and anti-rabbit IgG-FITC conjugate. Thin sections of wound-healing potato tissue which were stained with anti-peroxidase antibody and a goat anti-rabbit antibody-rhodamine conjugate exhibited a similar time course of development with a bright reddish-orange fluorescent layer observed on the inside wall of periderm cells. The production of this suberization-associated anionic peroxidase in wound-healing tissue was also demonstrated by an immunobinding dot blot assay which showed that the largest increase in the enzyme level occurred between 4 and 6 days of wound-healing. The present results support the hypothesis that this anionic peroxidase is involved in the deposition of the aromatic polymeric domain of suberin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal V. P., Kolattukudy P. E. Biochemistry of Suberization: omega-Hydroxyacid Oxidation in Enzyme Preparations from Suberizing Potato Tuber Disks. Plant Physiol. 1977 Apr;59(4):667–672. doi: 10.1104/pp.59.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal V. P., Kolattukudy P. E. Purification and characterization of a wound-induced omega-hydroxyfatty acid:NADP oxidoreductase from potato tuber disks (Solanum tuberosum L.). Arch Biochem Biophys. 1978 Dec;191(2):452–465. doi: 10.1016/0003-9861(78)90384-3. [DOI] [PubMed] [Google Scholar]
- Borchert R. Time course and spatial distribution of phenylalanine ammonia-lyase and peroxidase activity in wounded potato tuber tissue. Plant Physiol. 1978 Nov;62(5):789–793. doi: 10.1104/pp.62.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottle W., Kolattukudy P. E. Abscisic Acid stimulation of suberization : induction of enzymes and deposition of polymeric components and associated waxes in tissue cultures of potato tuber. Plant Physiol. 1982 Sep;70(3):775–780. doi: 10.1104/pp.70.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottle W., Kolattukudy P. E. Biosynthesis, deposition, and partial characterization of potato suberin phenolics. Plant Physiol. 1982 Feb;69(2):393–399. doi: 10.1104/pp.69.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B. B., Kolattukudy P. E. Biochemistry of Suberization: Incorporation of [1-C]Oleic Acid and [1-C]Acetate into the Aliphatic Components of Suberin in Potato Tuber Disks (Solanum tuberosum). Plant Physiol. 1977 Jan;59(1):48–54. doi: 10.1104/pp.59.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B. B., Kolattukudy P. E., Davis R. W. Chemical Composition and Ultrastructure of Suberin from Hollow Heart Tissue of Potato Tubers (Solanum tuberosum). Plant Physiol. 1977 May;59(5):1008–1010. doi: 10.1104/pp.59.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espelie K. E., Kolattukudy P. E. Purification and characterization of an abscisic acid-inducible anionic peroxidase associated with suberization in potato (Solanum tuberosum). Arch Biochem Biophys. 1985 Aug 1;240(2):539–545. doi: 10.1016/0003-9861(85)90060-8. [DOI] [PubMed] [Google Scholar]
- Hapner S. J., Hapner K. D. Rhodamine immunohistofluorescence applied to plant tissue. J Histochem Cytochem. 1978 Jun;26(6):478–482. doi: 10.1177/26.6.353187. [DOI] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Greengard P. A quantitative dot-immunobinding assay for proteins using nitrocellulose membrane filters. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1684–1687. doi: 10.1073/pnas.81.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy P. E., Dean B. B. Structure, gas chromatographic measurement, and function of suberin synthesized by potato tuber tissue slices. Plant Physiol. 1974 Jul;54(1):116–121. doi: 10.1104/pp.54.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy P. E., Kronman K., Poulose A. J. Determination of structure and composition of suberin from the roots of carrot, parsnip, rutabaga, turnip, red beet, and sweet potato by combined gas-liquid chromatography and mass spectrometry. Plant Physiol. 1975 Mar;55(3):567–573. doi: 10.1104/pp.55.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozuelo J. M., Espelie K. E., Kolattukudy P. E. Magnesium deficiency results in increased suberization in endodermis and hypodermis of corn roots. Plant Physiol. 1984 Feb;74(2):256–260. doi: 10.1104/pp.74.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R. G., Kolattukudy P. E. Evidence for Covalently Attached p-Coumaric Acid and Ferulic Acid in Cutins and Suberins. Plant Physiol. 1975 Nov;56(5):650–654. doi: 10.1104/pp.56.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijmons P. C., Kolattukudy P. E., Bienfait H. F. Iron Deficiency Decreases Suberization in Bean Roots through a Decrease in Suberin-Specific Peroxidase Activity. Plant Physiol. 1985 May;78(1):115–120. doi: 10.1104/pp.78.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsu L. Y., Espelie K. E., Kolattukudy P. E. Ultrastructural and chemical evidence that the cell wall of green cotton fiber is suberized. Plant Physiol. 1983 Oct;73(2):521–524. doi: 10.1104/pp.73.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]