Abstract
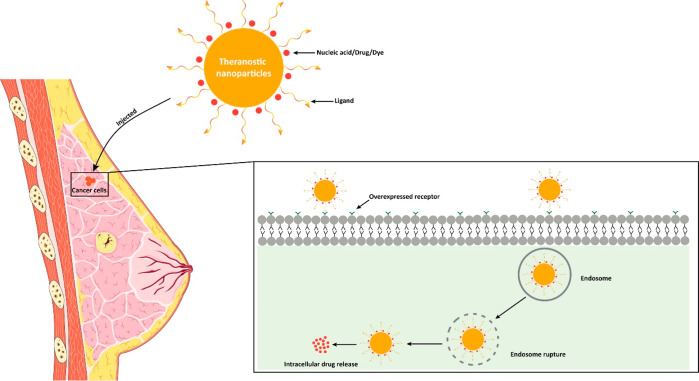
Breast cancer (BC) is a malignant neoplasm that begins in the breast tissue. After skin cancer, BC is the second most common type of cancer in women. At the end of 2040, the number of newly diagnosed BC cases is projected to increase by over 40%, reaching approximately 3 million worldwide annually. The hormonal and chemotherapeutic approaches based on conventional formulations have inappropriate therapeutic effects and suboptimal pharmacokinetic responses with nonspecific targeting actions. To overcome such issues, the use of nanomedicines, including liposomes, nanoparticles, micelles, hybrid nanoparticles, etc., has gained wider attention in the treatment of BC. Smaller dimensional nanomedicine (especially 50–200 nm) exhibited improved in vivo effectiveness, such as better tissue penetration and more effective tumor suppression through enhanced retention and permeation, as well as active targeting of the drug. Additionally, nanotechnology, which further extended and developed theranostic nanomedicine by incorporating diagnostic and imaging agents in one platform, has been applied to BC. Furthermore, hybrid and theranostic nanomedicine has also been explored for gene delivery as anticancer therapeutics in BC. Moreover, the nanocarriers’ size, shape, surface charge, chemical compositions, and surface area play an important role in the nanocarriers’ stability, cellular absorption, cytotoxicity, cellular uptake, and toxicity. Additionally, nanomedicine clinical translation for managing BC remains a slow process. However, a few cases are being used clinically, and their progress with the current challenges is addressed in this Review. Therefore, this Review extensively discusses recent advancements in nanomedicine and its clinical challenges in BC.
1. Introduction
Breast cancer (BC) is the second most frequently diagnosed cancer in women, surpassed only by skin cancer, and is categorized as a malignant neoplasm originating in the breast tissue.1 According to the GLOBOCAN 2020 report, 2.3 million BC cases have been reported worldwide, representing 11.7% of all cancer cases.2 The estimated incidence of BC was reported for the USA (119.2%),3 Germany (28.02%),4 Spain (30.72%),4 United Kingdom (27.55%),4 France (30.62%),4 Italy (30.09%),4 Poland (25.28%),4 Finland (32.74%),4 Norway (29.86%),4 Switzerland (32.47%),4 Sweden (28.73%),4 China (18.41%),5 and Japan (21.4%),6 and India (13.5).7 Furthermore, by the end of 2040, the number of newly diagnosed BC cases is projected to increase by over 40%, reaching approximately 3 million worldwide annually.3 The etiology of BC involves a complex interplay between modifiable and nonmodifiable factors. Genetic, hormonal, environmental, and nutritional factors determine the etiology of BC.8 The risk factors include a history of BC, a family history of the disease, obesity, height, smoking, drinking alcohol, early or late menstruation, a sedentary lifestyle, and hormone replacement therapy.9 Approximately 80% of patients with BC are over 50 years old, and the risk increases with age.10 The analysis of worldwide trends and projections regarding BC’s incidence and mortality rates reveals that metabolic issues are primary risk factors associated with BC-related fatalities globally.10 High body mass index and fasting glucose levels are potential risk factors for BC-related deaths.10 The relative contribution of these two risk factors varied considerably across the globe’s sociodemographic index (SDI) regions.11 The growth rate of BC is proportional to the increase in body mass index (BMI) in countries with a high SDI, while in regions with a low or middle SDI elevated BMI is leading to the fatalities of patients with BC.11 Alcohol consumption is one of the most significant risk factors for death in BC.8 Women showed double BC risk during pregnancy.12
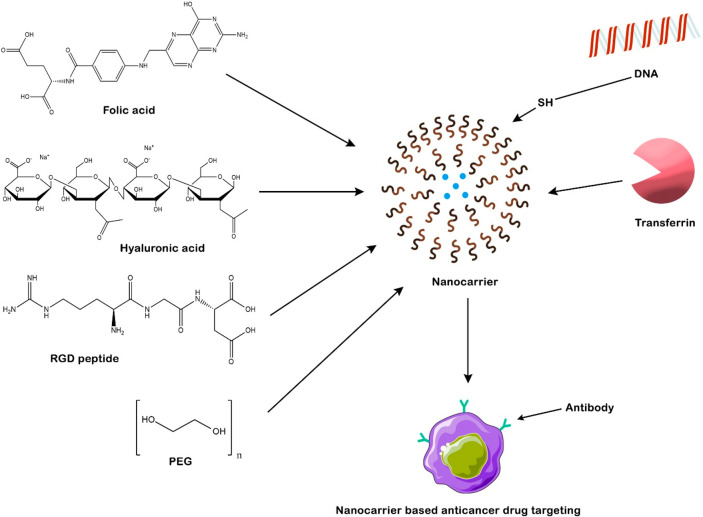
Previously, BC was linked to toxins including 2,3,7,8-tetrachlorodibenzo-p-dioxine (TCDD), bisphenol A (PFOA), and benzo[a]pyrene, as well as compounds like carbon tetrachloride (CCl4), benzene, formaldehyde, and styrene, which have been linked to an elevated risk of BC.13,14 Recent research has also identified possible connections with many pesticides.15 The polymorphism of the female gene CYP1A1 was more susceptible to polychlorinated biphenyls (PCBs).16 Current treatment strategies include radiology, hormonal therapy, and chemotherapy.17 Tamoxifen is the first-line chemotherapy treatment for BC, while hormone therapy involving the use of biologically active compounds uses aromatase inhibitors (AIs), gonadotropin hormone-releasing hormone (GnRHs), and selective estrogen receptor degraders (SERDs) to inhibit estrogen production and target BC.17 Nevertheless, some patients do not react to the hormone therapy due to their heterogeneity.17 In contrast, the hormonal and chemotherapy-based conventional formulations have inappropriate therapeutic effects and suboptimal pharmacokinetic response with nonspecific targeting action.18 To overcome such issues, nanomedicines gained wider attention in terms of effective drug delivery with optimized pharmacokinetics and pharmacodynamics.19 Nanomedicine is defined as the application of nanomaterials, which is used to address healthcare problems.19 As per the national nanotechnology initiative, the definition of nanomedicine for most pharmaceutical applications is defined as a size range of up to 1000 nm.20,21 The size of the anticancer drug has been established as the crucial factor for efficient absorption, biodistribution, penetration, and internalization at the targeted site and excretion. Consequently, the size attribute of an anticancer nanomedicine significantly affects the holistic efficacy of therapeutic interventions against cancer. The most recent studies have revealed that anticancer nanomedicines with smaller diameters have better efficacy with sizes at or below 50 nm, but the clinically approved nanomedicines for cancer treatment are found to be in the range of 100–200 nm. Several nanocarriers, such as liposomes, organic nanoparticles, micelles, dendrimers, nanotubes, inorganic nanoparticles, polymers, proteins, and combinations of these, are found to be therapeutically beneficial in the management of breast cancer, and few products are readily available in markets, e.g., Doxil (liposome-based nanoformulation) and Abraxane (nanoparticle formulation).22,23 Moreover, nanocarriers are also utilized in the effective delivery of nucleic acids to tumor sites, as further depicted in Figure 1. After all, apart from the anticancer drug delivery, nanomedicine can be utilized for diagnostics, imaging, and theranostic action in the management of BC. Therefore, this section summarizes the epidemiology of BC, its etiology, current therapeutic approaches with their importance and demerits, and the utilization of nanomedicine with its advantages in the management of BC.
Figure 1.
Representation of the nanocarriers and anchoring of ligands and anticancer drugs in the active drug targeting breast cancer.
2. Nanotechnology-Based Drug Targeting in the Management of BC
2.1. Passive Drug Targeting
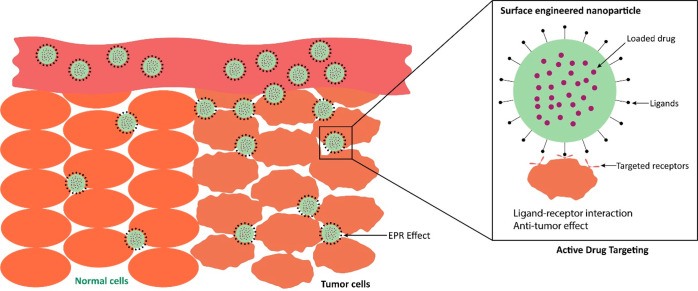
With their leaky vasculature and underdeveloped lymphatic drainage, tumors can accumulate material of a tiny size more readily than normal tissues.24 Enhanced permeability and retention (EPR) is an alternative term for passive diffusion, as depicted in Figure 2. Solid cancer, such as BC, can benefit from EPR’s ability to improve the specificity of drug delivery,25 whereas the nanocarriers may enhance the anticancer activity of encapsulated anticancer drugs at the tumor cellular level via EPR effects.25 Nanocarriers can penetrate tumor cells by passive endocytosis processes such as macropinocytosis to enhance the efficiency of drugs that target intracellular targets even if they do not involve receptor-mediated activities (e.g., RNA, paclitaxel, and doxorubicin).26 Additionally, even though passive targeting is often not a highly selective and efficient drug delivery approach, researchers use it to treat cancer, which is further discussed with examples in section 3.26
Figure 2.
Representation of the passive and active drug targeting of nanocarriers in breast cancer.
2.2. Targeted Therapy for BC Treatment
Targeted therapy delivers anticancer drugs and monoclonal antibodies acting via receptor targeting, as shown in Figures 1 and 2. The optimal delivery to target tissue should be defined by the following: (i) it should be comparatively higher than normal in tumors and (ii) it should be of absolute concentration to enable competent targeting.27 Transferrin receptors are an example of effective drug-targeting mechanisms in receptor-based ligands. The researcher reported potential drug release systems for treating multidrug-resistant BC.28 Epidermal growth factor receptors (EGFRs) are also found to be overexpressed in tumors. The percentage of solid tumors with EGFR overexpression was determined to be 30%. Immune-based nanoparticles loaded with docetaxel (DTX) reduced toxicity and precisely targeted EGFRs. Furthermore, results show intracellular drug release and enhanced drug targeting to the EGFRs in BC.28,29 Folate receptor (FR), estrogen receptor-negative, and human epidermal growth factor receptor 2 (HER2)-negative metastatic progesterone receptors are expressed in 50–86% of patients. Selectivity for MDA-MB-231 cells has been established with folate-conjugated liposomes and benzoporphyrin derivatives.30 Triple-negative breast cancer (TNBC) cells were remarkably inhibited by lipocalin-2 via siRNA silencing utilizing immune-based liposomes, suggesting that CXC chemokine receptor type 4 (CXCR-4) is a possible target of TNBC cells. A more comprehensive description is provided in the section below.
3. Therapeutic Interventions Involving Nanocarriers for Management of BC
Nanocarriers achieve the limited ability to significantly alter the oncology landscape through their target-specific action and overcome the conventional formulation challenges, including unnecessary dissemination, tumor tolerance, and extreme systemic adverse effects.31 The nanocarrier drug delivery system can boost therapeutic performance with fewer adverse effects and optimize pharmacogenetics.
3.1. Liposomes
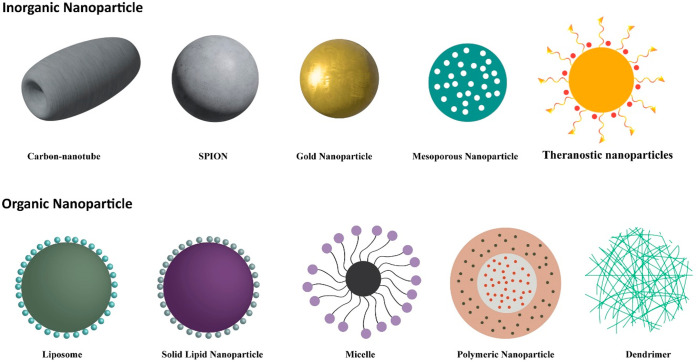
Liposomes are the most common nanoformulation to deliver anticancer drugs, as shown in Figure 3. They are a lipid bilayer system comprising biocompatible and biodegradable phospholipids and cholesterol.32 Doxil, the first US Food and Drug Administration (FDA)-approved chemotherapeutic nanosystem to be used therapeutically, contains doxorubicin (DOX). The PEGylated DOX liposomal formulation extends circulation time and prevents premature removal of the nanoformulation.33 For dual targeting, i.e., bone cancer and BC, Zhao and associates developed paclitaxel (PTX)-loaded liposomes.34 Samson et al. developed a 78 kDa glucose-regulated protein (GRP78)-siRNA/CLU (clusterin)-siRNA and camptothecin (CPT)-loaded liposomes to enhance the anticancer action of BC.35 This study revealed that the transfection performance of this liposomal formulation was significantly increased by threefold, with increased anticancer and gene-silence effects.35 Jiang and his team also made a liposome loaded with DOX to treat fibronectin in the blood vessels and stroma of BC tumors.36 DOX-loaded liposomes exhibit more pronounced antitumor and antimetastasis effects than free DOX, enhancing systemic circulation and efficacy.36 Mrugala and collaborators developed intrathecal cytarabine-loaded liposomes, now in a phase II clinical trial to diagnose BC. A preliminary test reported that they were also feasible in a few survival-encouraging patients in the phase III stage of the clinical trial.37 Schneeweiss and associates developed carboplatin-DOX-loaded liposomes for triple-negative BC.37 The study showed substantial improvement in cellular uptake via permeation and drug retention in the tumor cell.37 Scientists developed fusogenic liposomes (FL) loaded with α-tocopheryl succinate (α-TS) and deoxy ornithine (DO) as FL-α-TS-DO.38 FL were made to easily fuse with the plasma membrane and let the drug inside them enter the cytoplasm.39 This liposomal formulation showed adequate release with deformable formulation characteristics. Furthermore, the apoptotic, cell cycle, and nuclear morphology studies demonstrated that FL- α-TS-DO induces cell death more quickly. Moreover, it also exhibited higher DO cellular uptake with enhanced tumor accumulation.38 Therefore, the results concluded that better DO uptake and tumor accumulation exerted higher antitumor activity, which is further summarized in Table 1.
Figure 3.
Various examples of nanocarriers employed in breast cancer management.
Table 1. Examples of Anticancer Drug-Loaded Nanocarriers in the Management of Breast Cancer at the In Vitro Levela.
| drugs | nanocarriers | outcomes | ref |
|---|---|---|---|
| α-tocopheryl succinate (α-TS) and DOX | fusogenic liposomes (FL-TS-DOX) | this liposomal formulation demonstrated adequate release with deformable characteristics. further, FL-TS-DOX exhibited higher cellular uptake with enhanced tumor accumulation | (38) |
| β-carotene | SLNs | the formulation improved the bioavailability of β-carotene and extended blood circulation | (46) |
| PTX | SLNs | increased drug absorption by entering BC cells | (47) |
| pterostilbene (PTS) | SLNs | the in vitro drug release revealed regulated drug release; cytotoxicity of triple-negative MDA-MB-231 cell lines revealed a 2.63-fold decrease in IC50, with a greater antimigratory impact and cellular uptake compared to PTS solution | (59) |
| MTX | NLCs | the encapsulation efficiency of MTX was observed at 85%, and the in vitro release of MTX was sustained at 52% in 24 h | (65) |
| ribociclib (RBO) | NLCs | in vitro drug release study demonstrated 86.71% drug release. in addition, the in vitro hemolysis study found compatibility of NLCs with RBC as compared to the RBO suspension | (67) |
| tamoxifen (TF) + ganoderic acid A | polymeric NPs | it found a biphasic pattern of drug release with 60.01% in 6 h, followed by sustained release up to 94.2% in 24 h, and significantly reduced cell viability, with IC50 reaching a minimum value after 72 h | (78) |
| PTX and CUR | biodegradable polycaprolactone (PCEC) copolymer NPs | an in vitro cellular uptake study found the drug-loaded PCEC polymeric NPs were more readily taken up by tumor cells | (170) |
| baicalein | micelles | the baicalein-loaded micelles significantly enhanced cellular uptake and cytotoxicity against MDA-MB-231 cell lines compared to free baicalein | (91) |
| d-α-tocopheryl PEG 2000 succinate monoester (TPGS 2000)-DOX | micelles | the in vitro cellular uptake assays found that TPGS2000-DOX-loaded micelles enhanced the tumor targeting ability and cellular uptake of DOX | (93) |
| lithocholic bile acid | micelles | this study found higher inhibitory effect on the cell migration by LCA25 (44.5%) and LCA60 (41.7%) over the free LCA (64.7%) | (94) |
| carboxyl groups (SWCNT-COOH) and cisplatin | nanoconjugate | the in vitro cytotoxicity tests found that SWNT-COOH-cisplatin found a high cytotoxic effect with a time-dependent decrease in cell viability against MDA-MB-231 cell lines | (102) |
| RV | mesoporous silica NPs | RV-MSNPs inhibited the proliferation, invasion, and enhanced apoptosis of MCF-7 cells at the in vitro level | (108) |
| silymarin and metformin | mesoporous silica NPs | the coloaded MSNPs significantly inhibited BCRP mRNA expression, promoting apoptosis in MCF-7 cells | (111) |
| RBO | NLCs | in vitro hemolysis studies found compatibility with RBC, and confocal studies confirmed drug penetration into the intestine by RBO-NLCs to a higher extent than the RBO-suspension and 86.71% drug release in 0.1 N HCl over the RBO suspension. | (69) |
| dexibuprofen | NLCs | in vitro cytotoxicity studies showed the highest cytotoxic action against MDA-MB-468 cancer cell lines at a concentration of 3.4 M | (116) |
| psoralen | hybrid nanoparticles | in vitro drug release studies show burst release within the first 8 h and accumulated drug release of over 50%, possibly due to the free PSO attached to the NP surface | (117) |
| DTX | hybrid nanoparticles | this study found significant cytotoxicity and high cellular penetration at a lower IC50 of DTX against BC | (120) |
| exemestane | polymer–lipid hybrid nanoparticles | this study showed controlled drug release lasting up to 24 h in simulated gastrointestinal fluids | (141) |
| PDL1 siRNA | polymeric NPs | in vitro studies show that NPs enhance siRNA uptake specifically into TNBC MDA-MB-231 and BT-549 target cells, leading to endosomal release and suppression of PD-L1 expression within 90 min. overall, targeting PD-L1 in TNBC cells is achieved | (38) |
TNBC, triple-negative breast cancer; NPs, nanoparticles; DTX, docetaxel; BC, breast cancer;: NLC, nano-lipid carrier; SLN, solid lipid nanoparticles; DOX, doxorubicin; FA, folic acid; MTX, mthotrexate; PSO, psoralen; RBC, red blood cells; RV, resveratrol.
Recently, researchers developed an effective pH-responsive and biodegradable calcium orthophosphate (CaP) liposome for loading the hydrophobic drug PTX and the hydrophilic drug DO hydrochloride.39 The drug-loaded liposome has achieved 70% and 90% loading efficiencies.39 Further, the developed liposome achieved a particle size of 110 ± 20 nm.39 Physiological conditions negatively charge such liposome formulations. They are easily converted into positively charged compounds in a weakly acidic environment, leading to efficient tumor cell internalization.36 Furthermore, these liposomes had excellent biodegradability under an acidic pH of 5.5. In addition, the phenomenon of proton expansion within the endosome, coupled with the pH sensitivity of liposomes, enables the efficient release of nanoencapsulated drugs.39In vivo investigation revealed improved formulation efficiency and safety, inhibiting 76% of tumor development. Drug-loaded liposomes demonstrated targeting capabilities through the EPR effect, effectively inhibiting tumor growth and metastasis.39 Therefore, the combination of CaP with liposomes enhances the stability of liposomes.39 Thus, it was concluded that the developed liposomes may be utilized as smart drug-loaded nanocarriers for the management of BC.
Researchers used hydrogenated soy phosphatidylcholine (HSPC)/mPEG-2000 to develop DTX-loaded liposomes through a remote loading approach. Particle size (115 nm), polydispersity index (PDI, 0.2), drug encapsulation efficiency (up to 76%), and liposome stability were all optimized for DTX-loaded liposomes.40 The developed liposomes using remote loading achieved 50% more significant control in plasma over those using passive loading.40 While the biodistribution studies of DTX-loaded liposomes showed significantly higher accumulation in the tumor over the free DTX, this may be due to the EPR effect.40 Furthermore, in vivo research on BALB/c mice with 4T1 breast carcinoma tumors revealed a considerable reduction of tumor formation when delivered with DTX-loaded liposomes. Additionally, the survival duration of mice treated with DTX-loaded liposomes was higher than those of the control and Taxotere groups, all supplied at a comparable dose of 8 mg/kg.40 Therefore, it is concluded that liposomal formulations found higher antitumor action against BC management.
Conjugating monoclonal antibodies with liposomes is a potential strategy for improving liposome selectivity while minimizing the side effects associated with anticancer drugs.41 The overexpression of HER2 in HER2-positive BC cells can be targeted by encapsulating liposomes with a monoclonal antibody that targets HER2.41 Immunoliposomes that were loaded with calcein and DOX and functionalized with the monoclonal antibody trastuzumab (TRZ) were found to have higher cellular toxicity and enhanced drug uptake by the HER2+ human BC cell line (SK-BR-3).43 Therefore, this combination of formulations revealed a promising technique in the area of targeted drug delivery, with results that enhanced efficiency and reduced the cytotoxicity of anticancer drugs. There has been modest success using monoligand-directed liposomes; however, the dual-ligand-directed liposomes have been proven to show better improvement. Thus, for the purpose of creating a dual-targeted liposome for BC, scientists have synthesized a Y-shaped ligand that is covalently coupled to fructose and biotin (Fru-Bio-Chol).42 Fru–Bio liposomes showed better absorption by MCF-7 cells than Fru liposomes or Bio liposomes, and we found that they require energy for endocytosis. Fru-Bio liposomes loaded with PTX inhibited BC cell growth more effectively, resulting in a 1.7-fold higher apoptosis rate. They also outperformed both Fru liposomes and Bio liposomes in terms of tumor enrichment ability, as demonstrated by in vivo imaging.42 Thus, the Fru-Bio covalently modified liposomes enhanced the BC targeting ability and revealed great potential as a novel carrier system for BC.
Highly invasive BC cells have been proven to be resistant to standard therapies. Conversely, the vasculogenic mimicry (VM) channels produced by the surviving BC cells promoted tumor growth and metastasis. Daunorubicin and emodin, two drugs used to shut down the channels, were encapsulated in arginine-8-glycine-aspartic acid (R8GD)-targeted liposomes. Particle size and particle size distribution in the generated R8GD-modified daunorubicin liposomes were tiny and homogeneous, respectively, and the drug encapsulation efficiency was improved.43 Furthermore, the above-mentioned targeted liposomes destroyed the VM channels and inhibited tumor metastasis.43 Moreover, the R8GD-modified daunorubicin liposomes and R8GD-modified emodin liposomes inhibit VM channel growth and tumor cell propagation, exhibiting cytotoxic effects on MDA-MB-435S cells. They downregulate metastasis-related proteins like matrix metalloproteinase-2 (MMP-2), vascular endothelial cadherin (VE-cad), transforming growth factor-β (TGF-β1), and hypoxia-inducible factor 1α (HIF-1α). In vivo study showed targeted liposomes accumulated specifically at tumor sites, resulting in a distinct antitumor impact.43 Therefore, combining targeted daunorubicin liposomes with targeted emodin liposomes might be an effective new method of treating invasive BC. The membrane substance ginsenoside Rg-3 is used as a replacement for cholesterol, and it is an active ingredient found in traditional Chinese medicine.44 Liposomes based on ginsenoside Rg-3 are created and loaded with dihydroartemisinin and PTX. A spherical particle shape (107.81 nm), uniformity across the entire surface, and a zeta potential of 2.78 mV characterize the generated liposomes. The drug loading in liposomes was 4.46%, whereas the encapsulation efficiencies were 57.76% and 99.66% for dihydroartemisinin- and PTX-loaded liposomes, respectively.44 The accumulated drug release of dihydroartemisinin and PTX from the liposomes at pH 5 showed higher drug release over that at pH 7.4. Further, it revealed better stability.44 The administration of dihydroartemisinin- and PTX-loaded liposomes against MDA-MB-231 and 4T1 cells showed higher cytotoxicity and inhibited migration compared to the free drugs. Moreover, the liposomes showed active targeting and lysosome escape.44 Apart from this, the liposomes showed lower toxicity to H9c2 cells over the free drugs, which reduced cardiotoxicity.41 Therefore, the ginsenoside Rg-3-based liposomes with a combination of both drugs may a substitute for cholesterol in the development of liposomes and could be a better option in BC in the near future.
3.2. Solid Lipid Nanoparticles
Solid lipid nanoparticles (SLNs) are biocompatible and biologically degradable, providing efficient therapeutic lipid-based colloidal drug delivery, as shown in Figure 3. SLNs are the most commonly carriers of poorly water-soluble drugs to improve oral bioavailability.45 They have many advantages, such as ease of preparation, effective drug release, and prolonged stability.45 Jain et al. synthesized lipid β-carotene-loaded SLNs to treat BC. In vitro MTT test experiments revealed that free β-carotene had a substantial inhibitory effect on cancer cells. The formulation showed improved bioavailability for BC and extended blood circulation.46 Xu et al. developed PTX-loaded SLNs to increase drug absorption by entering BC cells.47 The anticancer action of the said formulation is further confirmed via in vitro cell line studies using MCF7 and multidrug-resistant (MDR) MCF7/ADR along with rhodamine dye.47 The SLNs demonstrated much more anticancer potential against MCF7/ADR as well as significantly greater cell penetration in MCF7/ADR via several endocytotic pathways.47
TNBC is the most lethal form of BC, with low estrogen receptor (ER), progesterone receptor (PR), and HER2 expression.48 Siddhartha et al. have developed diallyl disulfide SLNs (DADS-SLNs) that have been found to enhance apoptotic function in TNBC with receptors for advanced glycation end products (RAGE) receptors.48 The superficial DADS-SLNs variant with the RAGE antibody boosted the cytotoxic effect via intrinsic apoptotic signaling pathways in MDA-MB231-overexpressed RAGE cells. It is also established that RAGE is a suitable molecular target in TNBC.48 Zhang et al. synthesized a combination therapy of PTX and P53 mRNA-loaded amino lipid derivative nanoparticles (NPs) to diagnose TNBC.49In vivo studies of the orthotopic TNBC mouse model showed that BC cells are significantly inhibited due to their limited size.49 Eskiler and colleagues have studied the effects of tamoxifen (TF)-loaded SLNs on the MCF-7 cell line and observed higher cytotoxicity compared to free methotrexate (MTX).50
Niclosamide (Niclo), an anthelmintic drug licensed as an anticancer agent, was recently repurposed.51 Niclo inhibits the activation of the signal transducer and activator of transcription 3 (STAT3) via reducing STAT3’s phosphorylation. STAT3 was effectively suppressed by the medication, which offers significant antitumor benefits.51 The Niclo-induced breakdown of STAT3 boosted TNBC apoptosis and sensitized TNBC to radiation and chemotherapy.51,52 However, its therapeutic activity as an anticancer drug was constrained by poor water solubility, decreased bioavailability, nonspecific action, and side effects such as nausea, itching, and stomach discomfort.51,52 Researchers synthesized Niclo-SLNs modified with phenylboronic acid (PBA-Niclo-SLNs) and investigated their action against TNBC. To create PBA-Niclo-SLNs, PBA-associated stearyl amine (PBSA) was used as a lipid in an emulsion with solvent evaporation. Further cytotoxicity studies of the mentioned formulation revealed higher cytotoxicity over Niclo and Niclo-SLNs.53 Furthermore, the increased cytotoxicity can be attributed to increased cellular absorption by MDA-MB-231 cells. PBA-Niclo-SLNs were shown to be more effective in the destruction of tumor cells by increasing the likelihood of G0/G1 cell cycle arrest and apoptosis. They also suppress STAT3, the CD44+/CD24-TNBC stem cell subpopulation, and epithelial-mesenchymal transition markers. Furthermore, they accumulate at the tumor site, resulting in greater tumor shrinkage and increased survival in TNBC-bearing mice.54 In summary, the formulation of PBA-Niclo-SLNs is a viable alternative to overcoming traditional chemotherapy obstacles and treating TNBC radically.
Raloxifene (RLX) hydrochloride, a second-generation selective ER modulator, has been approved for the treatment of BC.55 Whereas it had poor (2%) oral bioavailability in humans, owing mostly to low water solubility, it demonstrated greater first-pass metabolism, p-gp efflux, and presystemic glucuronide conjugation.55 The RLX-loaded SLNs were created by the researcher and optimized using the Taguchi design, followed by the Box–Behnken design.55 Conversely, the optimized SLNs had a mean particle size of 109.7 nm, a PDI of 0.289, and a zeta potential of −13.7 mV. The in vitro drug release revealed fickian release with a release exponent of 0.137.55 A pharmacokinetic study revealed that RLX-SLNs greatly outperformed RLX alone in terms of biopharmaceutical performance, with a 4.06× increase in Cmax and a 1.22-fold increase in Tmax.55 Furthermore, the stability investigations demonstrated RLX-SLNs’ durability after three months.52 The findings of the various studies also show that the developed RLX-SLNs have the ability to combat cancer and that using SLNs as a drug delivery method is an excellent way to enhance RLX’s biopharmaceutical properties.
d-Tocopheryl polyethylene glycol 1000 succinate–resveratrol (RV)-loaded SLNs (TPGS-RV-SLNs) are being developed by researchers employing the solvent injection approach to combat MDR.56 Additionally, the characterization investigation showed that TPGS-RV-SLNs have a drug-loading of 32.4 ± 2.6% and a zeta potential of 25.6 ± 1.3 mV.56 Additionally, it was shown that TPGS-RV-SLNs significantly inhibited cell migration and invasion in SKBR3/PR cells compared to the free RV.56 Furthermore, TPGS-RV-SLNs increased cellular absorption, brought about mitochondrial malfunction, and increased tumor effectiveness by triggering apoptosis.56 The conclusion is that TPGS-RV-SLNs have substantial promise and are effective drug delivery systems for overcoming drug resistance in the therapy of BC.
A side chain lysyl (CH2)4NH2 and α-carboxylic acid group is a necessary building block in the amino acid lysine.57 As reported in the literature, it is is used in the surface modification of nanocarriers for targeted drug delivery. Epirubicin (EPI) was successfully delivered using lysine-conjugated SLNs, which researchers designed.57 The particle size is 148.5 nm, and an entrapment efficiency (EE) of 86.1% was observed. The scanning electron microscopy (SEM) findings support the formation of nanometer-size SLNs.57 Furthermore, differential scanning calorimetry (DSC) and Fourier transform infrared (FT-IR) analysis support a hyperlink of lysine to the free functional group of SLNs.57 Moreover, the in vitro drug release showed 68% EPI-L-SLN released in a controlled manner for 48 h.57 Noncoupled EPI-SLNs showed 48.5% drug release, while the cytotoxicity study showed a lower IC50 (3 μM) for l-conjugated EPI on the MCF-7 BC cell line.57 Thus, based on in vitro and in vivo results, it concluded that the EPI-L-SLNs have a remarkable anticancer effect and can be a good candidate for cancer-targeted therapy.
Radiolabeled TRZ is a humanized monoclonal antibody targeting HER2 in BC. It binds to the HER2 protein and prevents epidermal growth factor from entering cancer cells, preventing cell division and destruction of the immune system. TRZ can prolong the progression-free survival time of HER2-positive metastatic BC patients and is radiolabeled with 99 mTc for disease diagnosis.58 Using high-shear homogenization and sonication techniques, researchers developed radiolabeled TRZ-loaded SLNs found to be less than 100 nm in size with a negatively charged zeta potential. TRZ-SLNs were biocompatible and effectively induced apoptosis in the MCF-7 cells. The parenteral injection of TRZ-SLNs in rats uncovered their biocompatibility and sustained release profile in blood circulation compared to free drug solutions.58 Furthermore, based on characterizations, in vitro cytotoxicity, and in vivo investigations, the 99 mTc-radiolabeled TRZ-loaded SLNs could be a potential theranostic tool in treating BC.
Mitoxantrone (Mito), an anthracene-dione anticancer drug, inhibits DNA replication and has higher anticancer activity against advanced BC. However, systemic adverse effects like cardiotoxicity and myelosuppression restrict its usage.39 The subsequent exposure to Mito, which was caused by the upregulation of several drug efflux transporters, reduced its therapeutic effectiveness.39 BC faces challenges due to nonspecific targeted action and dose-limiting adverse side effects. Researchers developed Mito-loaded SLNs functionalized with disteroylphosphatidylethanolamine–poly(ethylene glycol)folic acid (DSPE-PEG-FA) ligands, which showed adequate size and formulation stability for up to six months. The conjugated SLNs improved circulation time and tumor selectivity and minimized systemic side effects. Furthermore, iv injection of conjugated SLNs at acidic pH led to rapid drug release, enhanced circulation time, tumor selectivity, and reduced systemic adverse effects.39 The study found that SLNs are nonhemolytic to human red blood cells (RBC) and possess the high anticancer activity of Mito, with improved cytotoxicity toward MCF-7 cells at minimal IC50 concentration. These effects are attributed to various cellular uptake mechanisms, including endocytosis, and can enter cells through passive or flip-flop diffusion.39 Additionally, increased intracellular drug delivery is achieved by FR-mediated endocytosis, which causes Mito to accumulate more within MCF-7 cells. These results were validated using confocal laser scanning microscopes (CLSM) and flow cytometry.39 This leads to the conclusion that functionalized SLNs are a remarkable drug delivery strategy because they enable targeted administration to cancer cells that express FR+ while decreasing nonspecific tissue distribution and systemic toxicity.
A phytomolecule with potential for effectiveness against BC is pterostilbene (PTS). The main drawbacks of it are its poor solubility, low bioavailability, and chemical instability. The researcher uses ultrasonication methods to develop PTS-loaded SLNs. In order to target active drugs, lactoferrin (Lf) and chondroitin sulfate (CS) are dual-functionalized to produce CS/Lf/PTS-SLNs.59 Additionally, a PDI of 0.33, a zeta potential of −11.85 mV, and a particle size of 223.42 nm were observed for these dual-functionalized SLNs. After 24 h, the in vitro drug release profile showed 72.93% release in a controlled manner. In contrast, the in vitro cytotoxicity of triple-negative MDA-MB-231 cell lines revealed a 2.63-fold decrease in IC50 with a greater antimigratory impact and cellular uptake compared to PTS solution.59 In addition to their in vivo antitumor activity in an orthotopic cancer model, the CS/Lf/PTS-SLNs reduced tumor growth by 2.4-fold when compared to the PTS solution.60 Moreover, the results are also summarized in Table 2. Furthermore, the immune-histochemistry experiment revealed that CS/Lf/PTS-SLNs had a greater antitumorogenic impact through a 5.87-fold reduction in Bcl-2 expression as compared to PTS solution.59 Therefore, it can be said that CS/Lf/PTS-SLNs show promise as a phytotherapeutic nanotargeted treatment against BC.
Table 2. Examples of Anticancer Drug-Loaded Nanomedicines in the Management of Breast Cancer at the In Vivo Levela.
| drug name | nanocarriers | outcomes | ref |
|---|---|---|---|
| DOX | Liposome | it exhibited extended systemic circulation compared to free DOX. further, it also exhibited more pronounced antitumor antimetastasis effects over the free DOX | (36) |
| PTX and DOX hydrochloride | calcium orthophosphate (CaP) liposome | it achieved EE’s of 70% and 90% for PTX and DOX, respectively. the conversion of negative charge to positive charge under weak acidic pH conditions promoted efficient cell internalization. the in vivo results demonstrated 76% tumor growth inhibition | (171) |
| PTX and P53 mRNA | SLNs | in vivo studies of the orthotopic TNBC mouse model showed that BC cells are significantly inhibited due to their limited size. | (49) |
| pterostilbene | SLNs | CS/Lf/PTS-SLNs reduced tumor growth by 2.4-fold when compared to the PTS solution | (59) |
| polyphyllin D | SLNs | in vivo studies showed maximum tumor inhibitory action on 4T1-implanted BALB/c mice | (60) |
| RBO | NLCs | in vivo pharmacokinetic study revealed that RBO-NLCs have 3.54× the bioavailability of RBO-suspension | (67) |
| MTX-CS | polymeric nanoparticles | in vivo pharmacokinetic studies showed enhanced plasma concentration, retention time, and decreased cellular clearance | (79) |
| PTX+CUR | polymeric NPs | in vivo antitumor effects were observed, with significant tumor growth inhibition, prolonged survival time, reduced side effects, and reduced Ki67 expression over the free combination drugs (PTX+CUR) in BALB/c nude mouse xenografted with MCF-7 cells | (170) |
| quercetin | micelles | in vivo experiments showed dHAD-QT inhibiting tumor growth by 91.8% in mice, prolonging survival time, and reducing drug toxicity to normal tissues | (95) |
| exemestane | polymer–lipid hybrid nanoparticles | in vivo investigations showed significantly enhanced antibreast cancer activity through oral administration of exemestane-loaded TPGS-PLHNPs and exemestane-PLHNPs, with tumor inhibition rates of 72.72% and 61.94%, respectively | (121) |
| DOX | theranostic nanoparticles | the in vivo experimental results show good biocompatibility and effective apoptosis, extending the life of animals in the murine breast cancer model | (135) |
| hypericin | polyester dendrimer NPs | it was found to be a highly efficient in MRI contrast enhancement with excellent tumor-inhibiting effects | (136) |
TNBC, triple-negative breast cancer; NPs, nanoparticles; DTX, docetaxel; BC, breast cancer; SLN, solid lipid nanoparticles; DOX, doxorubicin; FA, folic acid; MTX, methotrexate; PSO, psoralen.
A steroidal saponin called polyphyllin D (PD) is derived from the Paris polyphylla. In certain malignancies, it induces apoptosis.58 Its applications are limited because it causes hemolysis.60 Researchers developed and optimized PD-loaded SLNs and used them against BC cell lines. The IC50 values were 33.25 and 35.74 μg/mL for MCF-7 and MDA-MB-231 cells, respectively. Flow cytometry analysis showed a significant enhancement in apoptosis. PD-loaded SLNs downregulated B-cell lymphoma 2 (Bcl-2) and 70 kDa heat shock proteins (HSP70) while upregulating P-53, apoptotic protease activating factor 1 (Apaf-1), p-53, and Noxa proteins. In vivo studies showed maximum tumor inhibitory action on 4T1-implanted BALB/c mice.60 Therefore, it is concluded that PD-loaded SLNs may become promising in the management of BC without recognizable side effects.
Researchers developed vincristine-loaded SLNs with an average particle size of 160 ± 25 nm and a zeta potential of −34.00 mV, exhibiting higher cytotoxicity against MDA-MB-231 cell line.61 Therefore, based on in vitro cytotoxicity studies, it is concluded that vincristine-loaded SLNs could be a better approach in the management of BC. Maslinic acid (MA) is a natural pentacyclic triterpenoid with potential antitumor properties but limited applications due to poor solubility in water.62 Researchers developed MA-loaded SLNs using poloxamer 407 and dicarboxylic acid-poloxamer 407 as surfactants. These homogeneous particles had a 130 nm particle size and were found to be stable. In vitro cytotoxicity studies showed higher cytotoxicity against MCF-7 cancer cells, while Nile red loaded MA-SLNs showed higher cytotoxicity and quick uptake. No toxic effects were observed in oral or iv administration, and fluorescent MA-loaded SLNs showed homogeneous distribution in mice.62 Thus, based on the results in vitro and in vivo, MA-SLNs have great potential as nanocarriers for effective delivery of MA with specific targeted action.
3.3. Nanostructured Lipid Carriers (NLCs)
In 2000, NLCs were established in tumor sites in response to various drugs, including chemotherapy.63 This drug delivery system has successfully localized chemotherapeutic agent accumulation to the tumor site, where it can inhibit cancer cell proliferation.63 The most significant development of NLCs is the simple and improved entrapment of drug molecules with biodegradable and biocompatible excipients, including solid and liquid lipids, to construct a matrix with a large cavity.63 Researchers developed DTX-loaded NLCs and evaluated cytotoxicity against BT-474 and MDA-MB-468 HER2-positive and HER2-negative BC cell lines.64 The developed NLCs on BT-474 cells were more cytotoxic than those on MDA-MB-468 cells. Flow cytometric tests verified the improved cell penetration of the NLCs against the BT-474 cell line.64 Ellagic acid (EGA) is commonly used to prevent and treat cancer.33 A plethora of researchers have reported that surface-functionalized nanocarriers have been evaluated in tumor-targeted delivery of anticancer drugs.33 The researcher who developed the apigenin-functionalized EGA-loaded NLCs demonstrated a substantial cytotoxic impact, with an IC50 value of 5.472 μg/mL above that of the free drug reported at 9.09 μg/mL.33 The functionalized EGA-loaded NLCs effectively arrest MCF-7 cells in G2/M and S phases, exhibiting apoptotic activity by upregulating p53, bax, and casp-3 expression and downregulating bcl-2.33 Furthermore, tumor necrosis factor-α (TNF-α) expression was elevated. In contrast, nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) activity was reduced, which supported the considerable induction of apoptosis by functionalized EGF-NLCs.33 Therefore, functionalized EGA-NLCs have been successfully proposed as effective lipidic nanocarriers in managing BC.
Methotrexate (MTX) is a chemotherapeutic agent that is used in the management of BC. However, MTX has limited clinical applications due to its low solubility, nonspecific targeting, and adverse side effects.65 However, NLCs could be promising nanocarriers for the effective delivery of MTX in the management of BC. Researchers have developed MTX-loaded NLCs using biocompatible excipients such as glyceryl monostearate (GMS) and miglyol 812 (MI1). These NLCs exhibit spherical shapes, long-term stability, and a cubic crystalline structure. The encapsulation efficiency is 85%, and the in vitro release is 52% in 24 h.66 The biocompatibility of the NLCs is under a 10% tolerance limit, and the MTT results show significant cytotoxicity against the MCF-7 cell lines. Chlorambucil (CBL), including BC, is an alkylating agent in solid tumor management. However, CBL has side effects like nausea, vomiting, diarrhea, alopecia, oral ulcers, bone marrow suppression, and hypersensitivity reactions. Researchers have developed CBL-loaded NLCs functionalized with FA as a targeting probe. The optimized formulation had an EE of 79.9%, a particle size of 119 nm, a PDI of 0.3, and a zeta potential of −42 mV. The functionalized NLCs showed superior activity to nontargeted ones over a CBL solution with minimal systemic toxicity, confirming their effective targeting ability.66 Thus, the result finding concludes that using FA in NLCs provides targeted potential and effective drug release to the specific site of BC. Ribociclib (RBO), an FDA-approved anticancer agent, inhibits cyclin-dependent kinase activity in cell cycle pathways, reducing both retinoblastoma protein phosphorylation and CDK4 and CDK6 activity.67 It has low solubility and permeability and a high hepatometabolic rate. Researchers recently utilized the Box–Behnken design and produced RBO-NLCs via solvent evaporation.67 The optimized NLCs have a particle size of 114.23 nm and an EE of 87.7%. The transmission electron microscopy (TEM) study shows a spherical shape and a uniform size distribution, and the NLCs have shown potential in drug release studies; this is also summarized in Table 1. In vitro, hemolysis studies found compatibility with RBCs, and confocal studies confirmed drug penetration into the intestine by RBO-NLCs to a greater extent than the RBO suspension and 86.71% drug release in 0.1 N HCl over the RBO suspension. The IC50 was reduced in cell line studies against MCF-7.67 An In vivo pharmacokinetic study revealed that RBO-NLCs have 3.54× the bioavailability of the RBO suspension.67 As a consequence, the findings from in vitro, ex vivo, and in vivo studies demonstrated an efficient outcome with enhanced RBO bioavailability augmentation in the efficient management of BC.
NLCs are effective for the transdermal delivery of bioactive compounds by forming a lipid film upon contact with the stratum corneum.68 Tetrahydro-curcumin (TH-CUR) is a major metabolite of CUR, and literature has revealed that it has enhanced antioxidant and anticancer properties.68 Researchers developed TH-CUR-loaded chitosan (Ch)-coated NLCs using high-shear homogenization. The optimized TH-CUR-Ch-NLCs had a particle size of 244 nm, a zeta potential of −17.5 mV, and an EE of 76.6%.68 The in vitro drug release study found that the Korsmeyer–Peppas model with Fickian and non-Fickian diffusion at pH 7.4 and pH 5.5 improved in vitro skin penetration with sustained release. TH-CUR-Ch-NLCs showed enhanced skin permeation, cell uptake, and cytotoxicity toward MD-MBA-231 cell lines compared to nonencapsulated TH-CUR.68 Therefore, it is summarized that TH-CUR-Ch-NLCs have great potential as transdermal nanocarriers in the effective management of TNBC. Dexibuprofen (DXI) is the dextrorotatory isomer of ibuprofen (IBU), with nearly 160× higher anti-inflammatory activity and significantly lower toxicity as compared to the enantiomer R.69 Although DXI is usually well tolerated, it does have some of the negative effects that are common with NSAIDs.69 DXI is an antiproliferative therapy against tumors, but it has limited applications due to the side effects, with poor physiochemical characteristics. To overcome such issues, researchers developed NLCs for its effective delivery.69 A two-level factorial design was used to optimize DXI-NLCs, resulting in a particle size of 152.3 nm, a PDI below 0.2, and an EE of >99%. These NLCs showed prolonged drug release and stability for 2 months. Their spherical shape was observed by TEM. The cytotoxicity studies showed the highest cytotoxic action against MDA-MB-468 cancer cell lines at a concentration of 3.4 μM.69 In the last, it concludes that DXI-NLCs exhibit promising antiproliferative action and have been found effective for the management of BC.
3.4. Polymeric Nanoparticles
Polymeric nanoparticles (NPs) are a biocompatible and biodegradable material used to encapsulate various drugs, including chemotherapeutic agents, as shown in Figure 3. They are categorized into nanospheres and nanocapsules.70 Ch/polylactide (PLA)-NPs have been developed for treating TNBC.71 The particle size, in vitro drug release, and viability of the BC cells were evaluated. The drug was encapsulated, effectively released, and promoted cell death against BC.71,72 Biomimetic peptides were tested in vivo in a mouse model of MDA-MB-231orthotopic xenografts against TNBC.73 The researcher has developed AXT050-PLGA-block-PEG (AXT050–PLGA-PEG).73 Antitumor and antiangiogenic peptides were used as targets and therapeutic bioactive components. AXT050 NPs were designed to integrate the aVb3 receptor for BC cells.72 Nosrati et al. developed MTX-conjugated loaded l-lysine iron oxide magnetic NPs for MCF-7 BC cells.74 The particle size of <100 nm for the mentioned formulation found a major anticancer effect.74 Calcitriol polymeric nanocapsules were developed for BC in 2018 by Nicolas et al.75 The calcitriol-loaded nanocapsules have shown controlled drug release into the tumor cells with substantial calcitriol accumulation.75 The antitumor efficacy of CUR-loaded poly(lactic-co-glycolic acid) (PLGA)-NPs was assessed by another researcher in MDA-MB-231 cancer cells. The PLGA-NPs loaded with CUR were developed utilizing a modified solvent evaporation process, resulting in increased EE and prolonged drug release.76 Furthermore, the NPs notably decreased cellular viability, migration, and invasion to the MDA-MB-231 cell line.76
Treatment of TNBC poses significant obstacles in cancer treatment, mostly attributed to the limited availability of drug delivery methods capable of efficiently targeting malignant areas. The riboflavin analogue CSRf was loaded into PLGA-NPs as CSRf-PLGA-NPs. The NPs were synthesized and modified with a ligand and a photodynamic therapy agent to target MDA-MB-231 cancer cells.77 In contrast, the biocompatibility of the new CSRf-PLGA-NPs with BC cell lines was investigated. In vitro, cytotoxicity investigation found a sixfold increase in the cellular absorption of CSRf-PLGA-NPs in cancer cells compared to normal cellsm,77 while the photosensitizing property of Rf on the surface of the NPs was reduced in TNBC cells treated with DOX-loaded CSRf-PLGA-NPs and UV irradiation.77 Furthermore, in the TNBC model, the aforementioned NPs were excellent drug delivery carriers and therapeutic entities with improved photodynamic effects. Similarly, researchers developed TF-loaded polymeric NPs with ganoderic acid A (GA-A). They found an average particle size of 15.7 nm and PDI of 0.27, having excellent drug loading and an EE of 92.2%.78 The in vitro cytotoxicity study reported significantly reduced cell viability, with the IC50 at the lowest value after 72 h.78 In rats treated with 12-dimethylbenz[a]anthracene (DMBA), TF with GA-A-loaded polymeric NPs showed 11.7% tumor incidence and the lowest average tumor weight. This led to the best recovery and maximal restoration of hematological parameters, including mitochondrial enzymes, antioxidants, and inflammatory cytokines, in the DMBA generated rat breast tumor model. This suggests TF with GA-A-loaded polymeric NPs has a greater anticancer effect.78 A researcher developed MTX-Ch-NPs by using a single-step self-assembly method with ionic gelation. The Ch was cross-linked with MTX, achieving a 49% loading. The NPs had an average particle size of 143 nm with a zeta potential of 34 mV and an EE of 87%. In vitro drug release kinetics showed the Korsmeyer–Peppas model, while MTT assays showed an IC50 of 15 μg/mL in 24 h. Hemocompatibility studies showed biocompatibility, hemolysis of <2%, and higher cellular uptake. In vivo pharmacokinetic studies showed enhanced plasma concentration and retention time and decreased cellular clearance. The antitumor efficacy studies showed a reduction in tumor volume from 1414 to 385 mm3 by MTX-Ch-NPs and that from 1414 mm3 to 855 mm3 by free MTX.79 Therefore, the present research concludes that MTX-Ch-NPs could be effective in the management of BC.
For targeted cancer therapy, albumin enhances the therapeutic index of anticancer drugs, such as lapatinib (LAPA). Albumin-coated PLGA-NPs, prepared by an emulsification method, have a small spherical core, a drug-loading capacity of 9.65%, and an EE of 75.55% with enhanced stability and a pH-controlled drug release profile. The MTT study found significantly higher cancer cell growth inhibition by LAPA-albumin-PLGA-NPs compared to free LAPA and uncoated PLGA-LAPA-NPs, and the study has reported overexpression of albumin receptor as the progenitor of this effect. The LAPA-loaded albumin-PLGA-NPs also showed enhanced tumor accumulation, prolonged blood residence time, and potent tumor growth inhibition with superior biosafety. This suggests that albumin-coated PLGA-NPs could be a new strategy to improve the safety and efficacy of LAPA in BC management.80 As a result, the developed albumin-coated PLGA-NPs may be used as a new convenient technique to increase the safety and efficacy of LAPA in the treatment of BC. Researchers developed pH-responsive CS-based NPs loaded with DOX, resulting in a particle size of 277.4 nm, a zeta potential of −13.2 mV, homogeneous dispersion, and a low PDI. DOX release was pH-dependent, and DOX-laden NPs showed greater cell cytotoxicity against MCF-7 cancer cells.75 Therefore, the novel pH-sensitive NPs could be a promising nanocarrier for tumor-targeted delivery. Silibinin (SIL) is an anticancer polyphenolic flavonoid with potential. However, its bioavailability restricts its efficacy at tumor sites. To overcome this, we developed SIL-loaded PLGA-PEG-NPs with an average particle size of 220 nm and a zeta potential of −5.48 mV. In vitro cytotoxicity studies showed the cytotoxic effects of SIL-NPs were greater than those of pure SIL. SIL-NPs induced apoptosis in BC cells by upregulating caspase-3, caspase-7, p53, and Bax, as well as downregulating hTERT and surviving cyclin D1.81 Therefore, SIL-loaded PLGA–PEG-NPs can be used as a stable nanocarrier with higher cytotoxic effects. It can be a promising novel tool for the management of BC.81
Researchers have developed biodegradable polycaprolactone (PCEC) copolymer NPs for dual delivery of PTX and CUR against BC. These NPs have a particle size of 27.97 nm and a PDI of 0.197. They show slow release of PTX and CUR without burst effects. The NPs show dose-dependent cytotoxicity against MCF-7 cells, with a higher apoptosis rate of 64.29% compared with the free drug combination of PTX with CUR (34.21%). In vitro cellular uptake studies show drug-loaded PCEC polymeric NPs are more readily taken up by tumor cells. In vivo antitumor effects were observed, with significant tumor growth inhibition, prolonged survival time, reduced side effects, and reduced Ki67 expression over the free combination drugs (PTX + CUR) in a BALB/c nude mouse xenografted with MCF-7 cells,82 which is also summarized in Table 2. Therefore, the present work can be employed to effectively manage BC in the near future. FR expression in BC cells can be utilized as a ligand for conjugation to folate-functionalized NPs. Metformin (Met), has been used for anticancer effects. Researchers developed Met-loaded PLGA-PEG-NPs and evaluated them against MDA-MB-231 human BC cell lines. Met-loaded NPs showed cytotoxic effects in a dose-dependent manner, downregulating hTERT and Bcl-2 and upregulating caspase-7, caspase-3, Bax, and p53 gene expression.83 Thus, this suggests that the FA-functionalized PLGA-PEG-NPs are an appropriate approach to enhance the anticancer efficacy of Met against BC. Researchers have developed glucose-conjugated CPT-loaded glutenin nanoparticles (Glu-CPT-glutenin-NPs) as a promising anticancer drug delivery tool for targeting malignant cells without disturbing healthy cells. FT-IR and 13C-NMR spectra confirmed the conjugation. The Glu-CPT-glutenin NPs are spherical in shape, 200 nm in size, and have concentration-dependent cytotoxicity against MCF-7 cells (IC50 = 18.23 μg mL–1).84 The cellular uptake study shows enhanced endocytosis and effective delivery of CPT in MCF-7 cells. The NPs also target mitochondria, enhancing reactive oxygen species (ROS) levels and damaging mitochondrial membrane integrity.84 Thus, from the outcomes, it summarized that the wheat glutenin can be proposed as a delivery carrier in the enhancement of anticancer drugs.
3.5. Polymeric Micelles
Polymeric micelles are attractive for the delivery of anticancer drugs with amphiphilic block copolymers, i.e., a hydrophobic core for loading hydrophobic drugs, as shown in Figure 3. The nanostructured micelles contain a hydrophilic shell, which provides firmness.85 Further, the nanosized micelles can accommodate a large payload and extended duration of circulation, as well as greater drug permeability and higher tumor penetration for water-soluble anticancer drugs.85 Cholecalciferol (CCF)-loaded PEG containing nanomicelles for therapy against TNBC has been developed by Kutlehria et al.86 Furthermore, the MDA-MB-231, MDA-MB-468, and MDA-MB-231DR (DOX-resistant) cell lines showed 1.8×, 1.5×, and 2.9× greater cytotoxicity in response to the nanomicelles, respectively.86 Western Blotting analysis has shown that DOX-loaded PEG-CCF significantly reduced the mTOR, c-Myc, and BCl-xL antiapoptotic markers and the Bax proapoptotic marker.86 The improved chemical sensitization and apoptosis lead to reduced activity of P-gp, thus minimizing their adverse effects, and the micelles could also serve as a carrier for further chemotherapeutic drugs.86 Baidya et al. reported the enhanced bioavailability of the chrysin-loaded conjugated pluronic (PF127-F68) to human BC cells.87 This structure is used to target the FR in BC. An in vitro, in vivo, and cell line study for anticervical action has been evaluated. In the MCF-7 cell line, the formulation greatly increased the Cmax and AUC values by fivefold.87 Furthermore, this is also summarized in Table 1. Pawar et al. developed festin-loaded folate-functionalized pluronate micelles (FS-FA-PF). This formulation was developed to target the FA receptor of BC cells.88In vitro and pharmacokinetic studies were performed for the said formulation.88 Nanostructured FS-FA-PF micelles exhibit advanced Cmax, improved half-life, decreased clearance, long residual periods, moderate plasma removal, and low systemically toxic anticancer activity compared to free drugs.88 Farrokhi et al. have developed a DNAzyme targeting cyclodextrin nanosystem on c-Myc oncogenic cell expression.89 The formulation significantly prevented smooth muscle cell (SMC) and MCF-7 cell line growth by 30–80%. Phytomolecules are a promising alternative for BC management.90 In this regard, RV is a plant-derived polyphenolic phytoalexin with biological activity but low water solubility, restricting its therapeutic application.90 A researcher developed RV-loaded polymeric micelles using an emulsion technique with a pluronic PF127 block copolymer and vitamin E (d-α-tocopheryl) polyethylene glycol 1000 succinate (TPGS).90 The NPs were characterized by a particle size of 179 ± 22 nm, an EE of 73.2%, and a drug-loading capacity of 6.2%. Flow-cytometry showed higher uptake in BC cells than RV, while MTT studies showed RV-loaded NPs reduced cell viability.90 Therefore, this study concludes that NPs are excellent candidates for diagnosis and therapy to treat BC. In another study, the researcher developed baicalin-loaded mixed micelles to overcome the issues associated with the pharmacokinetic issues of Baicalein.91 Baicalein-loaded mixed micelles were synthesized by using TPGS and pluronic F127. These micelles had a particle size of 25.04 nm, a zeta potential of −4.01 ± 0.5 mV, an EE of 83.43%, and a sustained release profile at pH 7.4. FT-IR confirmed the drug’s successful entrapment, revealing baicalein’s characteristic peak.91 Baicalein-loaded micelles significantly increased cellular uptake and cytotoxicity against MDA-MB-231 cell lines, induced apoptosis, and arrested the cell cycle in the G0/G1 phase. They also induced mitochondrial-mediated apoptosis through ROS-dependent mechanisms.91 Therefore, the developed baicalein nanomicelles were found effective against BC.
Researchers developed polymeric micelles of N-(2 hydroxy propyl) methacrylamide (HPMA) and mPEG to deliver anticancer drugs. mPEG-b-HPMA-DOX-loaded micelles were developed using a different drug-to-polymer ratio through thin film hydration.92 The micelles were developed with spherical shapes with diameters of ∼20–100 nm, an EE of 63.3%, and a drug-loading capacity of 5.6%. They were found to be taken up by BC cell lines in a time-dependent manner when analyzed with confocal microscopy and showed lower half IC50 compared to free DOX. The formulation was safe in hemolysis studies and maintained its residence in circulation.92 Therefore, the developed formulation found served as a promising nanomedicine effective for the management of BC. To improve the capacity to target and overcome the MDR of tumor cells compared to DOX, TPGS2000-DOX prodrug micelles have been developed, and they are reported to release DOX into tumor tissues when the environment is somewhat acidic. Micelles loaded with TPGS 2000-DOX were found to have a particle size of less than 30 nm and exhibit stronger cytotoxicity in vitro with MCF-7/ADR cells and improved tumor targeting and absorption, with a stronger anticancer effect in MCF-7 tumor-bearing nude mice compared to free DOX.93 It was concluded that TPGS 2000-DOX micelles confirmed the reversal of MDR of tumor cells, enhanced anticancer efficacy, and reduced DOX toxicity.
There is evidence that lithocholic bile acid (LCA) can selectively kill cancer cells in a variety of tumor cell lines, including neuroblastoma and BC.94 LCA has been incorporated into micelles using methoxy poly(ethylene glycol)-block-poly(ε-caprolactone) copolymer (mPEG-b-PCL), allowing pH-sensitive release in acidic media. The micelles, LCA-60 and LCA-25, have particle sizes of 86.9 nm at 60 °C and 228.2 nm at 25 °C, with observed zeta potentials of −7.54 and −18.83 mV, respectively. These micelles release more LCA in acidic media than in a physiological medium of pH 7.4. Micelles containing a fluorescent dye, like coumarin-6, are effectively internalized in triple-negative MDA-MB-231 BC after 4 h. LCA-25 and LCA-60 have a greater inhibitory impact on cell migration than free LCA. LCA-conjugated micelles reduce lipogenic activity and increase the expression of apoptotic gene Bax (1.3-fold) and p53 (1.2-fold). Annexin V-FITC shows many apoptotic cells after applying micelles to MDA-MB-231 cells.94 Therefore, the developed micelles are unique in that they exhibit pH-sensitive LCA release while also displaying effective apoptosis on BC cells.94 Therefore, these micelles could have great potential for the management of BC and, in the future, require in vivo studies before clinical translation.95 Quercetin (QT) is a potent anticancer drug but has endured several drawbacks, including poor water solubility, low bioavailability, and nonspecific targeting, which have hindered its therapeutic uses. To overcome these challenges, researchers developed amphiphilic hyaluronic acid (HA) polymers (dHAD), which self-assemble with QT to create dHAD-QT micelles.95 The dHAD-QT micelles have a superior drug-loading capacity of 75.9% and improved CD44 targeting compared to unmodified HA. They show high cytotoxicity and apoptosis and rapid QT release under low pH conditions. In vivo experiments showed dHAD-QT inhibited tumor growth by 91.8% in mice, prolonging survival time and reducing drug toxicity to normal tissues.95 Furthermore, this is also summarized in Table 2. Thus, from the findings discussed above, it can be inferred that the developed dHAD-QT micelles have promising potential as effective nanocarriers for the treatment of BC. Combinational therapy is a new trend in biomedical sciences aiming to achieve higher drug response with lower adverse effects. Researchers have optimized DOX and dimethoxy curcumin (DiMC) coloaded nanomicelles at a ratio of 1:6, while mPEG2000-PLA5000 biocompatible diblock copolymer was considered at a fixed 9:1 ratio for both drugs. This approach addresses challenges in conventional cancer chemotherapy formulations.96 The developed nanomicelles had an average particle size of 30 nm, with stable drug loading of over 9% and an EE of 95%. The acid–base interaction enhances the drug binding with the copolymer, resulting in good colloidal stability and controlled drug release. A systematic evaluation of MCF-7 breast tumor-bearing nude mice showed enhanced anticancer potency with attenuated toxicity.96 Therefore, from the above finding, it is summarized that DOX/DiMC complex nanomicelles could be a better alternative to the conventional formulations of DOX. Since the glutathione reductase is heightened in BC cells, researchers developed a redox glutathione-sensitive micelle based on abietic acid cystamine-gellan gum (AB-ss-GG) for targeted delivery of ribociclib (RIB) to BC cells. Micelles were encapsulated with different RIB/polymer ratios, enhancing glutathione reductase levels in BC cells.97 Whereas with MTT and flow cytometry, the cell cytotoxicity and cellular uptake studies conducted on MCF-7 cells, RIB loaded AB-ss-GG micelles exhibited higher cytotoxicity and cellular uptake than free RIB.97 Therefore, AB-ss-GG micelles could be promising redox-sensitive micelles for effective delivery of RIB toward the management of BC.
3.6. Inorganic Nanoparticles
Inorganic NPs are reported as drug delivery carriers for the treatment of various disorders. Inorganic NPs play an effective role in drug delivery by simplifying the NP modification controlling drug release through multiple provocations and active distribution targets.98 Inorganic NPs strengthened the diagnostic and imaging action.98 Inorganic NPs such as metallic/nonmetallic NPs demonstrated higher permeability, light absorption plasma surface resonance, and infrared radiation interaction.98 Moreover, the various inorganic NPs are illustrated in Figure 3.
3.6.1. Carbon Nanotubes
Carbon atoms are arranged in tubular configurations to form the network-like structure termed carbon nanotubes. It imparts insolubility in aqueous solutions and various organic solvents because of its distinctive structural feature. This characteristic is important, since it is crucial to determining their potential toxicity in biological fluids.99 Additionally, Figure 3 illustrates the structure of carbon nanotubes (CNTs). Furthermore, chemical alteration of inorganic NPs resulted in water solubility, enhanced biocompatibility, and reduced toxic effects.99 The higher surface is suitable for high drug payloads and has special electrical and mechanical properties. Like an arrow, it is perfect and an asset to improving tumor targeting ability.99 Liu et al. observed the covalent binding of HA on amino-functionalized single-walled carbon nanotubes (NH2-SWCNTs) to load DOX.100 Furthermore, the amine-functionalized SWCNTs revealed that the intracellular availability of DOX in CD44-overexpressed cells of the MDA-MB-231 cell line resulted in a significant increase in the proliferation and activation of cell apoptosis.100 Therefore, SWCNTs-DOX-HA significantly exhibited cytotoxicity against MDA-MB-231 cells. Moreover, the impact on the development of cancer cell spheroids was considerably reduced by SWCNTs-DOX-HA.100 The researcher utilized annexin A5 (ANXA5) with SWCNTs for near-infrared photothermal ablation of primary orthotopic EMT6 breast tumors in synergetic BALB/cJ mice. The therapy increased survival by 55% at 100 days, with an average maximum temperature reaching 54 °C. Combinatorial photothermal ablation and immune stimulation also improved survival, as assessed by flow cytometric quantification of splenic antitumor immune effector cells and serum cytokine measurement.101 TNBC frequently dysregulated P13K/Akt signaling, targeting drug targets for drug control.101 In this regard, the researcher developed single-walled CNTs with carboxyl groups (SWCNT-COOH) and conjugated cisplatin to inhibit the P13K/Akt signaling pathway. These nanoconjugates showed remarkable cytotoxicity toward MDA-MB-231 cells, causing decreased viability and increased cell death at higher doses.102 In summary, nanoconjugates downregulate P13K/Akt signaling, promoting Akt protein degradation and inhibiting tumor cell migration by decreasing P13K and p-Akt expression. CNTs significantly decreased PI3K/Akt pathway activity and hindered tumor cell motility.
Multiwalled carbon nanotubes (MWCNTs) are characterized by elongated hollow cylindrical structures, which are highly sought after for drug delivery due to their large surface area and drug-loading capability. Their flexibility allows for precise and targeted delivery of anticancer drugs like DOX, enabling them to attach with bioactive ligands through covalent and noncovalent interactions.103 Researchers functionalized MWCNTs with carbohydrate ligands using lysine to improve drug delivery. DOX-loaded functionalized MWCNTs showed superior drug loading compared to that of carboxylated and lysinated MWCNTs. In vitro drug release showed higher release at pH 5.0 than physiological pH 7.4, demonstrating sustained release over 120 h. DOX-loaded galactosylated and mannosylated MWCNTs showed enhanced anticancer efficacy and cellular uptake against MDA-MB-231 and MCF-7 BC cell lines. Importantly, sugar-conjugated blank MWCNTs such as galactosylated (GA)-MWCNTs, mannosylated (MA)-MWCNTs, and lysinated MWCNTs displayed no significant toxicity in normal cells.104 Therefore, the result suggested that sugar-tethered anticancer drug-loaded MWCNTs, especially the DOX-GA-MWCNTs and DOX-MA-MWCNTs, may be used to enhance specific targeted delivery to BC cells.
3.6.2. Mesoporous Silica Nanoparticles (MSNPs)
Mesoporous silica nanoparticles (MSNPs) offer promising drug delivery for hydrophobic drugs, particularly in tumor spaces, through EPR effects. These nanocarrier systems improve drug delivery by decreasing toxicity, providing controlled drug release and stability, and increasing biocompatibility and biodegradability.104 Further, they have been illustrated to improve EE and drug-loading capacity via surface modification. MSNPs have significant curiosity and can effectively deliver drugs because of their special physicochemical characteristics.104 Surface structures ensure biocompatibility and denaturation protection that control cellular penetration of drug release and prevent untimely drug leakage, ensuring potent penetration and effective drug delivery within tissues.104 MSNPs have also been used for diagnosis and gene delivery in cancer management.104 Li and associates have developed sulfide mesoporous theranostic bismuth silica (Bi2S3@mPS) filled with a TRZ-paired DOX-loaded nanoformulation (antibody against expressed BC cells).105 Multiple desirable attributes have been identified for cancer theranostic (biocompatibility, drug-loading, accuracy, successful accumulation, and tumor targeting) by in vitro and in vivo studies.106 MTX is an antimetabolite agent that competes with dihydrofolate reductase receptors (DHFR) for binding, causing inhibition of cell division and proliferation.106 Researchers developed MTX-loaded MSNPs and modified them with 3-triethoxysilylpropylamine (APTES) and Ch through a covalent linkage involving glutaraldehyde. The MSNPs had a particle size of 100 nm, an EE of 12.2%, and an elevated release profile for MTX. Confocal microscopy showed enhanced cellular internalization of fluorescein isothiocyanate (FITC)-labeled MSNPs-APTES-Ch at a low dose and efficaciousness in cytotoxic delivery to BC cells.106 Thus, these NPs hold significant promise for BC treatment applications. Researchers developed pH-sensitive glucosamine-targeted polydopamine (PDA)-coated MSNPs. The optimized anderson-type polyoxomolybdate NPs exhibited pH-dependent drug release, an average particle size of 195 nm, a zeta potential of −18.9 mV, and a 45% drug loading capacity. The targeted NPs demonstrated enhanced anticancer activity against MDA-MB-231 BC cell lines, with the highest cellular uptake and apoptosis.107 Therefore, MSNPs could be clinically successful nanocarriers.
RV has had challenges in being used clinically for BC because of its poor solubility, pharmacokinetics, and stability issues. To overcome these difficulties, scientists developed MSNPs loaded with RV as RV-MSNPs.108 RV-MSNPs inhibited MCF-7 cell proliferation, invasion, and apoptosis in vitro, while in BC mouse models they showed better performance by inhibiting tumor growth via NF-κB signaling pathway inhibition.108 Therefore, this finding could be a new and safer use of phytochemicals against BC. Exemestane, an oral steroidal AI of the third generation, is used as an adjuvant therapy for postmenopausal women with hormonally responsive estrogen-receptor-positive BC. Despite this, its clinical effectiveness is hindered by its poor oral bioavailability (10%) and low water solubility.109 To surmount these challenges, researchers developed magnetic mesoporous silica nanoparticles (MMSNPs) to improve the delivery of exemestane delivery. These MMSNPs displayed a particle size of 137.2 nm and a PDI of 0.224, exhibiting a substantial drug-loading capacity of 37.7% and facilitating efficient sustained release, with up to 98% release. Magnetic resonance imaging studies show a concentration-dependent contrast effect, highlighting the potential of exemestane-loaded MSNPs.109 Therefore, based on results, it is concluded that exemestane-loaded MSNPs have good potential and can be used for the treatment of BC in the near future. In a recent development, researchers designed dual-loaded hollow mesoporous silica nanoparticles (HMSNPs) for BC management, combining PTX and 5-fluorouracil. The system, synthesized using the inverse microemulsion method, showed improved effects on MCF-7 cells with overexpressed FA receptors compared to individual drug administrations.110 Therefore, because the developed dual drug-loaded nanosystem selectively targets the tumor sites and the synergistic effect reduced its toxic effects on the healthy cells, it could be a promising option in the management of BC. Researchers developed silymarin (SLM) and Met-loaded MSNPs and evaluated them against MCF-7 cell lines. The coloaded MSNPs showed uniform size and shape, with a particle size of 100 nm and a pore size of 2 nm. In vitro cytotoxicity studies showed lower IC50 values for Met-MSNPs, SLM-MSNPs, and dual drug MSNPs than free-Met, free-SLM, and free-Met-SLM. The coloaded MSNPs significantly inhibited breast cancer resistance protein (BCRP) mRNA expression, promoting apoptosis in MCF-7 cells.111 Therefore, the finding concludes that both SLM and Met’s anticancer effects enhance the anticancer effect against BC. Recently, researchers developed CUR-loaded amine-functionalized MSNPs, which revealed high drug loading efficiency with sustained release when tested against MCF-7 cells.112 CUR-loaded functionalized MSNPs significantly decreased cell viability against MCF-7 compared to free CUR for 72 h. Confocal fluorescence microscopy showed higher cytotoxicity and significantly affected the mRNA and protein levels of caspase-9, Bcl-2, Bax, caspase-3, and hTERT compared to the free CUR treatment.112 Therefore, the above results suggested that amine-functionalized MSNPs drug delivery can be a promising alternative approach for CUR delivery against the management of BC.
3.7. Hybrid Nanoparticles
Mixing the nanoformulation with various organic and inorganic components allows for structural changes to produce hybrid NPs with the required effects.113 Effective action against tumor metastasis-resistant and tumor-guided metastasis therapy has been demonstrated with liposomes, micelles, dendrimers, metal oxides, gold oxide NPs, and quantum dots (QDs).113 The peptide enhances intracellular DOX antitumor action by stimulating BC mitochondria and nuclei.113 Zhang and co-workers have developed DOX and mitomycin C dual-targeted hybrid NPs, demonstrating a synergistic action against MDA-MB-231 cells.113 Albumin NPs codeliver DTX and gemcitabine (GEM) against BC, enhancing cellular uptake (nuclear colocation) through hNT, OATP1B3-independent clathrin-mediated internalization, and enhanced apoptosis in perinuclear and nuclear regions. Loading DTX into GEM-albumin-NPs increased AUC and T1/2 values by 6.12× and 3.27×, respectively, compared to taxotere and gemzars.114 Psoralen (PSO) is a phyto-based furocoumarin widely used in traditional medicine and obtained from Psoralea corylifolia.115 The therapeutic potential of PSO in BC has been well documented.115 PSO is a promising anticancer chemical. However, its therapeutic use is hindered by poor water solubility and decreased bioavailability, which limit its anticancer activity and make it difficult to use in therapeutic applications.115 To overcome such problems, lipid–polymer hybrid nanoparticles (LPHNPs) are carrier systems that offer advantages over liposomes and polymeric NPs. These carriers provide stability and inhibit drug diffusion, making them useful for treating BC. They enhance hydrophobic drug entrapment, control drug release, improve drug uptake, and prevent water-soluble drug leakage. PSO-loaded polymeric lipid NPs (PLNPs) are developed using the nanoprecipitation method and optimized using a central composite design response surface methodology. The characterizations of LPHNPs revealed a particle size of 93.44 nm with a zeta potential of −27.63 ± 0.31 mV. In vitro drug release studies show burst release within the first 8 h and accumulated drug release of over 50%, possibly due to the free PSO attached to the NP surface.116 The core–shell structure of PLNPs may retard PSO diffusion and control drug release, resulting in accumulated drug release exceeding 90% after 36 h.116 Therefore, PSO can be effectively prolonged through the extensive release time of said drug. The antitumor efficacy study measured changes in tumor volume and final tumor weight. The saline group did not cause tumor growth inhibition, while PSO-PLNPs showed maximum tumor inhibition. Nude mice treated with PSO-PLNPs exhibited a tumor inhibition rate of 78.10%, and that of mice treated with free DOX only was 46.69%, significantly different from the control group (p < 0.05). Body weight changes were also a key index in determining systemic adverse effects. PSO–PLNPs accumulated in the tumor via passive targeting, leading to enhanced PSO within the tumor over the free drug. PSO-PLNPs also had an extended circulation time for PSO and more efficient antitumor effects than other formulations. No significant histopathological changes were observed in the liver, spleen, lung, and kidney.116 Thus, from the above discussion, it is concluded that using PSO-PLNPs as carriers is a promising strategy to enhance the therapeutic efficacy of PSO against BC.
DTX-loaded LPHNPs have been developed by Jadon et al.117In vitro studies reported significant cytotoxicity and high cellular penetration at a lower IC50 of DTX against BC.117 Cucurbitacin (CuB), an oxygenated triterpene from the Cucurbitaceae family, has various effects, including cytotoxic, antitumoral, hepatoprotective, anti-inflammatory, antibacterial, antianthelmintic, cardiovascular, and antidiabetic actions. CuB is an interesting molecule in cancer research due to its anticancer activity. However, its poor water solubility and absorption have limited its clinical application. Ensuring adequate and proper drug access via nanoscale drug delivery technologies is essential.118 Researchers developed CuB-loaded LPHNPs using factorial design to balance the effects of total lipids/lecithin at a molar percentage ratio and used them against TNBC. The formulation effectively suppressed cell cycle development in the G0/G1 phase, with an annexin V-bound cell population of 20.66 ± 1.99%, whereas the depolarized cell population was higher in CuB-LPHNP treated cells. CuB-LPHNP-treated cells displayed a more depolarized cell population and higher levels of preapoptotic bax and cleaved poly(ADP-ribose) polymerase (PARP) while downregulating antiapoptotic Bcl-2 and NF-κB.119 Based on this evidence, therapy for BC using CuB-loaded LPHNPs seems successful. Recently, researchers have developed exemestane (EXE)-loaded TPGS-based polymer–lipid hybrid nanoparticles (EXE-TPGS-PLHNPs) using a single-step nanoprecipitation technique and Box-Behnken design optimization for the controlled delivery of EXE in BC treatment.120 The optimized PLHNPs have a particle size of 136.37 nm, a PDI of 0.110, and an EE of 88.56%. The encapsulated EXE was confirmed and validated by FT-IR, DSC, and powder X-ray diffraction (PXRD). The results show complete encapsulation of EXE in the hybrid matrix of PLHNPs, with no significant interaction between the drug and the excipient.120In vitro drug release studies showed controlled drug release lasting up to 24 h in simulated gastrointestinal fluids. Optimized EXE-loaded TPGS-PLHNPs showed remarkable stability under various storage conditions. Cellular uptake of EXE-loaded TPGS-PLHNPs increased over time, leading to dose-dependent cytotoxicity against MCF-7 cells. Oral administration of EXE-loaded TPGS-PLHNPs led to 3.58× higher bioavailability than the conventional EXE suspension, and no acute toxicity was observed. In vivo investigations showed significantly enhanced anti-BC activity through oral administration of EXE-loaded TPGS-PLHNPs and EXE-PLHNPs, with tumor inhibition rates of 72.72% and 61.94%, respectively. Histopathological assessments showed negligible changes in vital organs, and hematological studies confirmed the safety of administered PLHNPs.121 Thus, the results concluded that EXE-TPGS-PLHNPs are a promising platform for the effective and controlled delivery of EXE in the effective management of BC.
α-TS, a vitamin E derivative with robust anticancer properties, served as a foundation for further study. The researchers used a novel approach by developing hybrid NPs that are sensitive to changes in pH. These NPs consist of a core made of PLGA polymer and a shell composed of TPGS lipid. Additionally, the NPs were designed to include both DOX and TS. The lipid–polymer nanoparticles (LPNPs) exhibited a unique spherical shape.122In vitro studies showed a significant reduction in cell viability, migration and cell death in the 4T1 cell line compared to free DOX. TS-DOX-LPNPs were more effective in controlling tumor growth. The enhanced antitumor efficacy was due to the synergistic combination of DOX and TS, with high levels of DOX causing cellular internalization and pH-triggered payload release in the tumor area.122 Therefore, from the above data, it can be concluded that LPNPs could be novel option in the effective management of BC. Sunitinib malate (SM), a well-known anticancer drug used in the treatment of BC, was encapsulated in lipoid-90H, Ch, and lecithin-based LPHNPs. The LPHNPs were precisely produced using the emulsion solvent evaporation technique. Notably, four different LPHNPs, identified as LPHNP1–LPHNP4, were produced by changing the Ch concentrations. Through optimization efforts, SLPN4 emerged as the most promising, with a particle size of 439 nm, a PDI of 0.269, a zeta potential of +34 mV, and an excellent EE of 83.03%.123 An in vitro study found SLPN4 released 84.11% of its drug within 48 h, following the Korsemeyer–Peppas model with the Fickian mechanism. It also exhibited potent cytotoxicity against MCF-7 in the BC.123 Thus, this finding concludes that the SM-loaded LPHNPs could be a promising therapy option in the management of BC cells expressing IGF-1R, which activate intermittently through multiple pathways, causing neoplasm cell proliferation, apoptosis, survival, and resistance to chemotherapeutic agents. Suppressing these receptors could be a promising strategy to inhibit malignant phenotypes in BC cells.124 Researchers have hybridized methoxypoly(ethylene glycol)–poly(caprolactone) with dimethyldioctadecylammonium bromide (DDAB) cationic lipid (mPEG-PCL-DDAB) NPs to deliver lycopene and insulin-like growth factor-1 receptor-specific siRNA against MCF-7 cells. The mPEG-PCL-DDAB NPs loaded with anti-insulin-like growth factor-1 receptor siRNA and lycopene significantly induced apoptosis and arrested cell cycle in MCF-7 tumor cells.124 Therefore, this finding verifies the potency of mPEG-PCL-DDAB-NPs for the combinational delivery of siRNA and lycopene in the BC cell lines via the induction of apoptosis. The herb extract from Cordyceps militaris, which is high in the anticancer compound cordycepin, has shown remarkable effectiveness against BC. By considering this effect, HA surface-decorated LPHNPs were synthesized. Subsequently, MDA-MB-231 and MCF-7 cell lines were used to assess cordycepin-loaded LPHNPs. Notably, these LPHNPs displayed heightened cellular uptake facilitated through CD44 receptor-mediated endocytosis.125 Therefore, these results demonstrated that successful targeted delivery to the CD44 receptors of tumor cells by drug-loaded LPHNPs.125 Thus, the developed LPHNPs showed promising potential in the effective delivery of cordycepin against BC.125 However, in vivo results are required in the future for clinical translation.
3.8. Theranostic Nanoparticles
Imaging agents, drugs, and genes have been employed in nanoformulation development.126 Theranostic NPs in a single system imply “diagnostic and therapeutic substances”, as shown in Figure 4. It substantially impacts treating complicated illnesses such as cancer and proliferative tumors.127 In theranostics for anticancer drug delivery, QD-based nanohybrids are emerging, made using polysaccharides, lipids, proteins, and polymers. QDs are semiconductor crystals with photoluminescence characteristics.127 Further investigation on theranostic micelles of gold (Au)–silicon dioxide (SiO2)/QDs revealed the substantial lethal effects of free DOX in cells compared with Au-SiO2/QDs.128 The PTX-conjugated cadmium telluride (CdTe)/cadmium sulfide (CdS)/zinc sulfide (ZnS)-QDs demonstrated increased therapeutic effectiveness of the imaging action of the tumor detected.128 Yang and his collaborator developed Cd-free ZnS-QDs in optimally fluoresced fluorescent lipid vesicles that showed excellent colloidal stability, i.e., fluorescence/magnetic resonance-mediated diagnostics.129 Ghosh et al. developed dendrimer-functionalized QDs.130 Liu et al. developed 2D superparamagnetic tantalum carbide composite MXenes (Ta4C3-IONP-SPs), which showed efficient theranostic action with higher computed tomography imaging action in BC.131 The enzyme-sensitive theranostic NPs developed using PTX and enzyme, which degraded into lower molecular weight products (44 kDa), effectively released the anticancer drug into the cancer microenvironment and showed MRI activity, which improves therapeutic effectiveness and imaging skills and greatly increases tumor site conjugate accretion.131
Figure 4.
Representation of the theranostic nanocarriers and their fate inside breast tumor cells. This figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license (https://creativecommons.org/licenses/by/3.0/).
A single platform for tumor diagnosis and treatment using magnetic nanoparticles offers a novel perspective, offering superior magnetic properties, low toxic effects, biocompatibility, and biological degradability to potentially overcome conventional therapy problems.132 A researcher developed an innovative theranostic agent using soybean oil-based polystyrene-based graft copolymer with silver (Ag) box NPs for therapeutic and MRI-BC diagnosis. The drug could selectively bind and efficiently internalize HER2-conjugated magnetic PS-Ag-box-NPs in MDA-MB-231 and SKBR-3. Additionally, it revealed the ability to induce MRI activity.133 Thus, this combination nanosystem can be utilized as a theranostic agent for BC. Intercellular adhesion molecule-1 (ICAM1) is a biomarker against TNF-α, but its specific targeting limits its effectiveness. To address this issue, vesicular NPs with poly(ethylene glycol)-poly(ε-caprolactone) copolymers conjugate anti-ICAM1 antibodies using click chemistry. These NPs have shown enhanced diagnostic and therapeutic efficacy in tumor models, with extended circulation time, and additionally in vitro experiments exhibited specific targeting action specifically for TNBC.134 Theranostic NPs offer multiple therapeutic applications, including tumor-targeting imaging, focused ultrasound ablation, chemotherapy, and sonodynamic therapy. A multimodal synergistic therapy can enhance TNBC treatment efficacy and prognosis by enhancing imaging, guided ablation, and focused ultrasound (FUS)/chemotherapy effects. The AS1411-Apt-modified PEG@PLGA-NPs loaded with DOX and perfluorohexane (PFH) have a 306.03 nm particle size and a zeta potential of −4.05 mV. They bind to 4T1 cells and elevate ultrasound contrast agent (UCA) image after FUS exposure. Further, this results in a higher apoptosis rate and stronger inhibitory effect on 4T1 cell invasion. This multifunctional AS1411-DOX/PFH-PEG@PLGA-NPs enhances real-time contrast imaging, improving TNBC therapeutic efficacy and prognosis. Therefore, this NPs can be utilized with therapeutic outcomes against TNBC, which may provide a promising theranostic approach with MRI-guided therapy. TNBC is challenging, due to extremely metastatic and resistance to conventional chemotherapy. Combining chemotherapy and photothermal therapy (PTT) for enhanced anticancer effects is increasingly popular. DOX-loaded HMS-NPs are functionalized with Ch and copper sulfide (CuS) nanodots to create HMS-NPs-Ch-DOX@CuS. CuS acts as protective agent, sealing surface pores and preventing DOX release into systemic circulation. Further, it exhibits potent tumor-targeted photothermal properties and can induce DOX release through near-infrared laser irradiation. In vitro and in vivo experimental results show good biocompatibility and effective apoptosis, extending the life of animals in the murine BC model.135 Therefore, it can be concluded that the HMSNPs-Ch-DOX@CuS nanoplatform produced highly effective synergistic chemotherapeutic-PTT. Thus, this work reflects a new avenue for near-infrared (NIR) enhanced synergistic chemo-PTT against BC. Most recently, in the area of theranostic nanoplatforms in the management of BC, researchers developed manganese-based hypericin-loaded polyester dendrimer NPs (MHPD-NPs).136 The hypericin-loaded MHPD-NPs achieved MRI activity and enhanced photodynamic therapy and showed excellent biocompatibility.136 Therefore, this suggests that MHPD-NPs are highly efficient in MRI contrast enhancement with excellent tumor inhibiting effects.136 MHPD-NPs were concluded to be a novel, stable, and multifunctional nanotheranostic agent for the management of BC.
3.9. Nanomedicine for Gene Therapy
Gene silencing using RNA interference is a recently emerging tailored method for cancer therapy. Specific oncogenes may be silenced using the effector molecules such as siRNA and microRNA (miRNA).137 Now, therapeutically, the major limiting factor for siRNA and miRNA-based therapeutics is the lack of an efficient delivery method to prevent the degradation of nuclease RNA molecules and carry them into cancer cells for release on the target organelles.137 Several researchers have developed various NPs and attempted in vivo and cell-line experiments to solve the above difficulties for efficient gene therapy.137 HA-Ch-loaded NPs developed by Deng et al.138 further encapsulate miR-34a and DOX for simultaneous delivery against TNBC. Moreover, in vivo results revealed significant tumor inhibition by effective delivery and targeting of the BC cells. Additionally, the NPs also suppress the proto-oncogene BCl-2.138 Further extensive studies revealed that miR-34a inhibited BC cells via targeted Notch-1 signaling. Therefore, the synergistic impact of both drugs was strong for BC cell suppression.138 Juneja et al. developed protoporphyrin IX, CUR, and RNA-loaded NPs, and their simultaneous delivery found synergistic and significant suppression of TNBC via combined photothermal and chemotherapeutic and nucleic acid delivery.139 Using the simultaneous targeted delivery of DTX and insulin-like growth factor-1 (IGF-1) receptor siRNA, Jafari et al., have developed Apt-hybrid Ch-NPs against TNBC.140 The findings have shown that cell uptake increased and the cell viability of SKBR3 cells was dramatically reduced.140 Additionally, the genetic expression of IGF-1R, a signal transducer, transcription 3 activators, a metalloproteinase matrix, and vascular growth factors was drastically decreased by Apt-conjugated NPs. Polymeric NPs encapsulated with PD-L1 siRNA and conjugated with a new RNA-Apt are specific for TNBC and resistant to nucleases. In vitro studies show that NPs enhance siRNA uptake specifically into TNBC MDA-MB-231 and BT-549 target cells, leading to endosomal release and suppression of PD-L1 expression within 90 min. Overall, targeting PD-L1 in TNBC cells was achieved.141 Thus, the above results provide an effective siRNA-delivery system for TNBC treatment. A researcher developed Au-NPs modified with trimethyl-Ch (TM-Ch) to improve EGFR targeting, siRNA stability, and BC cell uptake. The AuNPs-TM-Ch complex, loaded with EGFR-siRNA at a 10:1 weight ratio, was tested against the MCF-7 BC cell line and found to have 67 nm spherical particles with a +45 mV zeta potential. Cy5-labeled AuNPs-TM-Ch increased cellular absorption after 4 h. After complexing with EGFR-siRNA, the nanocomplex had a particle size of 82 nm and zeta potential of +11 mV. Interestingly, the nanocomplex had negligible cytotoxicity, but EGFR-siRNA-loaded-AuNPs-TM-Ch had 50% cytotoxicity and cell viability loss. The nanocomplex knocked down EGFR gene expression (86%), causing increased apoptosis.142 Therefore, the above results suggested a promising nanocarrier for siRNA delivery and a potential therapeutic nanocarrier for managing BC. A researcher developed siRNA-loaded PEG disulfide bond-linked polyethylenimine (PEI) NPs that effectively inhibited protein kinase B (PKB or Akt), phosphoinositide-3-kinase (P13K), p-EGFR, EGFR, bromodomain-containing protein 4 (BRD4), and c-Myc expression in tumors without affecting hematological parameters, biochemical indices, or tissue morphology in treated mice.143 The siRNA-loaded PEG-SS-PEI demonstrated favorable antitumor effects against TNBC, with good targeting efficacy and biocompatibility.
RNAi-based technology is growing in neoplastic disease treatment, but in vivo applications are limited due to the lack of an efficient and safe carrier.144 A researcher developed Apt-protamine-siRNA-NPs that inhibited tumor growth and cell cycle in MCF-7-bearing nude mice. In receptor tyrosine-protein kinase (ErbB3)-positive BC xenograft mice, the nanocarrier effectively targeted ErbB3-positive tumor cells, silencing targeted gene expression. This technology has shown potential in both in vitro and in vivo applications.144 Niosomes containing lifeguard-specific siRNA are expected to be a promising therapeutic option for treating BC. However, siRNA cellular uptake is suboptimal, leading to nonsuitable accumulation in target tissues. Niosomes encapsulate siRNA, and iron oxide NPs are incorporated into the bilayer structure for enhanced uptake. Entrapment of siRNA in hybrid niosomes, administered alone or in combination with erlotinib or TRZ to BT-474 cells, leads to enhanced apoptosis and downregulation of the lifeguard (LFG) gene. This leads to improved anticancer therapeutic efficacy over single siRNAs. The external magnetic field promotes cellular internalization of siRNA and activation of apoptotic signaling pathways.145 The NP-Ch-xPEI composite has been developed by researchers, comprising an iron oxide core that PEI envelopes with a low molecular weight of 800 Da. Additionally, the PEI is cross-linked with Ch. The aforementioned NPs can compact DNA into smaller NPs measuring less than 100 nm. These NPs provide complete protection to the DNA, exhibit superior transfection effectiveness across several BC cell lines, and demonstrate reduced cytotoxicity in comparison to widely used commercial transfection agents such as lipofectamine 3000.146 Therefore, NP-Ch-xPEI can serve as a reliable vehicle to deliver DNA to BC. miRNA (i.e., miR-206) has problems with its delivery; it mimics the site of the tumor.147 Researchers developed Au-NPs modified with the PEG moiety to encapsulate miR-206, effectively delivering the compound and causing cytotoxicity in MCF-7 cells. This nanocomplex enhanced apoptosis by downregulating NOTCH3 and cell arrest in the G0-G1 phase.147 Au-NPs modified with PEG could be an emerging option for managing BC. Photochemical internalization (PCI) and photodynamic therapy (PDT) involve light-induced cell death and membrane disturbance. Two-photon excitation (TPE) is useful for PCI and PDT due to its spatiotemporal resolution and deeper penetration of NIR. Periodic mesoporous ion silica NPs (PMISNPs) complex with pro-apoptotic siRNA, leading to significant cell death in MDA-MB-231 BC cells. Preincubation with NPs in zebrafish embryos showed a synergistic effect,148 whereas it led to 90% MDA-MB-231 viable cell death. Therefore, PMINPs represent an interesting nanosystem application.
4. Factors Affecting the Pharmacokinetics of Nanomedicine Used in BC
Several of the most critical physicochemical properties of nanocarriers, including drug transport, cellular absorption, and toxicity, are described in the following section.149
4.1. Size and Surface Area
Nanocarrier size and surface area considerably influence how the nanocarriers are taken up by cells and distributed, how they accumulate at the tumor site, and how much of the drug they carry is released.149 The pharmacological properties of nanocarriers are determined by their large surface area and compact size. Because of their potential to access various biological systems, several studies reported that the toxicity increased with increasing size.150 When nanocarriers in the size range of 100–200 nm enter the reticuloendothelial system, their half-life is reduced and they are less able to target various tissues.150 Cellular absorption, cytotoxicity, and intracellular localization are all affected by the size of nanocarriers, and this is especially true for their interaction with living cells. The cellular absorption of nanocarriers necessitates a minimum particle size of 30–50 nm, regardless of content. The dispersion of nanocarriers was also significantly dependent on their size.151 PEG-linked nanocarriers are used to evaluate gemcitabine delivery, and there was an increase in effectiveness compared with the free drug. For nanocarriers, this study found that the conjugation of their surfaces provides them with the optimal size, facilitating their active cellular absorption.152 Small nanocarriers are more likely to be taken in the cells than larger sizes. Thus, it is concluded that the size and surface area of the nanocarriers played an important role in the nanocarriers’ stability to cellular absorption, cytotoxicity, cellular uptake, and toxicity.
4.2. Shape
One of the important aspects of targeted treatment is determining the nanocarriers. Spherical nanoparticles have shown that they are more easily absorbed into cells than rod-shaped nanoparticles. Additionally, it has been reported that the nonspherical nanoparticles, which are more likely to pass through capillaries than spheres, have demonstrated toxicity.153 Nanospheres are superior to nanorods, nanocages, and nanodiscs in tumor absorption and blood circulation.139 In most of the investigations, shape-dependent toxicity is also seen. There is greater evidence that nanospheres are more rapidly absorbed than Au nanorods.154 As a result, the NP form must also be considered when attempting to transport cancer cells to a specific tumor location, such as in the case of BC. Furthermore, this section concludes that shape can be a determinantal factor in absorption, tumor targeting, and toxicity.
4.3. Surface Charge
Their surface charge determines how nanocarriers interact with biological components. The surface charge of nanocarriers affects the absorption, plasma protein binding, colloidal nature, membrane permeability, and toxicity of nanocarriers.155 Cellular absorption of positively and negatively charged nanocarriers was shown to be much greater and quicker.156 The surface charge governs nanocarrier aggregation and its targeted delivery. The surface coating may alter the nanocarriers’ surface charge and can also help protect the nanocarriers from endosomal entrapment.157 It was shown in the study that the endosomal entrapment of surface-modified silica NPs with different charged functional groups was possible. According to the findings, the negatively charged nanocarriers could better escape endosomal entrapment.158 Researchers found that altering the surface charge can impact drug delivery to a specific tissue, which is believed to be due to the modification. Thus, the surface charge on the nanocarriers is an important factor in absorption, plasma protein binding, permeability, targeted drug delivery, escape endosomal entrapment, and toxicity.
4.4. Chemical Composition
The chemical composition of nanocarriers impacts their solubility, stability, and biocompatibility. Changes in these qualities can affect drug distribution to malignant tissue.159 Polymeric NPs may be more effective in delivering drugs than conventional NPs. The degradation and drug release kinetics of NPs may be controlled by the physical features of a polymer, such as molecular weight, crystallinity, dispersity index, and hydrophobicity.159 PLGA-based NPs loaded with tamoxifen citrate are more effective than free tamoxifen citrate.160 Another study found that transfection efficacy relies on the polymer’s properties when examining nanocomplexes’ biological and physicochemical aspects for gene delivery. An intermediate molecular weight, positively charged polymer with a greater degree of acetylation was shown to be able to carry the miRNA into the cytosol.161 Inorganic NPs with metallic compositions have been hazardous in the past. They can be used as drug delivery carriers because of their surface characteristics.161 Connecting the anticancer drugs to Au-NPs is possible using the thiol groups on their surface. The Au-NPs containing MTX were developed.162 They exerted a greater cytotoxic effect of MTX on BC cells than on either the free MTX or the Au-NPs. Finally, it is concluded that the chemical compositions of nanocarriers are determinantal factors in their crystallinity, hydrophobicity, solubility, stability, and biocompatibility.
5. Toxicological Aspect of Surface Engineered Nanocarriers
Biological barriers are more easily breached when nanocarriers are small enough to pass through. Toxicities of nanocarriers are the most serious concern.162 ROS are released when cells are under oxidative stress, leading to inflammation and cell damage. Studies have shown that the size, shape, and charge of NPs are the most important determinants of toxicity. The common method for lowering nanoparticle toxicity is surface modification or functionalization. The increased biomedical application of nanocarriers with surface functionalization results in less danger to cells and tissues.162 Chemical surface modification of nanocarriers promotes biocompatibility and affects binding to plasma proteins and the cytotoxicity of the NPs. The in vivo aggregation of CNTs determines their toxicity. The nonfunctionalized CNTs have been examined in several investigations for in vivo accumulation and toxicity. Although they accumulate in the liver, lungs, and spleen,162 Yang et al. observed elevated oxidative stress. Moreover, lactose dehydrogenase and alanine aminotransferase levels were elevated.163 Additionally, hepatic injury is to blame for the elevated markers in the blood. Malondialdehyde and glutathione levels in the lungs and liver have shown higher levels of oxidative stress. In several other investigations, SWCNTs and MWCNTs have also been studied for in vivo aggregation.163 A comparison of the in vivo compatibility of SWCNTs and MWCNTs was made by Faczek et al. They discovered that MWCNT aggregates were bigger than SWCNT aggregates.164 Several factors influence the toxicity of CNTs in general. Their length strongly influences the phagocytosis of CNT. For in vivo applications, SWCNT is preferred over MWCNT because of its shorter length and lower aggregation.165 To decrease the harmful effects of CNTs, surface functionalization is required. The functionalization of SWCNTs is intended to lessen harmful effects. There is, nevertheless, a need for extensive toxicity investigations of functionalized CNTs.165 Dendrimers are hazardous because of their surface charge and terminal groups. Nanoholes can form when positively charged dendrimers contact negatively charged membranes, causing cell lysis and membrane breakdown.166 There was evidence of dendrimer toxicity, including PPI (polypropyleneimine) and PAMAM (polyamide amine). It is possible to neutralize the surface charge of dendrimers by performing surface modifications such as PEGylation, acetylation, and peptide conjugations.166 The surface coating can minimize the toxicity, as in the case of metallic NPs, whereas in the case of QDs a secondary coating improves biocompatibility.166 Choosing the right coating agent is critical because a weak coating agent that can easily be degraded in biological environments might expose the metallic core, which may produce harmful effects or experience undesirable interactions with biological components.166 To ensure that NPs do not pose a health hazard, it is also crucial to evaluate the charge of the coating agent.167 By adding biological ligands to the surface of NPs, their toxicity to organs other than the intended target is reduced while their specificity increases.167 The toxicity of nontargeted NPs may also be decreased by functionalizing the intended location with ligands onto NPs. NPs may still be harmful even after the ligands have been attached to them.167 Thus, the size, shape, and surface charges are the key factors determining the toxicity of nanocarriers. The chemical surface modification of nanocarriers may enhance biocompatibility and minimize toxicity. Moreover, the surface coating can also be employed to minimize the toxicity attributed to the nanocarriers.
6. Current Challenges in the Clinical Translation of Nanomedicine: Poor Optimizations and Pharmacokinetics
Nanomedicine clinical translation for managing BC remains slow, although a few cases are being used clinically.168 However, various available products are on the market, and many clinical development stages against BC are summarized in Tables 3 and 4. However, the slow bench-to-bed conversion of nanomedicines is due to various factors, including difficulty in manufacturing, low reproducibility, high expenses, and lack of “quality by design” (QbD). The lack of predictability in relation to the success of anticancer nanomedicines is due to inappropriate pharmacokinetics, biodistribution, and release kinetics on the target location. Moreover, all these parameters differ greatly from animal models to human patients.128 Conversely, the nanocarriers can be modified to maximize safety based on the biodistribution or toxicity results.168 Lessons learned from current clinical applications of anticancer drugs show that a deeper understanding of tumor pathology dictates the incorporation of key nanomedicine design features and allows preclinical models to provide reliable pharmacological and toxicological data.168,169 The basis of “rational design” should also take account of well-known aspects that influence nanomedicine clinical success, including the EPR effect and the expression of disease-associated cell surface markers that permit cell identification, absorption, and the involvement of immune cells at the target location.168,169 Thus, this section concludes that challenges in the clinical translation of nanocarriers are manufacturing scale-up issues, low reproducibility, expenses, and a lack of quality control and stability. In addition, the in vivo challenges are suboptimal pharmacokinetics, biodistribution, and drug release kinetics at the target location.
Table 3. Anticancer-Loaded Nanomedicines Available on the Market for the Treatment of BC.
| brand name | drug | nanoformulations | indication | approved year | manufactured/marketed by | ref |
|---|---|---|---|---|---|---|
| Abraxane | paclitaxel | albumin | employed in breast cancer treatment when combination chemotherapy failed within 6 months | 2005 | Bristol-Myers Squibb | ABRAXANE® for Metastatic Breast Cancer |for HCPs (abraxanepro.com) |
| Genexol-PM/Cynviloq | paclitaxel | polymeric micelle | breast cancer, NSCLC, ovarian cancer | 2006 | Samyang Biopharmaceuticals Corporation, Sorrento Therapeutics | https://pdf.hres.ca/dpd_pm/00002333.PDF |
| Myocet | doxorubicin hydrochloride | liposome | first-line treatment for metastatic breast cancer in adult women | 2000 | Elan Pharmaceuticals, Sopherion Therapeutics | https://www.ema.europa.eu/en/documents/product-information/myocet-liposomal-previously-myocet-epar-product-information_en.pdf |
| Nanoxel M | paclitaxel | polymeric micelle | breast cancer | Samyang Biopharmaceuticals Corporation | https://samyangbiopharm.com/en/pharmaceuticals/product?idx=124&scrollpos=1333 | |
| Caelyx | doxorubicin | pegylated liposomal | monotherapy for patients with metastatic breast cancer | Janssen Pharmaceutica NV | https://www.ema.europa.eu/en/documents/product-information/caelyx-pegylated-liposomal-epar-product-information_en.pdf |
Table 4. Clinical Progress of Anticancer-Loaded Nanomedicines in the Management of Breast Cancer.
| trial ID | sponsors | trial objective | phases | interventions | delivery system | route of administration | indication | study designs | status | patient group | enrollment | study country |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT00629499 | SCRI Development Innovations, LLC/Celgene Corporation | phase II trial of nanoparticle albumin-bound (nab) paclitaxel/cyclophosphamide in early-stage breast cancer (with trastuzumab in HER2-positive patients) | II | nab paclitaxel, cyclophosphamide, trastuzumab | nanoparticle albumin-bound | intravenous | breast cancer | interventional, single group assignment, open-label | completed | 18 years and older | 63 | USA |
| NCT01644890 | Nippon Kayaku Co., Ltd. | multinational phase III clinical study comparing NK105 vs paclitaxel in patients with metastatic or recurrent breast cancer | III | NK105 | micellar nanoparticle | intravenous | breast cancer nos metastatic recurrent | interventional, randomized, parallel assignment, open-label | completed | 20 years to 74 years | 436 | Japan |
| NCT03739931 | ModernaTX, Inc./AstraZeneca | phase 1, open-label, multicenter, dose-escalation study of mRNA-2752, a lipid nanoparticle encapsulating mRNAs encoding human OX40L, IL-23, and IL-36γ, for intratumoral injection alone and in combination with immune checkpoint blockade | I | mRNA-2752 | lipid nanoparticle | intratumoral | triple-negative breast cancer | interventional, non-randomized, parallel assignment, open-label | recruiting | 18 years and older | 264 | USA, Australia, Israel |
| NCT03382340 | Immix Biopharma Australia Pty Ltd. | phase 1/2a open-label, dose-escalation/dose-expansion safety, tolerability, and pharmacokinetic study of IMX-110 in patients with advanced solid tumors | I/II | Imx-110 | PEG–PE polymeric micelle | –a | breast cancer | interventional, single group assignment, open-label | recruiting | 18 years and older | 70 | USA, Australia |
| NCT04640480 | SN BioScience | phase I, open-label, dose-finding study to evaluate the safety, tolerability, and pharmacokinetics of intravenously infused SNB-101(as SN-38) in patients with advanced solid tumors | I | SNB-101 | double core–shell micelle | intravenous | breast cancer | interventional, sequential assignment | recruiting | 19 years and older | 36 | Republic of Korea |
| NCT05001282 | Elucida Oncology | dose-escalation and expansion clinical study to evaluate the safety and efficacy of ELU001 in subjects who have advanced, recurrent or refractory FRα-overexpressing tumors | I/II | ELU001 | C-Dot | IV infusion | breast cancer, breast neoplasm, breast adenocarcinoma, breast cancer stage IV, metastatic breast cancer, invasive triple-negative breast neoplasms | interventional, single group assignment, open-label | recruiting | 18 years and older | 166 | USA |
Data not available.
7. Conclusion and Future Prospects
Significant progress has been made in the understanding of breast cancer (BC), and with the aid of nanotechnology large efforts have been made in the area of nanomedicine to effectively manage it with the least adverse effects. Developing nanomedicines based on passive and active drug targeting led to the elimination of multidrug resistance in BC, whereas the advancement in nanomedicine led to the development of hybrid and theranostic nanoparticles for drug-to-gene drug delivery in the management of BC. In addition, optimizing nanomedicines in terms of size, shape, surface charge, chemical compositions, etc., is an important consideration for enhancing biocompatibility and lowering toxicity. Moreover, fewer nanomedicine products for BC have been approved. However, the still slower bench-to-bed conversion of nanomedicine is due to the challenges of scaling up manufacturing, poor reproducibility, and a lack of implementation of quality control techniques. Therefore, in the end, it is concluded that the challenges in terms of pharmacokinetics, toxicology, and clinical progress are required in depth in the near future for the effective management of BC.
Acknowledgments
The authors deeply appreciate the Deanship of Scientific Research at King Khalid University for funding this study via the Research Group Project (Grant No. RGP1/245/44).
The authors declare no competing financial interest.
References
- Wilkinson L.; Gathani T. Understanding Breast Cancer as a Global Health Concern. British Journal of Radiology. 2022, 95, 1130. 10.1259/bjr.20211033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H.; Ferlay J.; Siegel R. L.; Laversanne M.; Soerjomataram I.; Jemal A.; Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71 (3), 209–249. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Arnold M.; Morgan E.; Rumgay H.; Mafra A.; Singh D.; Laversanne M.; Vignat J.; Gralow J. R.; Cardoso F.; Siesling S.; Soerjomataram I. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. 10.1016/j.breast.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Data Explorer. ECIS - European Cancer Information System. https://ecis.jrc.ec.europa.eu/explorer.php?$0-0 (accessed 2023-09-04).
- Cao W.; Chen H. Da; Yu Y. W.; Li N.; Chen W. Q. Changing Profiles of Cancer Burden Worldwide and in China: A Secondary Analysis of the Global Cancer Statistics 2020. Chin. Med. J. (Engl). 2021, 134 (7), 783–791. 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Global Cancer Observatory . Japan; International Agency for Research on Cancer: Lyon, France, 2021. https://gco.iarc.fr/today/data/factsheets/populations/392-japan-fact-sheets.pdf
- Mehrotra R.; Yadav K. Breast Cancer in India: Present Scenario and the Challenges Ahead. World J. Clin. Oncol. 2022, 13 (3), 209–218. 10.5306/wjco.v13.i3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap D.; Pal D.; Sharma R.; Garg V. K.; Goel N.; Koundal D.; Zaguia A.; Koundal S.; Belay A. Global Increase in Breast Cancer Incidence: Risk Factors and Preventive Measures. Biomed Res. Int. 2022, 2022, 9605439. 10.1155/2022/9605439. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Khan N. A. J.; Tirona M. An Updated Review of Epidemiology, Risk Factors, and Management of Male Breast Cancer. Medical Oncology. 2021, 38, 39. 10.1007/s12032-021-01486-x. [DOI] [PubMed] [Google Scholar]
- Bodewes F. T. H.; van Asselt A. A.; Dorrius M. D.; Greuter M. J. W.; de Bock G. H. Mammographic Breast Density and the Risk of Breast Cancer: A Systematic Review and Meta-Analysis. Breast 2022, 66, 62–68. 10.1016/j.breast.2022.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadnajafabad S.; Saeedi Moghaddam S.; Keykhaei M.; Shobeiri P.; Rezaei N.; Ghasemi E.; Mohammadi E.; Ahmadi N.; Ghamari A.; Shahin S.; Rezaei N.; Aghili M.; Kaviani A.; Larijani B.; Farzadfar F. Expansion of the Quality of Care Index on Breast Cancer and Its Risk Factors Using the Global Burden of Disease Study 2019. Cancer Med. 2023, 12 (2), 1729–1743. 10.1002/cam4.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starek-Świechowicz B.; Budziszewska B.; Starek A. Alcohol and Breast Cancer. Pharmacological Reports. 2023, 75, 69–84. 10.1007/s43440-022-00426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong X.; Liu Q.; Li W.; Wang L.; Feng Y.; Liu C.; Guo L.; Wang L.; Shi C.; Li P. Systematic Review and Meta-Analysis of Breast Cancer Risks in Relation to 2,3,7,8-Tetrachlorodibenzo-p-Dioxin and per- and Polyfluoroalkyl Substances. Environ. Sci. Pollut. Res. 2023, 30, 86540. 10.1007/s11356-023-28592-9. [DOI] [PubMed] [Google Scholar]
- Koual M.; Cano-Sancho G.; Bats A. S.; Tomkiewicz C.; Kaddouch-Amar Y.; Douay-Hauser N.; Ngo C.; Bonsang H.; Deloménie M.; Lecuru F.; Le Bizec B.; Marchand P.; Botton J.; Barouki R.; Antignac J. P.; Coumoul X. Associations between Persistent Organic Pollutants and Risk of Breast Cancer Metastasis. Environ. Int. 2019, 132, 105028. 10.1016/j.envint.2019.105028. [DOI] [PubMed] [Google Scholar]
- Koual M.; Tomkiewicz C.; Cano-Sancho G.; Antignac J. P.; Bats A. S.; Coumoul X. Environmental Chemicals, Breast Cancer Progression and Drug Resistance. Environ. Health 2020, 19, 117. 10.1186/s12940-020-00670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv F.; Huang W.; Wang Y. The CYP19A1 Rs700519 Polymorphism and Breast Cancer Susceptibility in China: A Case-Control Study and Updated Meta-Analysis. Genet. Test. Mol. Biomarkers 2021, 25 (7), 486–495. 10.1089/gtmb.2021.0032. [DOI] [PubMed] [Google Scholar]
- Barzaman K.; Moradi-Kalbolandi S.; Hosseinzadeh A.; Kazemi M. H.; Khorramdelazad H.; Safari E.; Farahmand L. Breast Cancer Immunotherapy: Current and Novel Approaches. Int. Immunopharmacol. 2021, 98, 107886. 10.1016/j.intimp.2021.107886. [DOI] [PubMed] [Google Scholar]
- Barchiesi G.; Mazzotta M.; Krasniqi E.; Pizzuti L.; Marinelli D.; Capomolla E.; Sergi D.; Amodio A.; Natoli C.; Gamucci T.; Vizza E.; Marchetti P.; Botti C.; Sanguineti G.; Ciliberto G.; Barba M.; Vici P. Neoadjuvant Endocrine Therapy in Breast Cancer: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 3528. 10.3390/ijms21103528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gote V.; Nookala A. R.; Bolla P. K.; Pal D. Drug Resistance in Metastatic Breast Cancer: Tumor Targeted Nanomedicine to the Rescue. Int. J. Mol. Sci. 2021, 22, 4673. 10.3390/ijms22094673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain V.; Kumar H.; Anod H. V.; Chand P.; Gupta N. V.; Dey S.; Kesharwani S. S. A Review of Nanotechnology-Based Approaches for Breast Cancer and Triple-Negative Breast Cancer. J. Controlled Release 2020, 326, 628–647. 10.1016/j.jconrel.2020.07.003. [DOI] [PubMed] [Google Scholar]
- Jain R. K.; Stylianopoulos T. Delivering Nanomedicine to Solid Tumors. Nature Reviews Clinical Oncology 2010, 7, 653–664. 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X.; Zhang Y.; Li F.; Lv T.; Li Z.; Chen H.; Jia L.; Gao Y. Challenges and Opportunities from Basic Cancer Biology for Nanomedicine for Targeted Drug Delivery. Curr. Cancer Drug Targets 2019, 19 (4), 257–276. 10.2174/1568009618666180628160211. [DOI] [PubMed] [Google Scholar]
- Fraguas-Sánchez A. I.; Martín-Sabroso C.; Fernández-Carballido A.; Torres-Suárez A. I. Current Status of Nanomedicine in the Chemotherapy of Breast Cancer. Cancer Chemotherapy and Pharmacology 2019, 84, 689–706. 10.1007/s00280-019-03910-6. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Zhi K.; Ding Z.; Sun Y.; Li S.; Li M.; Pu K.; Zou J. Emergence in Protein Derived Nanomedicine as Anticancer Therapeutics: More than a Tour de Force. Seminars in Cancer Biology 2021, 69, 77–90. 10.1016/j.semcancer.2019.11.012. [DOI] [PubMed] [Google Scholar]
- Wu J. The Enhanced Permeability and Retention (Epr) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Personalized Med. 2021, 11, 771. 10.3390/jpm11080771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R. P.; Gandhi V. V.; Singh B. G.; Kunwar A. Passive and Active Drug Targeting: Role of Nanocarriers in Rational Design of Anticancer Formulations. Curr. Pharm. Des. 2019, 25 (28), 3034–3056. 10.2174/1381612825666190830155319. [DOI] [PubMed] [Google Scholar]
- Alqaraghuli H. G. J.; Kashanian S.; Rafipour R. A Review on Targeting Nanoparticles for Breast Cancer. Curr. Pharm. Biotechnol. 2019, 20 (13), 1087–1107. 10.2174/1389201020666190731130001. [DOI] [PubMed] [Google Scholar]
- Malik Z.; Parveen R.; Abass S.; Irfan Dar M.; Husain S. A.; Ahmad S. Receptor-Mediated Targeting in Breast Cancer through Solid Lipid Nanoparticles and Its Mechanism. Curr. Drug Metab. 2022, 23 (10), 800–817. 10.2174/1389200223666220416213639. [DOI] [PubMed] [Google Scholar]
- Roskoski R. Small Molecule Inhibitors Targeting the EGFR/ErbB Family of Protein-Tyrosine Kinases in Human Cancers. Pharmacol. Res. 2019, 139, 395–411. 10.1016/j.phrs.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Pernas S.; Tolaney S. M. Targeting HER2 Heterogeneity in Early-Stage Breast Cancer. Current Opinion in Oncology 2020, 32, 545–554. 10.1097/CCO.0000000000000685. [DOI] [PubMed] [Google Scholar]
- Akhter M. H.; Rizwanullah M.; Ahmad J.; Ahsan M. J.; Mujtaba M. A.; Amin S. Nanocarriers in Advanced Drug Targeting: Setting Novel Paradigm in Cancer Therapeutics. Artificial Cells, Nanomedicine and Biotechnology 2018, 46, 873–884. 10.1080/21691401.2017.1366333. [DOI] [PubMed] [Google Scholar]
- Forouhari S.; Beygi Z.; Mansoori Z.; Hajsharifi S.; Heshmatnia F.; Gheibihayat S. M. Liposomes: Ideal Drug Delivery Systems in Breast Cancer. Biotechnology and Applied Biochemistry 2022, 69, 1867–1884. 10.1002/bab.2253. [DOI] [PubMed] [Google Scholar]
- Badr-Eldin S. M.; Aldawsari H. M.; Fahmy U. A.; Ahmed O. A. A.; Alhakamy N. A.; Al-hejaili O. D.; Alhassan A. A.; Ammari G. A.; Alhazmi S. I.; Alawadi R. M.; Bakhaidar R.; Alamoudi A. J.; Neamatallah T.; Tima S. Optimized Apamin-Mediated Nano-Lipidic Carrier Potentially Enhances the Cytotoxicity of Ellagic Acid against Human Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23 (16), 9440. 10.3390/ijms23169440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.; Zhao Y.; Xie C.; Chen C.; Lin D.; Wang S.; Lin D.; Cui X.; Guo Z.; Zhou J. Dual-Active Targeting Liposomes Drug Delivery System for Bone Metastatic Breast Cancer: Synthesis and Biological Evaluation. Chem. Phys. Lipids 2019, 223, 104785. 10.1016/j.chemphyslip.2019.104785. [DOI] [PubMed] [Google Scholar]
- Samson A. A. S.; Park S.; Kim S. Y.; Min D. H.; Jeon N. L.; Song J. M. Liposomal Co-Delivery-Based Quantitative Evaluation of Chemosensitivity Enhancement in Breast Cancer Stem Cells by Knockdown of GRP78/CLU. J. Liposome Res. 2019, 29 (1), 44–52. 10.1080/08982104.2017.1420081. [DOI] [PubMed] [Google Scholar]
- Jiang K.; Song X.; Yang L.; Li L.; Wan Z.; Sun X.; Gong T.; Lin Q.; Zhang Z. Enhanced Antitumor and Anti-Metastasis Efficacy against Aggressive Breast Cancer with a Fibronectin-Targeting Liposomal Doxorubicin. J. Controlled Release 2018, 271, 21–30. 10.1016/j.jconrel.2017.12.026. [DOI] [PubMed] [Google Scholar]
- Schneeweiss A.; Möbus V.; Tesch H.; Hanusch C.; Denkert C.; Lübbe K.; Huober J.; Klare P.; Kümmel S.; Untch M.; Kast K.; Jackisch C.; Thomalla J.; Ingold-Heppner B.; Blohmer J. U.; Rezai M.; Frank M.; Engels K.; Rhiem K.; Fasching P. A.; Nekljudova V.; von Minckwitz G.; Loibl S. Intense Dose-Dense Epirubicin, Paclitaxel, Cyclophosphamide versus Weekly Paclitaxel, Liposomal Doxorubicin (plus Carboplatin in Triple-Negative Breast Cancer) for Neoadjuvant Treatment of High-Risk Early Breast Cancer (GeparOcto—GBG 84): A Randomised Pha. Eur. J. Cancer 2019, 106, 181–192. 10.1016/j.ejca.2018.10.015. [DOI] [PubMed] [Google Scholar]
- Boratto F. A.; Lages E. B.; Loures C. M. C.; Sabino A. P.; Malachias A.; Townsend D. M.; Branco De Barros A. L.; Miranda Ferreira L. A.; Amaral Leite E. Alpha-Tocopheryl Succinate and Doxorubicin-Loaded Liposomes Improve Drug Uptake and Tumor Accumulation in a Murine Breast Tumor Model. Biomed. Pharmacother. 2023, 165, 115034 10.1016/j.biopha.2023.115034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granja A.; Nunes C.; Sousa C. T.; Reis S. Folate Receptor-Mediated Delivery of Mitoxantrone-Loaded Solid Lipid Nanoparticles to Breast Cancer Cells. Biomed. Pharmacother. 2022, 154, 113525. 10.1016/j.biopha.2022.113525. [DOI] [PubMed] [Google Scholar]
- Vakili-Ghartavol R.; Rezayat S. M.; Faridi-Majidi R.; Sadri K.; Jaafari M. R. Optimization of Docetaxel Loading Conditions in Liposomes: Proposing Potential Products for Metastatic Breast Carcinoma Chemotherapy. Sci. Rep. 2020, 10 (1), 5569. 10.1038/s41598-020-62501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elamir A.; Ajith S.; Sawaftah N. Al; Abuwatfa W.; Mukhopadhyay D.; Paul V.; Al-Sayah M. H.; Awad N.; Husseini G. A. Ultrasound-Triggered Herceptin Liposomes for Breast Cancer Therapy. Sci. Rep. 2021, 11 (1), 7545. 10.1038/s41598-021-86860-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.; Peng Y.; Pu Y.; Zhao Y.; Nie R.; Guo L.; Wu Y. Fructose and Biotin Co-Modified Liposomes for Dual-Targeting Breast Cancer. J. Liposome Res. 2022, 32 (2), 119–128. 10.1080/08982104.2021.1894171. [DOI] [PubMed] [Google Scholar]
- Fu M.; Tang W.; Liu J. J.; Gong X. Q.; Kong L.; Yao X. M.; Jing M.; Cai F. Y.; Li X. T.; Ju R. J. Combination of Targeted Daunorubicin Liposomes and Targeted Emodin Liposomes for Treatment of Invasive Breast Cancer. J. Drug Targeting 2020, 28, 245–258. 10.1080/1061186X.2019.1656725. [DOI] [PubMed] [Google Scholar]
- Liu H.; Liu Y.; Li N.; Zhang G.-Q.; Wang M. Ginsenoside Rg_3 Based Liposomes Target Delivery of Dihydroartemisinin and Paclitaxel for Treatment of Triple-Negative Breast Cancer. Zhongguo Zhong Yao Za Zhi 2023, 48 (13), 3472–3484. 10.19540/j.cnki.cjcmm.20230410.301. [DOI] [PubMed] [Google Scholar]
- Viegas C.; Patrício A. B.; Prata J. M.; Nadhman A.; Chintamaneni P. K.; Fonte P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15 (6), 1593. 10.3390/pharmaceutics15061593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A.; Sharma G.; Thakur K.; Raza K.; Shivhare U. S.; Ghoshal G.; Katare O. P. Beta-Carotene-Encapsulated Solid Lipid Nanoparticles (BC-SLNs) as Promising Vehicle for Cancer: An Investigative Assessment. AAPS PharmSciTech 2019, 20 (3), 100. 10.1208/s12249-019-1301-7. [DOI] [PubMed] [Google Scholar]
- Xu W.; Bae E. J.; Lee M. K. Enhanced Anticancer Activity and Intracellular Uptake of Paclitaxel-Containing Solid Lipid Nanoparticles in Multidrug-Resistant Breast Cancer Cells. Int. J. Nanomedicine 2018, 13, 7549–7563. 10.2147/IJN.S182621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddhartha V. T.; Pindiprolu S. K. S. S.; Chintamaneni P. K.; Tummala S.; Nandha Kumar S. RAGE Receptor Targeted Bioconjuguate Lipid Nanoparticles of Diallyl Disulfide for Improved Apoptotic Activity in Triple Negative Breast Cancer: In Vitro Studies.. Artif. Cells, Nanomedicine Biotechnol. 2018, 46 (2), 387–397. 10.1080/21691401.2017.1313267. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Zhang X.; Zhao W.; Zeng C.; Li W.; Li B.; Luo X.; Li J.; Jiang J.; Deng B.; McComb D. W.; Dong Y. Chemotherapy Drugs Derived Nanoparticles Encapsulating MRNA Encoding Tumor Suppressor Proteins to Treat Triple-Negative Breast Cancer. Nano Res. 2019, 12 (4), 855–861. 10.1007/s12274-019-2308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guney Eskiler G.; Cecener G.; Dikmen G.; Egeli U.; Tunca B. Solid Lipid Nanoparticles: Reversal of Tamoxifen Resistance in Breast Cancer. Eur. J. Pharm. Sci. 2018, 120, 73–88. 10.1016/j.ejps.2018.04.040. [DOI] [PubMed] [Google Scholar]
- Li R.; You S.; Hu Z.; Chen Z. G.; Sica G. L.; Khuri F. R.; Curran W. J.; Shin D. M.; Deng X. Inhibition of STAT3 by Niclosamide Synergizes with Erlotinib against Head and Neck Cancer. PLoS One 2013, 8 (9), e74670. 10.1371/journal.pone.0074670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L.; Gao Y.; Zhang X.; Wang J.; Ding D.; Zhang Y.; Zhang J.; Chen H. Niclosamide Sensitizes Triple-Negative Breast Cancer Cells to Ionizing Radiation in Association with the Inhibition of Wnt/β-Catenin Signaling. Oncotarget 2106, 7 (27), 42126–42138. 10.18632/oncotarget.9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Li P. K.; Roberts M. J.; Arend R. C.; Samant R. S.; Buchsbaum D. J. Multi-Targeted Therapy of Cancer by Niclosamide: A New Application for an Old Drug. Cancer Letters. 2014, 349, 8–14. 10.1016/j.canlet.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ss Pindiprolu S. K.; Krishnamurthy P. T.; Ghanta V. R.; Chintamaneni P. K. Phenyl Boronic Acid-Modified Lipid Nanocarriers of Niclosamide for Targeting Triple-Negative Breast Cancer. Nanomedicine 2020, 15 (16), 1551–1565. 10.2217/nnm-2020-0003. [DOI] [PubMed] [Google Scholar]
- Jain A.; Sharma T.; Kumar R.; Katare O. P.; Singh B. Raloxifene-Loaded SLNs with Enhanced Biopharmaceutical Potential: QbD-Steered Development, in Vitro Evaluation, in Vivo Pharmacokinetics, and IVIVC. Drug Delivery Transl. Res. 2022, 12 (5), 1136–1160. 10.1007/s13346-021-00990-x. [DOI] [PubMed] [Google Scholar]
- Wang W.; Zhou M.; Xu Y.; Peng W.; Zhang S.; Li R.; Zhang H.; Zhang H.; Cheng S.; Wang Y.; Wei X.; Yue C.; Yang Q.; Chen C. Resveratrol-Loaded TPGS-Resveratrol-Solid Lipid Nanoparticles for Multidrug-Resistant Therapy of Breast Cancer: In Vivo and In Vitro Study. Front. Bioeng. Biotechnol. 2021, 9, 762489. 10.3389/fbioe.2021.762489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayat P.; Pakravan P.; Salouti M.; Dolatabadi J. E. N. Lysine Decorated Solid Lipid Nanoparticles of Epirubicin for Cancer Targeting and Therapy. Adv. Pharm. Bull. 2021, 11 (1), 96–103. 10.34172/apb.2021.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgenc E.; Karpuz M.; Arzuk E.; Gonzalez-Alvarez M.; Sanz M. B.; Gundogdu E.; Gonzalez-Alvarez I. Radiolabeled Trastuzumab Solid Lipid Nanoparticles for Breast Cancer Cell: In Vitro and in Vivo Studies. ACS Omega 2022, 7 (34), 30015–30027. 10.1021/acsomega.2c03023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly S.; El-Kamel A. H.; Sheta E.; El-Habashy S. E. Chondroitin/Lactoferrin-Dual Functionalized Pterostilbene-Solid Lipid Nanoparticles as Targeted Breast Cancer Therapy. Int. J. Pharm. 2023, 642, 123163 10.1016/j.ijpharm.2023.123163. [DOI] [PubMed] [Google Scholar]
- Emami A.; Ghafouri H.; Sariri R. Polyphyllin D-Loaded Solid Lipid Nanoparticles for Breast Cancer: Synthesis, Characterization, in Vitro, and in Vivo Studies. Int. J. Pharm. 2023, 639, 122976. 10.1016/j.ijpharm.2023.122976. [DOI] [PubMed] [Google Scholar]
- Kostrzewa T.; Nowak I.; Feliczak-Guzik A.; Drzeżdżon J.; Jacewicz D.; Górska-Ponikowska M.; Kuban-Jankowska A. Encapsulated Oxovanadium(IV) and Dioxovanadium(V) Complexes into Solid Lipid Nanoparticles Increase Cytotoxicity Against MDA-MB-231 Cell Line. Int. J. Nanomedicine 2023, 18, 2507–2523. 10.2147/IJN.S403689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera-Garrido A.; Graván P.; Navarro-Marchal S. A.; Medina-O’Donnell M.; Parra A.; Gálvez-Ruiz M. J.; Marchal J. A.; Galisteo-González F. Maslinic Acid Solid Lipid Nanoparticles as Hydrophobic Anticancer Drug Carriers: Formulation, in Vitro Activity and in Vivo Biodistribution. Biomed. Pharmacother. 2023, 163, 114828. 10.1016/j.biopha.2023.114828. [DOI] [PubMed] [Google Scholar]
- Shamsuddin N. A. M.; Zulfakar M. H. Nanostructured Lipid Carriers for the Delivery of Natural Bioactive Compounds. Curr. Drug Delivery 2023, 20 (2), 127–143. 10.2174/1567201819666220324094234. [DOI] [PubMed] [Google Scholar]
- Varshosaz J.; Davoudi M. A.; Rasoul-Amini S. Docetaxel-Loaded Nanostructured Lipid Carriers Functionalized with Trastuzumab (Herceptin) for HER2-Positive Breast Cancer Cells. J. Liposome Res. 2018, 28 (4), 285–295. 10.1080/08982104.2017.1370471. [DOI] [PubMed] [Google Scholar]
- Rathee J.; Kanwar R.; Kumari L.; Pawar S. V.; Sharma S.; Ali M. E.; Salunke D. B.; Mehta S. K. Development of Nanostructured Lipid Carriers as a Promising Tool for Methotrexate Delivery: Physicochemical and in Vitro Evaluation. J. Biomol. Struct. Dyn. 2023, 41 (7), 2747–2758. 10.1080/07391102.2022.2037465. [DOI] [PubMed] [Google Scholar]
- Sahib A. S.; Wennas O. N.; Mahdi B. W.; Al abood R. M. In Vivo Antitumor Activity Study of Targeted Chlorambucil-Loaded Nanolipid Carrier for Breast Cancer. Pharmacia 2022, 69 (3), 631–636. 10.3897/pharmacia.69.e85390. [DOI] [Google Scholar]
- Sartaj A.; Annu; Biswas L.; Verma A. K.; Sahoo P. K.; Baboota S.; Ali J. Ribociclib Nanostructured Lipid Carrier Aimed for Breast Cancer: Formulation Optimization, Attenuating In Vitro Specification, and In Vivo Scrutinization. Biomed Res. Int. 2022, 2022, 6009309. 10.1155/2022/6009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong T. H.; Alcantara K. P.; Bulatao B. P. I.; Sorasitthiyanukarn F. N.; Muangnoi C.; Nalinratana N.; Vajragupta O.; Rojsitthisak P.; Rojsitthisak P. Chitosan-Coated Nanostructured Lipid Carriers for Transdermal Delivery of Tetrahydrocurcumin for Breast Cancer Therapy. Carbohydr. Polym. 2022, 288, 119401. 10.1016/j.carbpol.2022.119401. [DOI] [PubMed] [Google Scholar]
- Thiruchenthooran V.; Świtalska M.; Bonilla L.; Espina M.; García M. L.; Wietrzyk J.; Sánchez-López E.; Gliszczyńska A. Novel Strategies against Cancer: Dexibuprofen-Loaded Nanostructured Lipid Carriers. Int. J. Mol. Sci. 2022, 23 (19), 11310. 10.3390/ijms231911310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mughees M.; Wajid S. Herbal Based Polymeric Nanoparticles as a Therapeutic Remedy for Breast Cancer. Anticancer. Agents Med. Chem. 2021, 21 (4), 433–444. 10.2174/1871520620666200619171616. [DOI] [PubMed] [Google Scholar]
- Hembram K. C.; Prabha S.; Chandra R.; Ahmed B.; Nimesh S. Advances in Preparation and Characterization of Chitosan Nanoparticles for Therapeutics. Artif. Cells Nanomed. Biotechnol. 2016, 44, 305–314. 10.3109/21691401.2014.948548. [DOI] [PubMed] [Google Scholar]
- DeVeaux S. D.; Gomillion C. T. Assessing the Potential of Chitosan/Polylactide Nanoparticles for Delivery of Therapeutics for Triple-Negative Breast Cancer Treatment. Regen. Eng. Transl. Med. 2019, 5 (1), 61–73. 10.1007/s40883-018-0089-4. [DOI] [Google Scholar]
- Bressler E. M.; Kim J.; Shmueli R. B.; Mirando A. C.; Bazzazi H.; Lee E.; Popel A. S.; Pandey N. B.; Green J. J. Biomimetic Peptide Display from a Polymeric Nanoparticle Surface for Targeting and Antitumor Activity to Human Triple-Negative Breast Cancer Cells. J. Biomed. Mater. Res. - Part A 2018, 106 (6), 1753–1764. 10.1002/jbm.a.36360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosrati H.; Adibtabar M.; Sharafi A.; Danafar H.; Hamidreza Kheiri M. PAMAM-Modified Citric Acid-Coated Magnetic Nanoparticles as PH Sensitive Biocompatible Carrier against Human Breast Cancer Cells. Drug Dev. Ind. Pharm. 2018, 44 (8), 1377–1384. 10.1080/03639045.2018.1451881. [DOI] [PubMed] [Google Scholar]
- Nicolas S.; Bolzinger M. A.; Jordheim L. P.; Chevalier Y.; Fessi H.; Almouazen E. Polymeric Nanocapsules as Drug Carriers for Sustained Anticancer Activity of Calcitriol in Breast Cancer Cells. Int. J. Pharm. 2018, 550 (1–2), 170–179. 10.1016/j.ijpharm.2018.08.022. [DOI] [PubMed] [Google Scholar]
- Sharma A.; Hawthorne S.; Jha S. K.; Jha N. K.; Kumar D.; Girgis S.; Goswami V. K.; Gupta G.; Singh S.; Dureja H.; Chellappan D. K.; Dua K. Effects of Curcumin-Loaded Poly(Lactic-Co-Glycolic Acid) Nanoparticles in MDA-MB231 Human Breast Cancer Cells. Nanomedicine 2021, 16 (20), 1763–1773. 10.2217/nnm-2021-0066. [DOI] [PubMed] [Google Scholar]
- Mekseriwattana W.; Phungsom A.; Sawasdee K.; Wongwienkham P.; Kuhakarn C.; Chaiyen P.; Katewongsa K. P. Dual Functions of Riboflavin-Functionalized Poly(Lactic-Co-Glycolic Acid) Nanoparticles for Enhanced Drug Delivery Efficiency and Photodynamic Therapy in Triple-Negative Breast Cancer Cells. Photochem. Photobiol. 2021, 97 (6), 1548–1557. 10.1111/php.13464. [DOI] [PubMed] [Google Scholar]
- Barkat A.; Rahman M.; Alharbi K. S.; Altowayan W. M.; Alrobaian M.; Afzal O.; Altamimi A. S. A.; Alhodieb F. S.; Almalki W. H.; Choudhry H.; Singh T.; Beg S. Biocompatible Polymeric Nanoparticles for Effective Codelivery of Tamoxifen with Ganoderic Acid A: Systematic Approach for Improved Breast Cancer Therapeutics. J. Clust. Sci. 2023, 34 (3), 1483–1497. 10.1007/s10876-022-02332-4. [DOI] [Google Scholar]
- Verma R.; Singh V.; Koch B.; Kumar M. Evaluation of Methotrexate Encapsulated Polymeric Nanocarrier for Breast Cancer Treatment. Colloids Surfaces B Biointerfaces 2023, 226, 113308. 10.1016/j.colsurfb.2023.113308. [DOI] [PubMed] [Google Scholar]
- Iqbal H.; Razzaq A.; Khan N. U.; Rehman S. U.; Webster T. J.; Xiao R.; Menaa F. PH-Responsive Albumin-Coated Biopolymeric Nanoparticles with Lapatinab for Targeted Breast Cancer Therapy. Biomater. Adv. 2022, 139, 213039. 10.1016/j.bioadv.2022.213039. [DOI] [PubMed] [Google Scholar]
- Pourgholi A.; Dadashpour M.; Mousapour A.; Amandi A. F.; Zarghami N. Anticancer Potential of Silibinin Loaded Polymeric Nanoparticles against Breast Cancer Cells: Insight into the Apoptotic Genes Targets. Asian Pacific J. Cancer Prev. 2021, 22 (8), 2587–2596. 10.31557/APJCP.2021.22.8.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koual M.; Cano-Sancho G.; Bats A. S.; Tomkiewicz C.; Kaddouch-Amar Y.; Douay-Hauser N.; Ngo C.; Bonsang H.; Deloménie M.; Lecuru F.; Le Bizec B.; Marchand P.; Botton J.; Barouki R.; Antignac J. P.; Coumoul X. Associations between Persistent Organic Pollutants and Risk of Breast Cancer Metastasis. Environ. Int. 2019, 132, 105028. 10.1016/j.envint.2019.105028. [DOI] [PubMed] [Google Scholar]
- Jafari-Gharabaghlou D.; Dadashpour M.; Khanghah O. J.; Salmani-Javan E.; Zarghami N. Potentiation of Folate-Functionalized PLGA-PEG Nanoparticles Loaded with Metformin for the Treatment of Breast Cancer: Possible Clinical Application. Mol. Biol. Rep. 2023, 50 (4), 3023–3033. 10.1007/s11033-022-08171-w. [DOI] [PubMed] [Google Scholar]
- Rajeshkumar R. R.; Pavadai P.; Panneerselvam T.; Deepak V.; Pandian S. R. K.; Kabilan S. J.; Vellaichamy S.; Jeyaraman A.; Kumar A. S. K.; Sundar K.; Kunjiappan S. Glucose-Conjugated Glutenin Nanoparticles for Selective Targeting and Delivery of Camptothecin into Breast Cancer Cells. Naunyn. Schmiedebergs. Arch. Pharmacol. 2023, 396, 2571. 10.1007/s00210-023-02480-y. [DOI] [PubMed] [Google Scholar]
- Junnuthula V.; Kolimi P.; Nyavanandi D.; Sampathi S.; Vora L. K.; Dyawanapelly S. Polymeric Micelles for Breast Cancer Therapy: Recent Updates, Clinical Translation and Regulatory Considerations. Pharmaceutics 2022, 14, 1860. 10.3390/pharmaceutics14091860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlehria S.; Behl G.; Patel K.; Doddapaneni R.; Vhora I.; Chowdhury N.; Bagde A.; Singh M. Cholecalciferol-PEG Conjugate Based Nanomicelles of Doxorubicin for Treatment of Triple-Negative Breast Cancer. AAPS PharmSciTech 2018, 19 (2), 792–802. 10.1208/s12249-017-0885-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baidya D.; Kushwaha J.; Mahadik K.; Patil S. Chrysin-Loaded Folate Conjugated PF127-F68 Mixed Micelles with Enhanced Oral Bioavailability and Anticancer Activity against Human Breast Cancer Cells. Drug Dev. Ind. Pharm. 2019, 45 (5), 852–860. 10.1080/03639045.2019.1576726. [DOI] [PubMed] [Google Scholar]
- Pawar A.; Singh S.; Rajalakshmi S.; Shaikh K.; Bothiraja C. Development of Fisetin-Loaded Folate Functionalized Pluronic Micelles for Breast Cancer Targeting. Artif. Cells, Nanomedicine Biotechnol. 2018, 46 (sup1), 347–361. 10.1080/21691401.2018.1423991. [DOI] [PubMed] [Google Scholar]
- Farrokhi F.; Karami Z.; Esmaeili-Mahani S.; Heydari A. Delivery of DNAzyme Targeting C-Myc Gene Using β-Cyclodextrin Polymer Nanocarrier for Therapeutic Application in Human Breast Cancer Cell Line. J. Drug Delivery Sci. Technol. 2018, 47, 477–484. 10.1016/j.jddst.2018.08.015. [DOI] [Google Scholar]
- Gregoriou Y.; Gregoriou G.; Yilmaz V.; Kapnisis K.; Prokopi M.; Anayiotos A.; Strati K.; Dietis N.; Constantinou A. I.; Andreou C. Resveratrol Loaded Polymeric Micelles for Theranostic Targeting of Breast Cancer Cells. Nanotheranostics 2021, 5 (1), 113–124. 10.7150/ntno.51955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S.; Kumar A.; Yadav P. K.; Kumar M.; Mathew J.; Pandey A. C.; Chourasia M. K. Formulation and Performance Evaluation of Polymeric Mixed Micelles Encapsulated with Baicalein for Breast Cancer Treatment. Drug Dev. Ind. Pharm. 2021, 47 (9), 1512–1522. 10.1080/03639045.2021.2007394. [DOI] [PubMed] [Google Scholar]
- Bobde Y.; Patel T.; Paul M.; Biswas S.; Ghosh B. PEGylated N-(2 Hydroxypropyl) Methacrylamide Polymeric Micelles as Nanocarriers for the Delivery of Doxorubicin in Breast Cancer. Colloids Surfaces B Biointerfaces 2021, 204, 111833. 10.1016/j.colsurfb.2021.111833. [DOI] [PubMed] [Google Scholar]
- Tang L.; Jiang W.; Wu L.; Yu X.; He Z.; Shan W.; Fu L.; Zhang Z.; Zhao Y. TPGS2000-DOX Prodrug Micelles for Improving Breast Cancer Therapy. Int. J. Nanomedicine 2021, 16, 7875–7890. 10.2147/IJN.S335405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isik G.; Kiziltay A.; Hasirci N.; Tezcaner A. Lithocholic Acid Conjugated MPEG-b-PCL Micelles for PH Responsive Delivery to Breast Cancer Cells. Int. J. Pharm. 2022, 621, 121779. 10.1016/j.ijpharm.2022.121779. [DOI] [PubMed] [Google Scholar]
- Sun J.; Li M.; Lin K.; Liu Z.; Wang Z.; Wang W.; Zhao Y.; Zhen Y.; Zhang S. Delivery of Quercetin for Breast Cancer and Targeting Potentiation via Hyaluronic Nano-Micelles. Int. J. Biol. Macromol. 2023, 242, 124736. 10.1016/j.ijbiomac.2023.124736. [DOI] [PubMed] [Google Scholar]
- Sohail M.; Yu B.; Sun Z.; Liu J.; Li Y.; Zhao F.; Chen D.; Yang X.; Xu H. Complex Polymeric Nanomicelles Co-Delivering Doxorubicin and Dimethoxycurcumin for Cancer Chemotherapy. Drug Delivery 2022, 29 (1), 1523–1535. 10.1080/10717544.2022.2073403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirani S.; Varshosaz J.; Rostami M.; Mirian M. Redox Responsive Polymeric Micelles of Gellan Gum/Abietic Acid for Targeted Delivery of Ribociclib. Int. J. Biol. Macromol. 2022, 215, 334–345. 10.1016/j.ijbiomac.2022.06.095. [DOI] [PubMed] [Google Scholar]
- Núñez C.; Estévez S. V.; del Pilar Chantada M. Inorganic Nanoparticles in Diagnosis and Treatment of Breast Cancer. Journal of Biological Inorganic Chemistry 2018, 23, 331–345. 10.1007/s00775-018-1542-z. [DOI] [PubMed] [Google Scholar]
- Srivastava N.; Mishra Y.; Mishra V.; Ranjan A.; Tambuwala M. M. Carbon Nanotubes in Breast Cancer Treatment: An Insight into Properties, Functionalization, and Toxicity. Anticancer. Agents Med. Chem. 2023, 23, 1606. 10.2174/1871520623666230510094850. [DOI] [PubMed] [Google Scholar]
- Liu D.; Zhang Q.; Wang J.; Fan L.; Zhu W.; Cai D. Hyaluronic Acid-Coated Single-Walled Carbon Nanotubes Loaded with Doxorubicin for the Treatment of Breast Cancer. Pharmazie 2019, 74 (2), 83–90. 10.1691/ph.2019.8152. [DOI] [PubMed] [Google Scholar]
- McKernan P.; Virani N. A.; Faria G. N. F.; Karch C. G.; Prada Silvy R.; Resasco D. E.; Thompson L. F.; Harrison R. G. Targeted Single-Walled Carbon Nanotubes for Photothermal Therapy Combined with Immune Checkpoint Inhibition for the Treatment of Metastatic Breast Cancer. Nanoscale Res. Lett. 2021, 16 (1), 9. 10.1186/s11671-020-03459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea M. A.; Balas M.; Prodana M.; Cojocaru F. G.; Ionita D.; Dinischiotu A. Carboxyl-Functionalized Carbon Nanotubes Loaded with Cisplatin Promote the Inhibition of PI3K/Akt Pathway and Suppress the Migration of Breast Cancer Cells. Pharmaceutics 2022, 14 (2), 469. 10.3390/pharmaceutics14020469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur C. K.; Neupane R.; Karthikeyan C.; Ashby C. R.; Babu R. J.; Boddu S. H. S.; Tiwari A. K.; Moorthy N. S. H. N. Lysinated Multiwalled Carbon Nanotubes with Carbohydrate Ligands as an Effective Nanocarrier for Targeted Doxorubicin Delivery to Breast Cancer Cells. Molecules 2022, 27 (21), 7461. 10.3390/molecules27217461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poonia N.; Lather V.; Pandita D. Mesoporous Silica Nanoparticles: A Smart Nanosystem for Management of Breast Cancer. Drug Discovery Today 2018, 23, 315–332. 10.1016/j.drudis.2017.10.022. [DOI] [PubMed] [Google Scholar]
- Li L.; Lu Y.; Jiang C.; Zhu Y.; Yang X.; Hu X.; Lin Z.; Zhang Y.; Peng M.; Xia H.; Mao C. Actively Targeted Deep Tissue Imaging and Photothermal-Chemo Therapy of Breast Cancer by Antibody-Functionalized Drug-Loaded X-Ray-Responsive Bismuth Sulfide@Mesoporous Silica Core–Shell Nanoparticles. Adv. Funct. Mater. 2018, 28 (5), 1704623. 10.1002/adfm.201704623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeran Z.; Keyhanfar M.; Varshosaz J.; Sutherland D. S. Biodegradable Nanocarriers Based on Chitosan-Modified Mesoporous Silica Nanoparticles for Delivery of Methotrexate for Application in Breast Cancer Treatment. Mater. Sci. Eng., C 2021, 118, 111526. 10.1016/j.msec.2020.111526. [DOI] [PubMed] [Google Scholar]
- Ramezani-Aliakbari M.; Varshosaz J.; Mirian M.; Khodarahmi G.; Rostami M. PH-Responsive Glucosamine Anchored Polydopamine Coated Mesoporous Silica Nanoparticles for Delivery of Anderson-Type Polyoxomolybdate in Breast Cancer. J. Microencapsul. 2022, 39 (5), 433–451. 10.1080/02652048.2022.2096139. [DOI] [PubMed] [Google Scholar]
- Gu Y.; Fei Z. Mesoporous Silica Nanoparticles Loaded with Resveratrol Are Used for Targeted Breast Cancer Therapy. J. Oncol. 2022, 2022, 8471331. 10.1155/2022/8471331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laranjeira M. S.; Ribeiro T. P.; Magalhães A. I.; Silva P. C.; Santos J. A. M.; Monteiro F. J. Magnetic Mesoporous Silica Nanoparticles as a Theranostic Approach for Breast Cancer: Loading and Release of the Poorly Soluble Drug Exemestane. Int. J. Pharm. 2022, 619, 121711. 10.1016/j.ijpharm.2022.121711. [DOI] [PubMed] [Google Scholar]
- Yin H.; Yan Q.; Liu Y.; Yang L.; Liu Y.; Luo Y.; Chen T.; Li N.; Wu M. Co-Encapsulation of Paclitaxel and 5-Fluorouracil in Folic Acid-Modified, Lipid-Encapsulated Hollow Mesoporous Silica Nanoparticles for Synergistic Breast Cancer Treatment. RSC Adv. 2022, 12 (50), 32534–32551. 10.1039/D2RA03718A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harandi Z. R.; Heidari R.; Reiisi S. Co-Delivery of Silymarin and Metformin Dual-Loaded in Mesoporous Silica Nanoparticles Synergistically Sensitizes Breast Cancer Cell Line to Mitoxantrone Chemotherapy. IEEE Trans. Nanobiosci. 2023, 22, 872. 10.1109/TNB.2023.3242912. [DOI] [PubMed] [Google Scholar]
- Mohebian Z.; Babazadeh M.; Zarghami N. In Vitro Efficacy of Curcumin-Loaded Amine-Functionalized Mesoporous Silica Nanoparticles against MCF-7 Breast Cancer Cells. Adv. Pharm. Bull. 2023, 13 (2), 317–327. 10.34172/apb.2023.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.; Prasad P.; Cai P.; He C.; Shan D.; Rauth A. M.; Wu X. Y. Dual-Targeted Hybrid Nanoparticles of Synergistic Drugs for Treating Lung Metastases of Triple Negative Breast Cancer in Mice. Acta Pharmacol. Sin. 2017, 38 (6), 835–847. 10.1038/aps.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwah V.; Katiyar S. S.; Dora C. P.; Kumar Agrawal A.; Lamprou D. A.; Gupta R. C.; Jain S. Co-Delivery of Docetaxel and Gemcitabine by Anacardic Acid Modified Self-Assembled Albumin Nanoparticles for Effective Breast Cancer Management. Acta Biomater. 2018, 73, 424–436. 10.1016/j.actbio.2018.03.057. [DOI] [PubMed] [Google Scholar]
- Aekrungrueangkit C.; Wangngae S.; Kamkaew A.; Ardkhean R.; Thongnest S.; Boonsombat J.; Ruchirawat S.; Khotavivattana T. Novel Psoralen Derivatives as Anti-Breast Cancer Agents and Their Light-Activated Cytotoxicity against HER2 Positive Breast Cancer Cells. Sci. Rep. 2022, 12 (1), 13487. 10.1038/s41598-022-17625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M.; Ouyang Y.; Meng F.; Zhang X.; Ma Q.; Zhuang Y.; Liu H.; Pang M.; Cai T.; Cai Y. Polymer-Lipid Hybrid Nanoparticles: A Novel Drug Delivery System for Enhancing the Activity of Psoralen against Breast Cancer. Int. J. Pharm. 2019, 561, 274–282. 10.1016/j.ijpharm.2019.03.006. [DOI] [PubMed] [Google Scholar]
- Jadon R. S.; Sharma M. Docetaxel-Loaded Lipid-Polymer Hybrid Nanoparticles for Breast Cancer Therapeutics. J. Drug Delivery Sci. Technol. 2019, 51, 475–484. 10.1016/j.jddst.2019.03.039. [DOI] [Google Scholar]
- Clericuzio M.; Mella M.; Vita-Finzi P.; Zema M.; Vidari G. Cucurbitane Triterpenoids from Leucopaxillus Gentianeus. J. Nat. Prod. 2004, 67 (11), 1823–1828. 10.1021/np049883o. [DOI] [PubMed] [Google Scholar]
- Bakar-Ates F.; Ozkan E.; Sengel-Turk C. T. Encapsulation of Cucurbitacin B into Lipid Polymer Hybrid Nanocarriers Induced Apoptosis of MDAMB231 Cells through PARP Cleavage. Int. J. Pharm. 2020, 586, 119565. 10.1016/j.ijpharm.2020.119565. [DOI] [PubMed] [Google Scholar]
- Rizwanullah M.; Perwez A.; Mir S. R.; Rizvi M. M. A.; Amin S. Exemestane Encapsulated Polymer-Lipid Hybrid Nanoparticles for Improved Efficacy against Breast Cancer: Optimization, in Vitro Characterization and Cell Culture Studies. Nanotechnology 2021, 32 (41), 415101. 10.1088/1361-6528/ac1098. [DOI] [PubMed] [Google Scholar]
- Rizwanullah M.; Perwez A.; Alam M.; Ahmad S.; Mir S. R.; Rizvi M. M. A.; Amin S. Polymer-Lipid Hybrid Nanoparticles of Exemestane for Improved Oral Bioavailability and Anti-Tumor Efficacy: An Extensive Preclinical Investigation. Int. J. Pharm. 2023, 642, 123136. 10.1016/j.ijpharm.2023.123136. [DOI] [PubMed] [Google Scholar]
- Fernandes R. S.; Arribada R. G.; Silva J. O.; Silva-Cunha A.; Townsend D. M.; Ferreira L. A. M.; Barros A. L. B. In Vitro and In Vivo Effect of PH-Sensitive PLGA-TPGS-Based Hybrid Nanoparticles Loaded with Doxorubicin for Breast Cancer Therapy. Pharmaceutics 2022, 14 (11), 2394. 10.3390/pharmaceutics14112394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M. M.; Anwer M. K.; Fatima F.; Aldawsari M. F.; Alalaiwe A.; Alali A. S.; Alharthi A. I.; Kalam M. A. Boosting the Anticancer Activity of Sunitinib Malate in Breast Cancer through Lipid Polymer Hybrid Nanoparticles Approach. Polymers (Basel) 2022, 14 (12), 2459. 10.3390/polym14122459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennati A.; Rostamizadeh K.; Manjili H. K.; Fathi M.; Danafar H. Co-Delivery of SiRNA and Lycopene Encapsulated Hybrid Lipid Nanoparticles for Dual Silencing of Insulin-like Growth Factor 1 Receptor in MCF-7 Breast Cancer Cell Line. Int. J. Biol. Macromol. 2022, 200, 335–349. 10.1016/j.ijbiomac.2021.12.197. [DOI] [PubMed] [Google Scholar]
- Suksiriworapong J.; Pongprasert N.; Bunsupa S.; Taresco V.; Crucitti V. C.; Janurai T.; Phruttiwanichakun P.; Sakchaisri K.; Wongrakpanich A. CD44-Targeted Lipid Polymer Hybrid Nanoparticles Enhance Anti-Breast Cancer Effect of Cordyceps Militaris Extracts. Pharmaceutics 2023, 15 (6), 1771. 10.3390/pharmaceutics15061771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandghanooni S.; Eskandani M.; Barar J.; Omidi Y. Recent Advances in Aptamer-Armed Multimodal Theranostic Nanosystems for Imaging and Targeted Therapy of Cancer. European Journal of Pharmaceutical Sciences 2018, 117, 301–312. 10.1016/j.ejps.2018.02.027. [DOI] [PubMed] [Google Scholar]
- Liu R.; Hu C.; Yang Y.; Zhang J.; Gao H. Theranostic Nanoparticles with Tumor-Specific Enzyme-Triggered Size Reduction and Drug Release to Perform Photothermal Therapy for Breast Cancer Treatment. Acta Pharm. Sin. B 2019, 9 (2), 410–420. 10.1016/j.apsb.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Volsi A.; Fiorica C.; D’Amico M.; Scialabba C.; Palumbo F. S.; Giammona G.; Licciardi M. Hybrid Gold/Silica/Quantum-Dots Supramolecular-Nanostructures Encapsulated in Polymeric Micelles as Potential Theranostic Tool for Targeted Cancer Therapy. Eur. Polym. J. 2018, 105, 38–47. 10.1016/j.eurpolymj.2018.05.013. [DOI] [Google Scholar]
- Yang W.; Guo W.; Gong X.; Zhang B.; Wang S.; Chen N.; Yang W.; Tu Y.; Fang X.; Chang J. Facile Synthesis of Gd-Cu-In-S/ZnS Bimodal Quantum Dots with Optimized Properties for Tumor Targeted Fluorescence/MR in Vivo Imaging. ACS Appl. Mater. Interfaces 2015, 7 (33), 18759–18768. 10.1021/acsami.5b05372. [DOI] [PubMed] [Google Scholar]
- Ghosh S.; Ghosal K.; Mohammad S. A.; Sarkar K. Dendrimer Functionalized Carbon Quantum Dot for Selective Detection of Breast Cancer and Gene Therapy. Chem. Eng. J. 2019, 373, 468. 10.1016/j.cej.2019.05.023. [DOI] [Google Scholar]
- Liu Z.; Lin H.; Zhao M.; Dai C.; Zhang S.; Peng W.; Chen Y. 2D Superparamagnetic Tantalum Carbide Composite MXenes for Efficient Breast-Cancer Theranostics. Theranostics 2018, 8 (6), 1648–1664. 10.7150/thno.23369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Z.; Yang M.; Feng X.; Liao H.; Zhang Z.; Du Y. Multifunctional Theranostic Nanoparticles for Enhanced Tumor Targeted Imaging and Synergistic FUS/Chemotherapy on Murine 4T1 Breast Cancer Cell. Int. J. Nanomedicine 2022, 17, 2165–2187. 10.2147/IJN.S360161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karahaliloğlu Z.; Kilicay E.; Hazer B. Herceptin-Conjugated Magnetic Polystyrene-Agsbox Nanoparticles as a Theranostic Agent for Breast Cancer. J. Biomater. Appl. 2022, 36 (9), 1599–1616. 10.1177/08853282211065085. [DOI] [PubMed] [Google Scholar]
- Chen J.; Lv M.; Su X.; Wang S.; Wang Y.; Fan Z.; Zhang L.; Tang G. ICAM1-Targeting Theranostic Nanoparticles for Magnetic Resonance Imaging and Therapy of Triple-Negative Breast Cancer. Int. J. Nanomedicine 2022, 17, 5605–5619. 10.2147/IJN.S374293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu S.; Zhang X.; Williams G. R.; Wu J.; Gao F.; Fu Z.; Chen X.; Lu S.; Zhu L. M. Hollow Mesoporous Silica Nanoparticles Gated by Chitosan-Copper Sulfide Composites as Theranostic Agents for the Treatment of Breast Cancer. Acta Biomater. 2021, 126, 408–420. 10.1016/j.actbio.2021.03.024. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Xu X.; Hu Q.; Wu Y.; Yu F.; He C.; Qian Y.; Han Y.; Tang J.; Hu H. Novel Manganese and Polyester Dendrimer-Based Theranostic Nanoparticles for MRI and Breast Cancer Therapy. J. Mater. Chem. B 2023, 11 (3), 648–656. 10.1039/D2TB01855A. [DOI] [PubMed] [Google Scholar]
- Ashrafizadeh M.; Zarrabi A.; Bigham A.; Taheriazam A.; Saghari Y.; Mirzaei S.; Hashemi M.; Hushmandi K.; Karimi-Maleh H.; Nazarzadeh Zare E.; Sharifi E.; Ertas Y. N.; Rabiee N.; Sethi G.; Shen M. (Nano)Platforms in Breast Cancer Therapy: Drug/Gene Delivery, Advanced Nanocarriers and Immunotherapy. Med. Res. Rev. 2023, 43, 2115. 10.1002/med.21971. [DOI] [PubMed] [Google Scholar]
- Deng X.; Cao M.; Zhang J.; Hu K.; Yin Z.; Zhou Z.; Xiao X.; Yang Y.; Sheng W.; Wu Y.; Zeng Y. Hyaluronic Acid-Chitosan Nanoparticles for Co-Delivery of MiR-34a and Doxorubicin in Therapy against Triple Negative Breast Cancer. Biomaterials 2014, 35 (14), 4333–4344. 10.1016/j.biomaterials.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Juneja R.; Lyles Z.; Vadarevu H.; Afonin K. A.; Vivero-Escoto J. L. Multimodal Polysilsesquioxane Nanoparticles for Combinatorial Therapy and Gene Delivery in Triple-Negative Breast Cancer. ACS Appl. Mater. Interfaces 2019, 11 (13), 12308–12320. 10.1021/acsami.9b00704. [DOI] [PubMed] [Google Scholar]
- Jafari R.; Zolbanin N. M.; Majidi J.; Atyabi F.; Yousefi M.; Jadidi-Niaragh F.; Aghebati-Maleki L.; Shanehbandi D.; Zangbar M. S. S.; Rafatpanah H. Anti-Mucin1 Aptamer-Conjugated Chitosan Nanoparticles for Targeted Co-Delivery of Docetaxel and IGF-1R SiRNA to SKBR3Metastatic Breast Cancer Cells. Iran. Biomed. J. 2019, 23 (1), 21–33. 10.29252/ibj.23.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camorani S.; Tortorella S.; Agnello L.; Spanu C.; D’Argenio A.; Nilo R.; Zannetti A.; Locatelli E.; Fedele M.; Comes Franchini M.; Cerchia L. Aptamer-Functionalized Nanoparticles Mediate PD-L1 SiRNA Delivery for Effective Gene Silencing in Triple-Negative Breast Cancer Cells. Pharmaceutics 2022, 14 (10), 2225. 10.3390/pharmaceutics14102225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghani L.; Noroozi Heris N.; Khonsari F.; Dinarvand S.; Dinarvand M.; Atyabi F. Trimethyl-Chitosan Coated Gold Nanoparticles Enhance Delivery, Cellular Uptake and Gene Silencing Effect of EGFR-SiRNA in Breast Cancer Cells. Front. Mol. Biosci. 2022, 9, 871541. 10.3389/fmolb.2022.871541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X.; Sun R.; Bao Y.; Zhang C.; Wu Y.; Gong Y. In Vivo Delivery of SiRNAs Targeting EGFR and BRD4 Expression by Peptide-Modified Redox Responsive PEG-PEI Nanoparticles for the Treatment of Triple-Negative Breast Cancer. Mol. Pharmaceutics 2021, 18 (11), 3990–3998. 10.1021/acs.molpharmaceut.1c00282. [DOI] [PubMed] [Google Scholar]
- Xu X.; Li L.; Li X.; Tao D.; Zhang P.; Gong J. Aptamer-Protamine-SiRNA Nanoparticles in Targeted Therapy of ErbB3 Positive Breast Cancer Cells. Int. J. Pharm. 2020, 590, 119963. 10.1016/j.ijpharm.2020.119963. [DOI] [PubMed] [Google Scholar]
- Maurer V.; Altin S.; Seleci D. A.; Zarinwall A.; Temel B.; Vogt P. M.; Strauß S.; Stahl F.; Scheper T.; Bucan V.; Garnweitner G. In-Vitro Application of Magnetic Hybrid Niosomes: Targeted Sirna-Delivery for Enhanced Breast Cancer Therapy. Pharmaceutics 2021, 13 (3), 394. 10.3390/pharmaceutics13030394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G.; Huang J.; Zhang M.; Chen S.; Zhang M. Chitosan-Crosslinked Low Molecular Weight PEI-Conjugated Iron Oxide Nanoparticle for Safe and Effective DNA Delivery to Breast Cancer Cells. Nanomaterials 2022, 12 (4), 584. 10.3390/nano12040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari R.; Nasra S.; Meghani N.; Kumar A. MiR-206 Conjugated Gold Nanoparticle Based Targeted Therapy in Breast Cancer Cells. Sci. Rep. 2022, 12 (1), 4713. 10.1038/s41598-022-08185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezghrani B.; Ali L. M. A.; Cubedo N.; Rossel M.; Hesemann P.; Durand J. O.; Bettache N. Periodic Mesoporous Ionosilica Nanoparticles for Dual Cancer Therapy: Two-Photon Excitation SiRNA Gene Silencing in Cells and Photodynamic Therapy in Zebrafish Embryos. Int. J. Pharm. 2023, 641, 123083. 10.1016/j.ijpharm.2023.123083. [DOI] [PubMed] [Google Scholar]
- Powers K. W.; Palazuelos M.; Moudgil B. M.; Roberts S. M. Characterization of the Size, Shape, and State of Dispersion of Nanoparticles for Toxicological Studies. Nanotoxicology 2007, 1, 42–51. 10.1080/17435390701314902. [DOI] [Google Scholar]
- Olerile L. D.; Liu Y.; Zhang B.; Wang T.; Mu S.; Zhang J.; Selotlegeng L.; Zhang N. Near-Infrared Mediated Quantum Dots and Paclitaxel Co-Loaded Nanostructured Lipid Carriers for Cancer Theragnostic. Colloids Surfaces B Biointerfaces 2017, 150, 121–130. 10.1016/j.colsurfb.2016.11.032. [DOI] [PubMed] [Google Scholar]
- Mozar F. S.; Chowdhury E. H. Surface-Modification of Carbonate Apatite Nanoparticles Enhances Delivery and Cytotoxicity of Gemcitabine and Anastrozole in Breast Cancer Cells. Pharmaceutics 2017, 9 (2), 21. 10.3390/pharmaceutics9020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. H.; Chhowalla M.; Iqbal Z.; Sesti F. Single-Walled Carbon Nanotubes Are a New Class of Ion Channel Blockers. J. Biol. Chem. 2003, 278 (50), 50212–50216. 10.1074/jbc.M310216200. [DOI] [PubMed] [Google Scholar]
- Black K. C. L.; Wang Y.; Luehmann H. P.; Cai X.; Xing W.; Pang B.; Zhao Y.; Cutler C. S.; Wang L. V.; Liu Y.; Xia Y. Radioactive 198Au-Doped Nanostructures with Different Shapes for in Vivo Analyses of Their Biodistribution, Tumor Uptake, and Intratumoral Distribution. ACS Nano 2014, 8 (5), 4385–4394. 10.1021/nn406258m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chithrani B. D.; Ghazani A. A.; Chan W. C. W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006, 6 (4), 662–668. 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- Hoshino A.; Fujioka K.; Oku T.; Suga M.; Sasaki Y. F.; Ohta T.; Yasuhara M.; Suzuki K.; Yamamoto K. Physicochemical Properties and Cellular Toxicity of Nanocrystal Quantum Dots Depend on Their Surface Modification. Nano Lett. 2004, 4 (11), 2163–2169. 10.1021/nl048715d. [DOI] [Google Scholar]
- Goodman C. M.; McCusker C. D.; Yilmaz T.; Rotello V. M. Toxicity of Gold Nanoparticles Functionalized with Cationic and Anionic Side Chains. Bioconjugate Chem. 2004, 15 (4), 897–900. 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- Slowing I.; Trewyn B. G.; Lin V. S. Y. Effect of Surface Functionalization of MCM-41-Type Mesoporous Silica Nanoparticles on the Endocytosis by Human Cancer Cells. J. Am. Chem. Soc. 2006, 128 (46), 14792–14793. 10.1021/ja0645943. [DOI] [PubMed] [Google Scholar]
- Alexis F.; Pridgen E. M.; Langer R.; Farokhzad O. C.. Nanoparticle Technologies for Cancer Therapy. In Handbook of Experimental Pharmacology; Springer, 2010; pp 55–86. 10.1007/978-3-642-00477-3_2. [DOI] [PubMed] [Google Scholar]
- Maji R.; Dey N. S.; Satapathy B. S.; Mukherjee B.; Mondal S. Preparation and Characterization of Tamoxifen Citrate Loaded Nanoparticles for Breast Cancer Therapy. Int. J. Nanomedicine 2014, 9 (1), 3107–3118. 10.2147/IJN.S63535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Carballal B.; Aaldering L. J.; Ritzefeld M.; Pereira S.; Sewald N.; Moerschbacher B. M.; Götte M.; Goycoolea F. M. Physicochemical and Biological Characterization of Chitosan-MicroRNA Nanocomplexes for Gene Delivery to MCF-7 Breast Cancer Cells. Sci. Rep. 2015, 5, 13567. 10.1038/srep13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherukuri P.; Gannon C. J.; Leeuw T. K.; Schmidt H. K.; Smalley R. E.; Curley S. A.; Weisman R. B. Mammalian Pharmacokinetics of Carbon Nanotubes Using Intrinsic Near-Infrared Fluorescence. Proc. Natl. Acad. Sci. U. S. A. 2006, 103 (50), 18882–18886. 10.1073/pnas.0609265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. T.; Guo W.; Lin Y.; Deng X. Y.; Wang H. F.; Sun H. F.; Liu Y. F.; Wang X.; Wang W.; Chen M.; Huang Y. P.; Sun Y. P. Biodistribution of Pristine Single-Walled Carbon Nanotubes in Vivo. J. Phys. Chem. C 2007, 111 (48), 17761–17764. 10.1021/jp070712c. [DOI] [Google Scholar]
- Yang S. T.; Wang X.; Jia G.; Gu Y.; Wang T.; Nie H.; Ge C.; Wang H.; Liu Y. Long-Term Accumulation and Low Toxicity of Single-Walled Carbon Nanotubes in Intravenously Exposed Mice. Toxicol. Lett. 2008, 181 (3), 182–189. 10.1016/j.toxlet.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Fraczek A.; Menaszek E.; Paluszkiewicz C.; Blazewicz M. Comparative in Vivo Biocompatibility Study of Single- and Multi-Wall Carbon Nanotubes. Acta Biomater. 2008, 4 (6), 1593–1602. 10.1016/j.actbio.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Hirano S.; Kanno S.; Furuyama A. Multi-Walled Carbon Nanotubes Injure the Plasma Membrane of Macrophages. Toxicol. Appl. Pharmacol. 2008, 232 (2), 244–251. 10.1016/j.taap.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Stasko N. A.; Johnson C. B.; Schoenfisch M. H.; Johnson T. A.; Holmuhamedov E. L. Cytotoxicity of Polypropylenimine Dendrimer Conjugates on Cultured Endothelial Cells. Biomacromolecules 2007, 8 (12), 3853–3859. 10.1021/bm7008203. [DOI] [PubMed] [Google Scholar]
- Daniels T. R.; Delgado T.; Helguera G.; Penichet M. L. The Transferrin Receptor Part II: Targeted Delivery of Therapeutic Agents into Cancer Cells. Clinical Immunology 2006, 121, 159–176. 10.1016/j.clim.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Ioannidis J. P. A.; Kim B. Y. S.; Trounson A. How to Design Preclinical Studies in Nanomedicine and Cell Therapy to Maximize the Prospects of Clinical Translation. Nat. Biomed. Eng. 2018, 2 (11), 797–809. 10.1038/s41551-018-0314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nino-Pariente A.; Nebot V. J.; Vicent M. J. Relevant Physicochemical Descriptors of “Soft Nanomedicines” to Bypass Biological Barriers. Curr. Pharm. Des. 2016, 22 (9), 1274–1291. 10.2174/1381612822666151216152143. [DOI] [PubMed] [Google Scholar]
- Xiong K.; Zhang Y.; Wen Q.; Luo J.; Lu Y.; Wu Z. X.; Wang B. Q.; Chen Y.; Zhao L.; Fu S. Z. Co-Delivery of Paclitaxel and Curcumin by Biodegradable Polymeric Nanoparticles for Breast Cancer Chemotherapy. Int. J. Pharm. 2020, 589, 119875. 10.1016/j.ijpharm.2020.119875. [DOI] [PubMed] [Google Scholar]
- Chen X.; He H.; Guo X.; Hou M.; Zhang X.; Li S.; Wang C.; Zhao G.; Li W.; Zhang X.; Hong W. Calcium Orthophosphate in Liposomes for Co-Delivery of Doxorubicin Hydrochloride/Paclitaxel in Breast Cancer. Mol. Pharm. 2023, 20, 3914. 10.1021/acs.molpharmaceut.3c00015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Data Explorer. ECIS - European Cancer Information System. https://ecis.jrc.ec.europa.eu/explorer.php?$0-0 (accessed 2023-09-04).