Abstract
Introduction
Chronic bronchitis (CB), a phenotype of chronic obstructive pulmonary disease (COPD) characterised by persistent cough and mucus hypersecretion, is associated with poor outcomes despite guideline-based treatment. Bronchial rheoplasty (BR) with the RheOx system delivers non-thermal pulsed electric fields to the lower airway epithelium and submucosa to reduce mucus producing cells. Early phase clinical trials including 1-year follow-up have demonstrated reduction in airway goblet cell hyperplasia and improvement in CB symptoms.
Methods
The current multicentre observational BR study enrolled 21 patients with CB at six centres in the USA, with bilateral treatment and 2-year follow-up. Entry criteria included elevated cough and sputum scores from COPD Assessment Test (CAT) and forced expiratory volume in one second<80% predicted. Safety was assessed by serious adverse event (SAE) incidence through 24 months. Clinical utility was evaluated using changes in the CAT, the St. George’s Respiratory Questionnaire (SGRQ) and by comparing exacerbation rates before and following intervention.
Results
No procedure-related or device-related SAEs occurred. Mean (SD) changes from baseline in CAT at 12 and 24 months were −9.0 (6.7) (p<0.0001) and −5.6 (7.1) (p<0.0047) and in SGRQ were −16.6 (13.2) (p<0.0001) and −11.8 (19.2) (p<0.0227), respectively. There was a 34% reduction in moderate and a 64% reduction in severe COPD exacerbation events compared with the year prior to treatment.
Conclusions
This study extends the findings from previous feasibility studies, demonstrating that BR can be performed safely and may significantly improve symptoms and health-related quality of life for patients with CB through 24 months.
Trail registration number
Keywords: COPD Exacerbations; Airway Epithelium; Bronchoscopy; COPD epidemiology; Cough/Mechanisms/Pharmacology; Pulmonary Disease, Chronic Obstructive
What is already known on this topic
Chronic bronchitis (CB) in chronic obstructive pulmonary disease is associated with poor outcomes including increased risk of death despite guideline-based treatment.
What this study adds
This study demonstrates that bronchial rheoplasty (BR) can be performed safely and may significantly improve symptoms and quality of life for patients with CB.
How this study might affect research, practice or policy
A randomised controlled trial confirming safety and effectiveness of BR may provide an additional and more effective treatment to add to existing guidelines.
Introduction
Chronic bronchitis (CB) is a phenotype of chronic obstructive pulmonary disease (COPD) associated with the persistent cough and sputum production.1 The presence of CB in patients with COPD is associated with poor outcomes including reduced quality of life, greater rate of lung function decline, more frequent exacerbations and increased mortality compared with patients with COPD without CB.2 3 The prevalence of CB has been reported from 3.4% to 22.0% in the general population and up to 74.1% in patients with COPD.4–6 CB is associated with pathologic presence of airway inflammation, goblet cell and mucosal gland metaplasia and hyperplasia and altered mucous composition leading to mucous accumulation in large airways and mucous plugging of peripheral airways.7–10 The clinical symptoms and pathologic findings of CB may occur independent of spirometric airflow obstruction severity.11
While existing maintenance therapies for patients with COPD with or without CB have demonstrated improvements in lung function and reductions in exacerbation rate, the impact of these therapies is poor with respect to cough and sputum symptoms.12 Because of its heterogeneity, COPD aetiology is challenging to define and precision-based therapies are needed.13 To this point, while CB is defined by patient-reported symptoms, few clinical trials have focused on these.14 15 Thus, despite guideline-directed medical therapy, many patients with CB remain significantly symptomatic.
Bronchial rheoplasty (BR) is a bronchoscopic procedure, intended for patients with a CB phenotype of COPD, which uses the RheOx system to deliver a non-thermal pulsed electric field (PEF) to the airway epithelium and submucosa via an endobronchial catheter electrode. PEF induces cell death by disrupting cellular homeostasis, leading to processes such as osmotic swelling and apoptosis while leaving extracellular matrix components and collagenous structures within the treatment zone unaffected.16 17 A proof of concept clinical trial demonstrated safety and symptomatic improvement at 1 year.17 Airway biopsy studies in this trial supported preclinical studies demonstrating regeneration of a normalised epithelium with a reduction in goblet cell hyperplasia.17 The current multicentre, prospective single-arm observational study evaluates the safety and clinical utility of BR in a US cohort of CB patients with follow-up through 2 years.
Methods
Study design
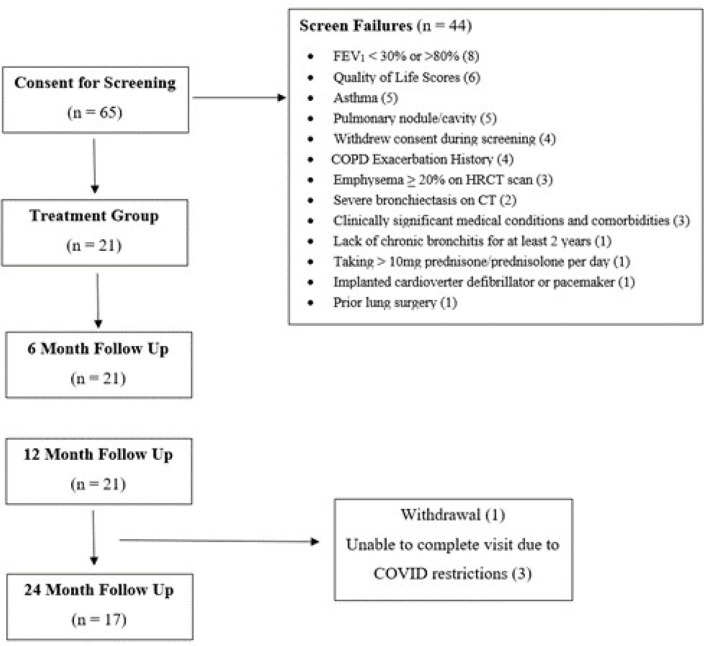
The study is a prospective, multicentre, single-arm clinical study conducted in the USA (NCT03631472) in patients with moderate-to-severe CB (figure 1). All patients provided written informed consent prior to undergoing study specific screening (figure 1).
Figure 1.
Consolidated Standards of Reporting Trials diagram. COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; HRCT, high-resolution CT scan.
Patients were recruited between November 2018 and December 2019 at six study sites. Eligible patients were at least 40 years of age, had a smoking history of at least 10 pack-years and were diagnosed with CB. Key inclusion criteria included sum of COPD Assessment Test (CAT) question 1 (cough) and question 2 (phlegm) score of ≥7, (each question range 0–5), postbronchodilator forced expiratory volume in one second (FEV1) from ≥30% to ≤ 80% predicted and a history of at least one exacerbation of any severity in the prior year. To establish a predominantly bronchitic phenotype, baseline high-resolution CT (HRCT) scans were performed. Key exclusion criteria were emphysema≥20% on HRCT scan, need for daily oral steroid use (>10 mg/day), active respiratory infection at time of procedure, a history of arrhythmia within the past 2 years, presence of implanted cardiac devices, prior lung surgery, a history of asthma before age 30 years and current smoking (within 6 months of treatment). A full list of inclusion and exclusion criteria can be found in the online supplemental file.
bmjresp-2023-001710supp001.pdf (942.7KB, pdf)
Neither patients nor the public were involved in the design, or conduct, or reporting, or dissemination plans of this research.
Procedure description
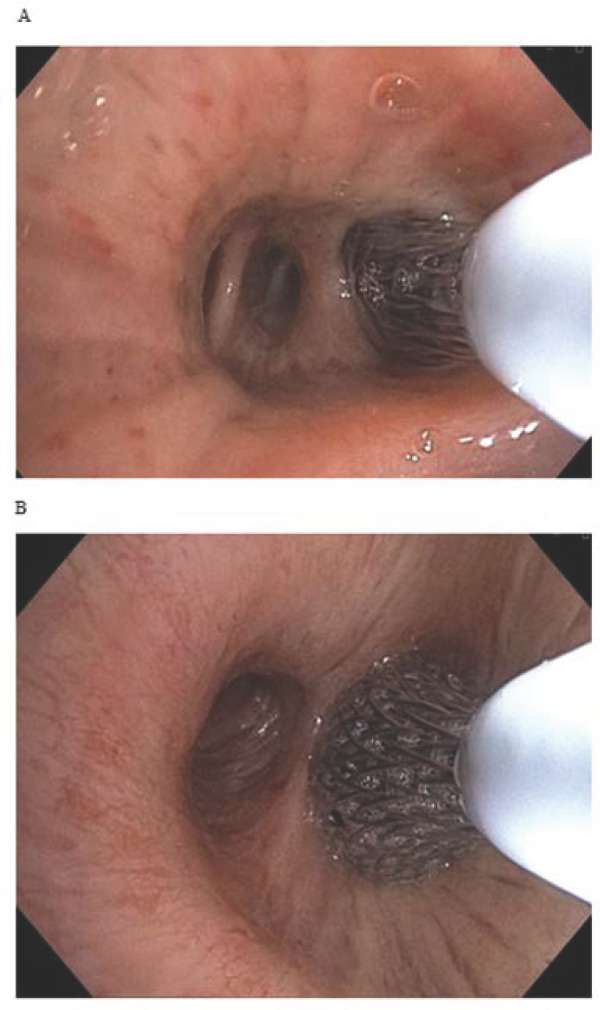
The BR procedure was performed under general anaesthesia using a therapeutic bronchoscope with at least 2.8 mm working channel under direct visualisation. The bronchoscope was navigated to the desired treatment area, a catheter with a distal self-expanding basket electrode with shape memory was advanced through the bronchoscope into the target location, expanded to circumferentially contact the airway wall (figure 2) and activated via a foot pedal to deliver pulsed electric current in synchronisation with the patient’s cardiac cycle over 5 s. This process was repeated until all accessible bronchial segments and subsegments of the target lung were treated. Both lungs were treated in separate sessions, approximately one month apart.
Figure 2.
Procedural images taken from the bronchoscope during a right lung procedure show the RheOx catheter during an activation in a subsegment of the right lover lobe (A) and then right bronchus intermedius (B).
Patients continued to receive standard-of-care pharmacologic treatment per institution and society guidelines throughout the study. Study follow-up visits are at 1 week following each treatment and at 1 month, 3 months, 6 months, 12 months and annually at years 2–5 following the completion of the second BR procedure (online supplemental table S–1).
Outcomes
The primary outcome of the study was safety, as assessed by the incidence of serious adverse events (SAEs) through 12 months. Adverse events of interest were death, COPD exacerbation requiring hospitalisation, pneumothorax within 2 days of procedure, pneumonia within 7 days of procedure, respiratory failure or arrhythmia requiring intervention. Non-SAEs were also assessed. Events were defined as occurring during the treatment recovery period if they occurred within 30 days after either treatment. Events thereafter were defined as occurring during the 3-month, 6-month, 12-month or 2-year periods which were calculated from the second treatment. COPD exacerbation rates (excluding the treatment recovery period) were also calculated and assessed by severity as defined by the 2020 Global Initiative for Obstructive Lung Disease (GOLD) guidelines.15 Preprocedure and postprocedure spirometry (FEV1 and forced vital capacity (FVC)) was incorporated as an additional safety measure.
The secondary outcome of the study was clinical utility as determined by the CAT and the St. George’s Respiratory Questionnaire (SGRQ) total scores. Outcome data were collected at baseline and at 3, 6, 12 and 24 months after the second procedure. Responder rates were calculated by using the established minimally clinically important difference (MCID) thresholds, a reduction of 4 points for the SGRQ and 2 points for the CAT.18 19
Other and exploratory outcomes included frequency of moderate (outpatient treated) and severe (hospitalised) exacerbations and the Cough and Sputum Questionnaire (CASA-Q). The CASA-Q is specifically designed and validated to measure cough and sputum symptoms and their impact on patients with CB. The instrument has four domains assessing cough symptoms, cough impacts, sputum symptoms and sputum impacts. Each domain is scored using a 0–100 scale, with lower scores indicating worse quality of life.
Statistical methods
Since this is an early feasibility study, sample size was targeted for 30 patients based on clinical judgement prior to limitations imposed by the pandemic. Descriptive statistics and graphical representations were used to summarise the data. For categorical variables including adverse events, counts and percentages were calculated. For continuous variables, means, SDs and, when appropriate, 95% CI for the mean, assuming a normal distribution, were calculated. All calculations were based on available data; no imputations or extrapolations were made to replace missing values. P values for longitudinal secondary outcome measures (CAT and SGRQ) were calculated from a Sign test corresponding to non-parametric approach to test whether the median differs from zero and CIs are reported. The creation of analysis datasets and all statistical analyses were performed using SAS V.9.4 (SAS Institute).
Results
Though the sample size was originally set at up to 30 patients, due to limitations during the pandemic, only 65 were screened, yielding a total of 21 eligible patients who were enrolled (online supplemental table S–2) and received treatment (figure 1). Follow-up to 12 and 24 months after the second study treatment was completed for 21 and 20 patients, respectively (figure 1). In total, 1 patient withdrew prior to completing a 24-month visit and 3 patients were unable to complete the 24-month follow-up testing due to COVID-19-related restrictions.
Treated patients had a mean (SD) age of 66.1 (5.2) years, a smoking history of 53.6 (39.0) pack-years, postbronchodilator FEV1% predicted of 53.3 (15.8) (range 32–78), CAT Score of 26.9 (4.9) (range 17–34) points and SGRQ Score of 60.1 (15.5) (range 26.6–81.0) points, indicating a high symptom burden despite most patients receiving beta2-agonist and/or long-acting muscarinic agonist treatment (90.5%) and/or an inhaled corticosteroid (80.9%). In total, 9 patients (42.9%) were GOLD stage II and 10 (47.6%) were in GOLD stage III and 2 patients had preserved ratio impaired spirometry (FEV1/FVC>0.7 despite FEV1<80% predicted) (table 1).20
Table 1.
Baseline demographics, clinical characteristics and medications
| Variable | Value (n=21 patients) |
| Age (years) | 66.1 (5.2) |
| Male, n (%) | 12 (57.1) |
| BMI (kg/m2) | 31.7 (4.5) |
| Smoking history (pack-years) | 53.6 (39.0) |
| FEV1% predicted* | 53.3 (15.8) |
| FEV1/FVC* | 51.2 (14.3) |
| Airflow obstruction†, n (%) | |
| GOLD II | 9 (42.85) |
| GOLD III | 10 (47.6) |
| RV% predicted* | 137.2 (44.3) |
| RV/TLC* | 48.9 (8.2) |
| % emphysema (−950 HU) | 4.8 (5.2) |
| 6MWT (m) | 298.8 (83.5) |
| CAT total score | 26.9 (4.9) |
| CAT phlegm score | 3.8 (0.7) |
| CAT cough score | 3.9 (0.9) |
| SGRQ total score | 60.1 (15.5) |
| COPD medications, n (%) | |
| LABA and/or LAMA | 19 (90.5) |
| Inhaled corticosteroid | 17 (80.9) |
| COPD exacerbation history‡ | |
| Moderate | 1.05 (1.67) |
| Severe | 0.14 (0.36) |
Values are mean (SD) unless otherwise noted.
*Lung function parameters are post bronchodilator.
†2 patients had FEV1/FVC>0.70 despite FEV1<80%.
‡Exacerbation data presented as event rate per patient per year in the 12 months prior to the first RheOx treatment.
BMI, body mass index; CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; GOLD, Global Initiative for Obstructive Lung Disease; LABA, Long-acting beta agonist; LAMA, Long-acting beta anti-muscarinic (LAMA); 6MWT, 6-minute walk test; RV, residual volume; SGRQ, St. George’s Respiratory Questionnaire; TLC, total lung capacity.
All 21 enrolled patients completed both BR treatments for a total of 42 procedures. Patients tolerated the procedure well with a mean (SD) of 64.6 (25.0) activations applied to each lung (online supplemental table S–3). Most patients (15 of the 21 patients; 30 of the 42 procedures; 71%) were discharged from the hospital the same day as the procedure. All 12 procedures (6 patients) in which patients were held overnight occurred at a single centre, reflecting that institution’s standard of care.
Safety assessment
A total of 6 SAEs were reported in 3 patients through 12 months which included pneumonia,2 COPD exacerbation,1 hyponatremia,1 stress cardiomyopathy1 and hip fracture.1 An additional 4 SAEs were reported in two patients between 12 and 24 months which included COPD exacerbation,2 acute pulmonary embolism1 and worsening dyspnoea1 (table 2). None of the SAEs reported were judged by the investigator to be related to the investigational device or procedure. No unanticipated adverse events were reported.
Table 2.
Serious adverse events
| MedDRA lower-level term (n events) |
Treatment recovery period* (n=21) |
3 months† (n=21) |
6 months‡ (n=21) |
12 months§ (n=21) |
24 months¶ (n=20) |
| Worsening dyspnoea | 0 | 0 | 0 | 0 | 1 |
| COPD exacerbation | 0 | 0 | 0 | 1 | 2 |
| Hyponatremia | 1 | 0 | 0 | 0 | 0 |
| Hip fracture | 0 | 0 | 1 | 0 | 0 |
| Pneumonia | 0 | 1 | 1 | 0 | 0 |
| Pulmonary embolism | 0 | 0 | 0 | 0 | 1 |
| Stress cardiomyopathy | 0 | 1 | 0 | 0 | 0 |
| Total | One event in one patient |
Two events in two patients |
Two events in two patients |
One event in one patient |
Four events in two patients |
None were related to investigational device or procedure.
*Defined as the 30 days following either RheOx procedure.
†Defined as the follow-up period through 3 months post treatment 2, excluding the treatment recovery period.
‡Defined as the follow-up period between 3 months and 6 months after treatment 2.
§Defined as the follow-up period between 6 months and 12 months after treatment 2.
¶ Defined as the follow-up period between 12 months and 24 months after treatment 2.
COPD, chronic obstructive pulmonary disease.
The most frequently reported non-SAEs through 12 months (online supplemental table S–4) were COPD exacerbation (25 events in 11 patients), cough (15 events in 9 patients), wheezing (6 events in 5 patients), sore throat (5 events in 5 patients) and headache (6 events in 4 patients). Of the 25 non-serious COPD exacerbations reported through 12 months, 7 were considered mild (treated with short acting medication only) and 18 were considered moderate (patient was prescribed oral corticosteroid, antibiotics or both).
Pulmonary function remained stable throughout the follow-up period and there were no significant changes from baseline to months 6, 12 and 24 in FEV1 or FEV1/FVC ratio (online supplemental table S–5).
Clinical utility
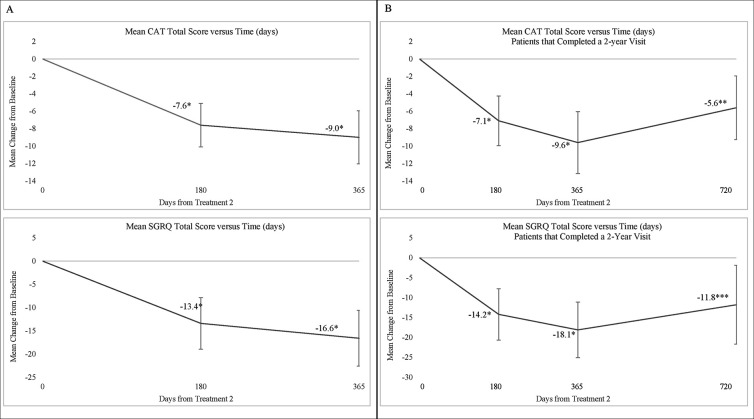
Statistically significant changes in patient symptoms and health-related quality of life were observed at all timepoints. Mean (SD) changes in CAT from baseline to months 6 and 12 were −7.6 (5.1) points (p<0.0001) and −9.0 (6.7) points (p<0.0001), respectively (figure 3). Mean (SD) change in CAT from baseline to month 24 for the 17 patients who completed a questionnaire at the 24-month visit was −5.6 (7.1) points (p=0.005). Mean (SD) change in SGRQ from baseline to 12 months was −16.6 (13.2) points (p<0.0001) depicted in figure 3. Mean (SD) change in SGRQ from baseline to month 24 for the 17 patients who completed the 24-month visit was −11.8 (19.2) points (p=0.0227). Responder rates for the CAT and SGRQ at 12 months were each 85.7% (18 of the 21). At 24 months responder rates for the 17 patients who completed the visit were 64.7% (11/17) and 58.8% (10/17) for CAT and SGRQ, respectively.
Figure 3.
Change from baseline in patient-reported CAT and SGRQ total sores (A) for all 21 subjects through 12 months and (B) through 24 months for those 17 subjects that completed 2-year follow-up. Data are presented as mean change from baseline±95% CI. Visits are measured from the second treatment. *P value<0.001. **P value<0.01. ***P value<0.05. CAT, Chronic Obstructive Pulmonary Disease Assessment Test; SGRQ, St. George’s Respiratory Questionnaire.
For the CASA-Q, at 12 months, cough impacts improved by a mean (SD) 31.7 (23.1) points (p<0.0001), cough symptoms improved 29.3 (20.3) points (p<0.0001), sputum impacts improved 28.8 (22.2) points (p<0.0001) and sputum symptoms improved 25.0 (21.2) points (p<0.0001) (online supplemental table S–6). At 24 months, cough impacts improved by a mean (SD) 25.5 (26.5) points (p=0.0011), cough symptoms improved 23.5 (24.9) points (p=0.0013), sputum impacts improved 23.1 (28.6) points (p=0.0042) and sputum symptoms improved 14.0 (22.0) points (p=0.0223) (online supplemental table S–6).
Exacerbation rates are provided in table 3. At baseline, moderate and severe COPD exacerbation rates, defined as the number of events per patient per year of follow-up, were 1.05 (SD 1.66) and 0.14 (0.36), respectively, in the 12 months prior to the treatment. Through 12 months following treatment, COPD exacerbation rates were 0.69 (1.33) and 0.05 (0.25), respectively, excluding the treatment recovery period, representing a relative reduction of 34% for the moderate COPD exacerbation rate and a relative reduction of 64% for the severe COPD exacerbation rate. Event rates in the 12-month to 2-year period also demonstrated reductions in moderate and severe COPD exacerbation rates from baseline.
Table 3.
Moderate and severe COPD exacerbation rates
| COPD exacerbation rate (events/patient/year) |
Baseline* (n=21) |
12 months post treatment† (n=21) |
12 months post treatment (excluding the treatment recovery period)‡ (n=21) |
Post 12 months through 24 months§ (n=20) |
| All (moderate+severe) | 1.19±1.60 | 0.99±1.46 | 0.75±1.32 | 0.88±1.91 |
| Moderate | 1.05±1.66 | 0.94±1.48 | 0.69±1.33 | 0.79±1.74 |
| Severe | 0.14±0.36 | 0.05±0.21 | 0.05±0.25 | 0.10±0.44 |
Values are mean±SD (number of events).
*Defined as the 12 months prior to treatment 1.
†Defined as the follow-up period from treatment 1 through 12 months after treatment 2.
‡Defined as the follow-up period from treatment 1 through 12 months after treatment 2 excluding the 30 days following either RheOx procedure.
§Defined as the follow-up period post 12 months through 24 months after treatment 2.
COPD, chronic obstructive pulmonary disease.
Discussion
In a multicentre observational study of BR in patients with COPD and CB, the procedure was shown to be safe with no procedure-related SAEs. Clinically significant improvements in CAT symptom and SGRQ, which exceeded the minimal clinically important difference for each instrument, persisted through 24 months.
This study addresses a group of patients with varying levels of airflow obstruction, selected for moderate-to-severe CB symptoms of cough and sputum, as determined by the first two items of the CAT instrument, persisting despite guideline-based therapy and excluded patients with a significant emphysema phenotype. The absence of serious device-related or procedure-related adverse events through 24 months confirms the feasibility of BR in this CB population in which there remains a significant unmet therapeutic need to relieve their persistent symptom burden. Non-SAEs reported were consistent with expectations for patients with COPD undergoing bronchoscopy alone. Our study confirms the safety findings observed in a previously published 12-month clinical trial and extends these finding through 24 months.17
Notably, this population exhibited a high symptomatic burden as assessed by the CAT and SGRQ despite moderate levels of airflow obstruction. Baseline characteristics were similar to the prior study, except for lower FEV1, lower six-minute walk test distance and higher body mass index in this US cohort. In total, 2 of the 21 patients with CB enrolled had preserved FEV1/FVC ratio but reduced FEV1%. This preserved ratio impaired spirometric phenotype has been linked to an airway dominant disease pattern and has been observed to be a precursor to traditional reduced ratio COPD. Further, the risk for exacerbations and limitation in activity is greater in this group with lack of any effective evidence-based therapeutic options.21 22
Symptom and quality of life improvements in treated patients were both statistically significant and clinically meaningful at the 6-month, 12-month and 24-month follow-up. Importantly, the mean improvement in both CAT and SGRQ persisted at greater than twice the minimally important differences at 24 months with 64.7% of the 17 patients who completed a 24-month visit exceeding the MCID for CAT and 58.8% for SGRQ.19 23 These improvements corresponded with significant improvements in the CASA-Q cough and sputum symptom and impact scores. While there was a small drop in responder rates between 12 months and 2 years, this is potentially attributable to disease progression and may be an opportunity for retreatment for certain patients in the future. These findings are particularly notable given the consistent absence of clinically meaningful improvement in symptoms and quality of life found in pharmacologic trials in patients with COPD.12 24 25 Thus, the clinically significant improvements in symptoms and quality of life following BR experienced by a significant majority of treated patients and maintained through 2 years in most patients have the potential to address a significant unmet need in the treatment of patients with the chronic bronchitic phenotype.
BR is an endoscopic device-based therapy that uses the RheOx system to directly target the cells responsible for mucus hypersecretion and therefore CB symptoms. Initial mechanistic studies demonstrated a 39% histologic reduction in goblet cell hyperplasia with 84% of patients demonstrating improvement.17 This sparing of extracellular matrix components and collagenous structures within the treatment zone can be attributed to the nature of PEF energy. Further, spirometric and HRCT scan assessments have shown that BR does not trigger airway stenosis or negatively impact lung function. Impacts on non-goblet cell epithelial function, airway microbiome and mucus composition, known to be important in CB pathogenesis, have not yet been described. This technology contrasts with other bronchoscopic systems which deliver thermal energy (heat or cold) to induce cell death and modify the airway pathology. Bronchial thermoplasty (BT) is US Food and Drug Administration approved for the treatment of asthma and uses radiofrequency heat energy to reduce airway smooth muscle mass through bronchoscopy and is not targeted at epithelial remodelling or to impact goblet cell hyperplasia. SAEs occurred more frequently in BT-treated patients than in patients receiving a sham bronchoscopic procedure and/or standard care during the 12-week treatment period.26 This procedure, however, has not been studied in COPD. Recently, metered cryospray has been tested in patients with CB. The system delivers liquid nitrogen to the airways to ablate abnormal epithelium and facilitates healthy mucosal regeneration. In total, 5 out of the 11 patients scheduled to undergo surgery for lung cancer underwent this endoscopic approach, followed by lung resection and assessments of airway histology at 2 weeks.25 Like previous results of BR,17 re-epithelialisation at the treatment site was observed in that report.
The prespecified secondary outcome of moderate and severe COPD exacerbations resulting in hospitalisation was compared in the 12 and 24 months following BR treatment to rates from the year prior to treatment. The rates of moderate and severe COPD exacerbations in the current study were, respectively, 0.69 and 0.05 events per patient per year in the 12 months following treatment compared with 1.05 and 0.14 in the 12 months prior to treatment. Reducing exacerbation frequency and subsequent healthcare utilisation is an important outcome target in the treatment of COPD.27 28 While the interpretation of the results are limited due to the lack of a control group and ongoing pandemic during the follow-up period (14 of the 21 patients completed the 6-month visit prior to March 2020), this is a promising early signal that should be assessed in future randomised trials.
The strengths of this paper include the duration of follow-up, the consistent and durable magnitude of symptom, quality of life improvement that exceeds the signal found in the treatment group of any previous drug or device study in COPD and the excellent safety signal.24 29 30The primary limitations to this study are the small sample size, incomplete follow-up data for a few subjects due to the COVID-19 pandemic and lack of sham control group preventing exclusion of a placebo effect on patient-reported outcomes. Notably, response rates within control groups of other bronchoscopic device studies in COPD do not demonstrate meaningful changes in symptom or quality of life scales.31 32Further, it is difficult to assess the impact that follow-up of patients during the unique conditions of the COVID-19 pandemic may have had on COPD exacerbation rates.
In summary, this study extends the evidence of the feasibility, safety and clinical outcomes of BR in symptomatic patients with COPD and CB. We identified clinically meaningful reduction in CB symptom burden and improved quality of life following treatment that is maintained through 24 months, demonstrating promise for a CB population with persistent symptoms despite current guideline-based treatment. Further study in a randomised setting is currently underway to confirm these findings.
Footnotes
Contributors: FCS (primary author): design and composition of manuscript. MTD, VK, NM, AC, DKH and AM (coauthors): guidance and editing.
Funding: Gala Therapeutics (sponsor) provided funding for the investigation as well as administrative and logistical support in co-ordinating the study across the study sites.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by Western IRB ethics committee (ID#1-1185822-1). Participants gave informed consent to participate in the study before taking part.
References
- 1.Dotan Y, So JY, Kim V. Chronic Bronchitis: where are we now Chronic Obstr Pulm Dis 2019;6:178–92. 10.15326/jcopdf.6.2.2018.0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martínez-García MÁ, Faner R, Oscullo G, et al. Chronic bronchial infection is associated with more rapid lung function decline in chronic obstructive pulmonary disease. Annals ATS 2022;19:1842–7. 10.1513/AnnalsATS.202108-974OC [DOI] [PubMed] [Google Scholar]
- 3.Miravitlles M. Cough and Sputum production as risk factors for poor outcomes in patients with COPD. Respir Med 2011;105:1118–28. 10.1016/j.rmed.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 4.Burgel P-R, Nesme-Meyer P, Chanez P, et al. Cough and Sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest 2009;135:975–82. 10.1378/chest.08-2062 [DOI] [PubMed] [Google Scholar]
- 5.Kim V, Crapo J, Zhao H, et al. Comparison between an alternative and the classic definition of chronic Bronchitis in Copdgene. Annals ATS 2015;12:332–9. 10.1513/AnnalsATS.201411-518OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Oca MM, Halbert RJ, Lopez MV, et al. The chronic Bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J 2012;40:28–36. 10.1183/09031936.00141611 [DOI] [PubMed] [Google Scholar]
- 7.Boucher RC, Drazen JM, editor. N Engl J Med . Muco-obstructive lung diseases. N Engl J Med 2019;380:1941–53. 10.1056/NEJMra1813799 [DOI] [PubMed] [Google Scholar]
- 8.Andelid K, Öst K, Andersson A, et al. Lung Macrophages drive mucus production and steroid-resistant inflammation in chronic Bronchitis. Respir Res 2021;22:172. 10.1186/s12931-021-01762-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kesimer M, Ford AA, Ceppe A, et al. Airway Mucin concentration as a marker of chronic Bronchitis. N Engl J Med 2017;377:911–22. 10.1056/NEJMoa1701632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–53. 10.1056/NEJMoa032158 [DOI] [PubMed] [Google Scholar]
- 11.Woodruff DP. Sketching as a tool for numerical linear algebra. TCS 2014;10:1–157. [Google Scholar]
- 12.Riley CM, Sciurba FC. Diagnosis and outpatient management of chronic obstructive pulmonary disease: A review. JAMA 2019;321:786. 10.1001/jama.2019.0131 [DOI] [PubMed] [Google Scholar]
- 13.Montuschi P. Pharmacological treatment of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2006;1:409–23. 10.2147/copd.2006.1.4.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pauwels RA, Buist AS, Ma P, et al. GOLD scientific committee. global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: national heart, lung, and blood Institute and world health organization global initiative for chronic obstructive lung disease (GOLD): executive summary. Respir Care 2001;46:798–825. [PubMed] [Google Scholar]
- 15.Global initiative for chronic obstructive lung disease, Inc. global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2020. Available: https://goldcopd.org/gold-reports/
- 16.Kodama H, Vroomen LG, Ueshima E, et al. Catheter-based Endobronchial Electroporation is feasible for the focal treatment of Peribronchial tumors. J Thorac Cardiovasc Surg 2018;155:2150–9. 10.1016/j.jtcvs.2017.11.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valipour A, Fernandez-Bussy S, Ing AJ, et al. Bronchial Rheoplasty for treatment of chronic Bronchitis. twelve-month results from a multicenter clinical trial. Am J Respir Crit Care Med 2020;202:681–9. 10.1164/rccm.201908-1546OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones PW, Quirk FH, Baveystock CM. The St George’s respiratory questionnaire. Respir Med 1991;85 Suppl B:25–31; 10.1016/s0954-6111(06)80166-6 [DOI] [PubMed] [Google Scholar]
- 19.Kon SSC, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD assessment test: a prospective analysis. Lancet Respir Med 2014;2:195–203. 10.1016/S2213-2600(14)70001-3 [DOI] [PubMed] [Google Scholar]
- 20.Wan ES, Castaldi PJ, Cho MH, et al. Epidemiology, Genetics, and Subtyping of preserved ratio impaired Spirometry (Prism) in Copdgene. Respir Res 2014;15:89. 10.1186/s12931-014-0089-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodruff PG, Couper D, Han MK. Clinical significance of symptoms in Smokers with preserved pulmonary function. N Engl J Med 2016;375:896–7. 10.1056/NEJMc1608235 [DOI] [PubMed] [Google Scholar]
- 22.Young KA, Strand MJ, Ragland MF, et al. n.d. Pulmonary subtypes exhibit differential global initiative for chronic obstructive lung disease Spirometry stage progression: the Copdgene® study. J COPD F;6:414–29. 10.15326/jcopdf.6.5.2019.0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones PW. George’s respiratory questionnaire: MCID. COPD: Journal of Chronic Obstructive Pulmonary Disease 2005;2:75–9. 10.1081/COPD-200050513 [DOI] [PubMed] [Google Scholar]
- 24.Kew KM, Dias S, Cates CJ. Long‐Acting inhaled therapy (Beta‐Agonists, Anticholinergics and steroids) for COPD: a network Meta‐Analysis. Cochrane Database Syst Rev 2014:CD010844. 10.1002/14651858.CD010844.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017;9:CD002309. 10.1002/14651858.CD002309.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Anci KE, Lynch MP, Leas BF, et al. Effectiveness and safety of bronchial Thermoplasty in management of asthma. In: Agency for Healthcare Research and Quality (AHRQ). 2017. Available: https://effectivehealthcare.ahrq.gov/topics/asthma-nonpharmacologic-treatment/thermoplasty-systematic-review [PubMed] [Google Scholar]
- 27.Bourbeau J. Activities of life: the COPD patient. COPD 2009;6:192–200. 10.1080/15412550902902638 [DOI] [PubMed] [Google Scholar]
- 28.Marchetti N, Criner GJ, Albert RK. Preventing acute exacerbations and hospital admissions in COPD. Chest 2013;143:1444–54. 10.1378/chest.12-1801 [DOI] [PubMed] [Google Scholar]
- 29.Sciurba FC, Criner GJ, Strange C, et al. Effect of Endobronchial coils vs usual care on exercise tolerance in patients with severe emphysema: the RENEW randomized clinical trial. JAMA 2016;315:2178–89. 10.1001/jama.2016.6261 [DOI] [PubMed] [Google Scholar]
- 30.Criner GJ, Sue R, Wright S, et al. A multicenter randomized controlled trial of Zephyr Endobronchial valve treatment in heterogeneous emphysema (LIBERATE). Am J Respir Crit Care Med 2018;198:1151–64. 10.1164/rccm.201803-0590OC [DOI] [PubMed] [Google Scholar]
- 31.Shah P, Slebos D-J, Cardoso P, et al. Bronchoscopic lung-volume reduction with exhale airway Stents for emphysema (EASE trial): randomised, sham-controlled, Multicentre trial. The Lancet 2011;378:997–1005. 10.1016/S0140-6736(11)61050-7 [DOI] [PubMed] [Google Scholar]
- 32.Coxson HO, Nasute Fauerbach PV, Storness-Bliss C, et al. Computed tomography assessment of lung volume changes after bronchial valve treatment. Eur Respir J 2008;32:1443–50. 10.1183/09031936.00056008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2023-001710supp001.pdf (942.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request.