Abstract
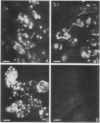
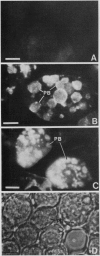
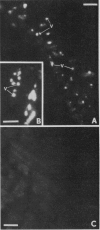
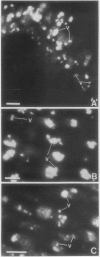
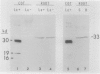
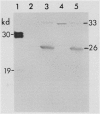
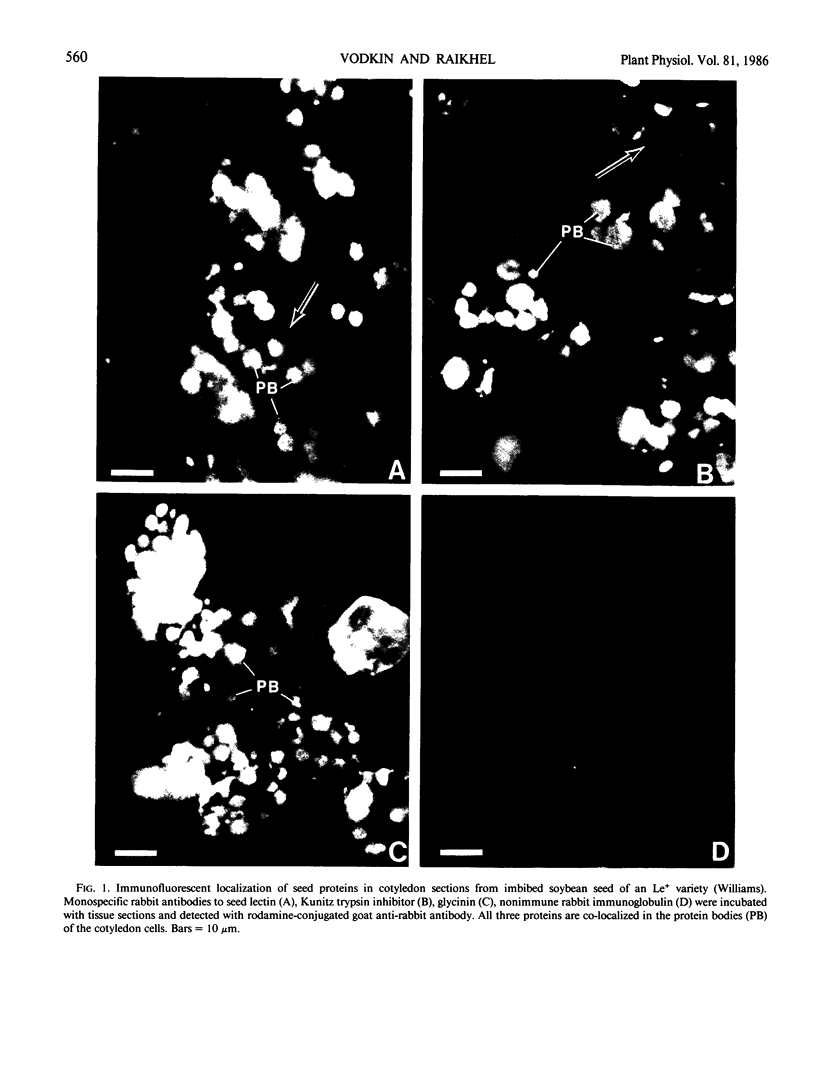
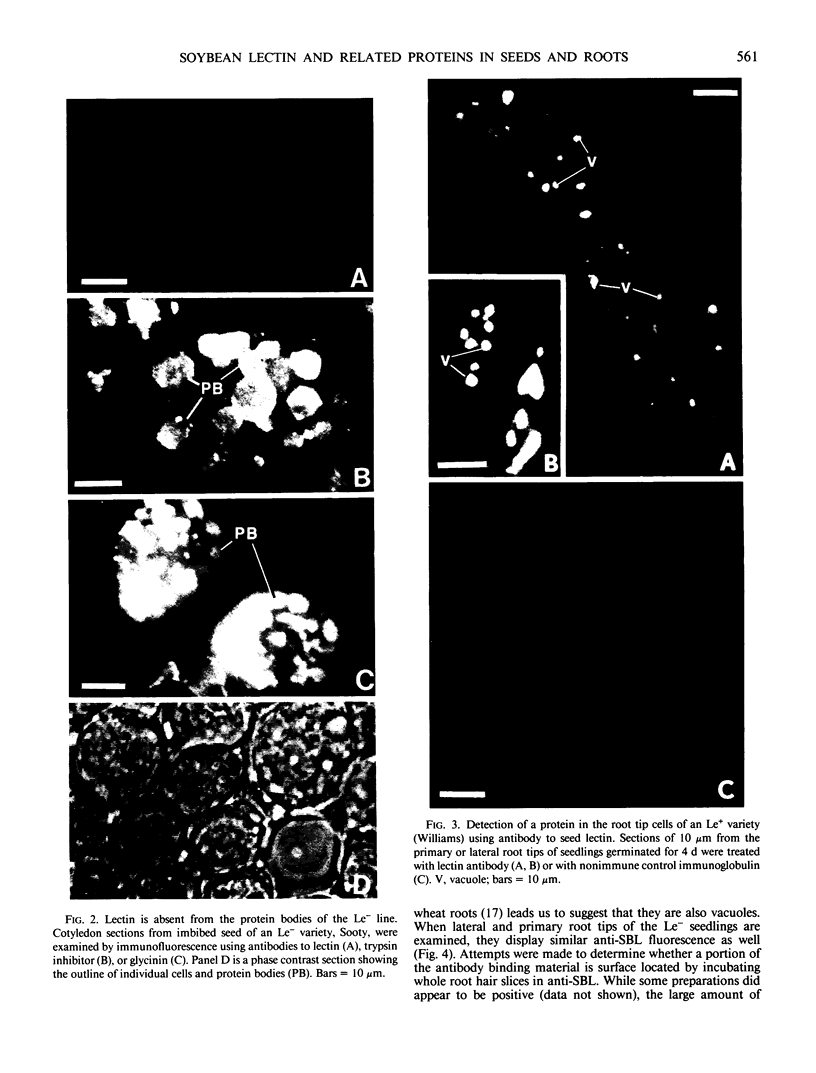
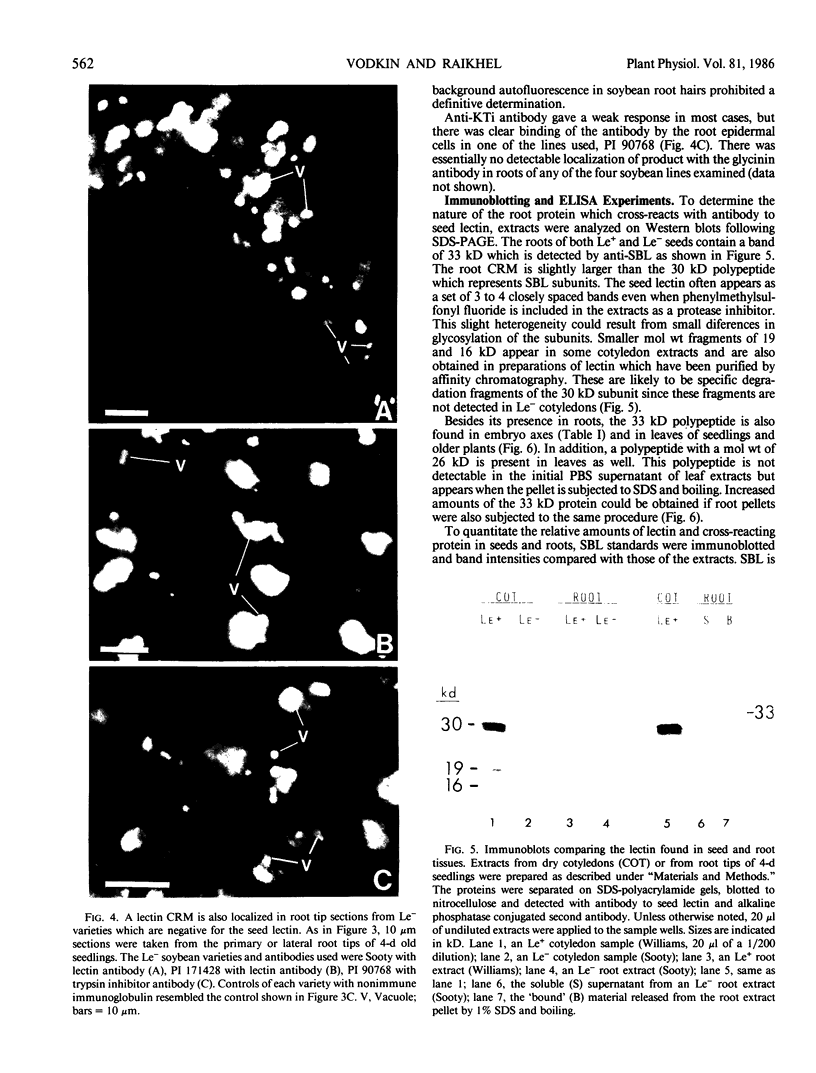
The localizations of soybean lectin (SBL) and antigenically related proteins in cotyledons and roots of lectin positive (Le+) and lectin negative (Le−) soybean cultivars were compared by light level immunocytochemistry using antibodies produced against the 120 kilodalton (kD) native seed lectin tetramer or its subunits. Lectin is present in the protein bodies of cotyledons cells as are two other seed proteins, the Kunitz trypsin inhibitor and the storage protein glycinin. Analysis of single seed extracts by immunoblotting of sodium dodecyl sulfate-polyacrylamide gels using the same antibodies, reveals up to 4 milligrams of the 30 kD seed lectin protein is present per seed in the Le+ varieties. There is no detectable lectin in the protein bodies of Le− cotyledons as determined by immunocytochemistry and immunoblotting. Enzyme-linked immunosorbent assay confirmed this result to a sensitivity of less than 20 nanograms per seed. In contrast, the roots of both Le+ and Le− plants bind the seed lectin antibody during immunocytochemistry, with fluorescence mainly localized in vacuole-like bodies in the epidermis. Root extracts contain a 33 kD polypeptide that binds anti-SBL antibody at an estimated minimal level of 20 nanograms per 4-day seedling, or 2.0 nanograms per primary root tip. This polypeptide is also present in the embryo axis and in leaves. The latter also contain a 26 kD species that binds seed lectin antibody. The 30 kD seed lectin subunit, however, is not detectable in roots or leaves.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrispeels M. J., Vitale A., Staswick P. Gene Expression and Synthesis of Phytohemagglutinin in the Embryonic Axes of Developing Phaseolus vulgaris Seeds. Plant Physiol. 1984 Nov;76(3):791–796. doi: 10.1104/pp.76.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campillo E., Shannon L. M. An alpha-Galactosidase with Hemagglutinin Properties from Soybean Seeds. Plant Physiol. 1982 Mar;69(3):628–631. doi: 10.1104/pp.69.3.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrink-Kurtzman M. A., Dick W. E., Jr, Burton K. A., Cadmus M. C., Slodki M. E. A soybean lectin having 4-O-methyl-D-glucuronic acid specificity. Biochem Biophys Res Commun. 1983 Mar 29;111(3):798–803. doi: 10.1016/0006-291x(83)91369-4. [DOI] [PubMed] [Google Scholar]
- Fett W. F., Sequeira L. A New Bacterial Agglutinin from Soybean: I. ISOLATION, PARTIAL PURIFICATION, AND CHARACTERIZATION. Plant Physiol. 1980 Nov;66(5):847–852. doi: 10.1104/pp.66.5.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gade W., Jack M. A., Dahl J. B., Schmidt E. L., Wold F. The isolation and characterization of a root lectin from soybean (Glycine max (L), cultivar Chippewa). J Biol Chem. 1981 Dec 25;256(24):12905–12910. [PubMed] [Google Scholar]
- Goldberg R. B., Hoschek G., Vodkin L. O. An insertion sequence blocks the expression of a soybean lectin gene. Cell. 1983 Jun;33(2):465–475. doi: 10.1016/0092-8674(83)90428-2. [DOI] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Host recognition in the Rhizobium-soybean symbiosis : evidence for the involvement of lectin in nodulation. Plant Physiol. 1985 Mar;77(3):621–625. doi: 10.1104/pp.77.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M., Vonlanthen M. Ultrastructural localization of soybean agglutinin on thin sections of Glycine max (soybean) var. Altona by the gold method. Histochemistry. 1980 Feb;65(2):181–186. doi: 10.1007/BF00493167. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lotan R., Siegelman H. W., Lis H., Sharon N. Subunit structure of soybean agglutinin. J Biol Chem. 1974 Feb 25;249(4):1219–1224. [PubMed] [Google Scholar]
- Mishkind M., Raikhel N. V., Palevitz B. A., Keegstra K. Immunocytochemical localization of wheat germ agglutinin in wheat. J Cell Biol. 1982 Mar;92(3):753–764. doi: 10.1083/jcb.92.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira M. A., Hermodson M. A., Larkins B. A., Nielsen N. C. Partial characterization of the acidic and basic polypeptides of glycinin. J Biol Chem. 1979 Oct 10;254(19):9921–9926. [PubMed] [Google Scholar]
- Pueppke S. G. Examination of Le and lele Genotypes of Glycine max (L.) Merr. for Membrane-Bound and Buffer-Soluble Soybean Lectin. Plant Physiol. 1981 Oct;68(4):905–909. doi: 10.1104/pp.68.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pull S. P., Pueppke S. G., Hymowitz T., Orf J. H. Soybean lines lacking the 120,000-dalton seed lectin. Science. 1978 Jun 16;200(4347):1277–1279. doi: 10.1126/science.200.4347.1277. [DOI] [PubMed] [Google Scholar]
- Su L. C., Pueppke S. G., Friedman H. P. Lectins and the soybean-Rhizobium symbiosis. I. Immunological investigations of soybean lines, the seeds of which have been reported to lack the 120 000 dalton soybean lectin. Biochim Biophys Acta. 1980 May 7;629(2):292–304. doi: 10.1016/0304-4165(80)90102-6. [DOI] [PubMed] [Google Scholar]
- Talbot C. F., Etzler M. E. Development and Distribution of Dolichos biflorus Lectin as Measured by Radioimmunoassay. Plant Physiol. 1978 May;61(5):847–850. doi: 10.1104/pp.61.5.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin L. O. Isolation and Characterization of Messenger RNAs for Seed Lectin and Kunitz Trypsin Inhibitor in Soybeans. Plant Physiol. 1981 Sep;68(3):766–771. doi: 10.1104/pp.68.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin L. O., Rhodes P. R., Goldberg R. B. cA lectin gene insertion has the structural features of a transposable element. Cell. 1983 Oct;34(3):1023–1031. doi: 10.1016/0092-8674(83)90560-3. [DOI] [PubMed] [Google Scholar]