Abstract
Intracellular accumulation of sucrose in response to lowered water activity seems to occur only in photosynthetic organisms. Here we demonstrate, for the first time, the potent ability of this common sugar, supplied exogenously, to reduce growth inhibition of Sinorhizobium meliloti cells in media of inhibitory osmolarity. Independently of the nature of the growth substrates and the osmotic agent, sucrose appears particularly efficient in promoting the recovery of cytoplasmic volume after plasmolysis. Surprisingly, sucrose is not accumulated by the bacteria at an osmotically efficient level. Instead, it strongly stimulates the accumulation of the main endogenous osmolytes glutamate and N-acetylglutaminylglutamine amide (NAGGN). Examining cell volume changes during the hyperosmotic treatment, we found a close correlation between the enhancement of the osmotically active solute pool and the increase in cell volume. Sucrose shares several features with ectoine, another nonaccumulated osmoprotectant for S. meliloti. Overall, osmoregulation in S. meliloti appears to be strongly divergent from that in most bacteria.
Water availability is crucial for the development of all living cells. Various physical and chemical parameters such as desiccation and hyperosmotic stress generate cellular dehydration. The mechanisms of cellular adaptation preventing water loss under hyperosmotic conditions (osmoregulation) have been extensively studied in bacteria, fungi, algae, plants, and animals (4, 6, 10, 13, 36, 37). A consensus strategy of osmoregulation leads to the intracellular accumulation of inorganic and/or organic low-molecular-weight compounds known as compatible solutes. As a rule, the working capacity of the metabolic machinery is limited by the ionic strength in the cytosolic medium. This latter must be maintained at a relatively low level. However, one exception is provided by the halophilic organisms, which must accumulate high cellular levels of inorganic ions to cope with extremely high salt concentrations in their environment (16).
For most osmotically stressed cells, turgor adjustment is achieved via the cytoplasmic accumulation of relatively few organic solutes: carbohydrates (sugars and polyols such as trehalose and glycerol), amino acids or imino acids (glutamate, proline, pipecolate, and ectoine), and betaines and their analogues (5, 9, 22). Most of these solutes behave as noncharged compounds at physiological pH and are highly soluble in aqueous solutions. Some of them have been demonstrated to protect cell macromolecular structures against the destabilizing effects of salts and urea (27, 37).
Compatible solutes are accumulated by de novo biosynthesis in many organisms subjected to an elevated osmolarity, and their intracellular content remains at a high level as long as the stressing conditions are maintained. After a sudden decrease in osmolarity or cell decay, accumulated compatible compounds may be liberated into the surrounding environment and subsequently taken up, via an active transport process, by other organisms under osmotic stress (5, 18, 20). Such organic compounds, taken up and accumulated by organisms unable to synthesize them de novo and able to improve growth under inhibitory osmolarities, are called osmoprotectants. Hence, in natural environments, the concept of osmoprotectant supposes an ecological cycle in which the compatible solutes are shuttled from producers to consumers injured by a sudden change in the osmotic strength of their medium. Glycine betaine (GB) provides substantial evidence supporting this concept. GB is synthesized by only a few organisms (mainly plants, algae, and cyanobacteria) (12, 28, 36) and is actively transported and accumulated as an osmoprotectant by a large variety of cells (6). It is generally accepted that an osmoprotectant must be accumulated durably within the cell to be effective. However, this concept, which was established on the basis of studies of members of the family Enterobacteriaceae and several gram-positive and gram-negative bacteria, is not entirely applicable to Sinorhizobium meliloti, which catabolizes most of the osmoprotectants known to date, including GB and ectoine (2, 34). Indeed, ectoine is a compatible solute that is synthesized de novo by many halotolerant bacteria (1, 9); it acts as a potent osmoprotectant for both Escherichia coli and S. meliloti but displays highly contrasting behaviors in these two bacteria: ectoine is accumulated at high intracellular concentrations in enteric bacteria but is never accumulated by stressed cells of S. meliloti (33). This intriguing observation led us to hypothesize that ectoine may belong to a new class of nonaccumulated osmoprotectants that are not detectable by the usual methods, such as natural-abundance 13C nuclear magnetic resonance (NMR) spectroscopy.
Previous work demonstrated that high concentrations of sucrose were much less effective than iso-osmotic concentrations of NaCl in inhibiting the growth of S. meliloti (25). In the present report, we provide evidence that sucrose acts as a powerful osmoprotectant for S. meliloti in media of inhibitory osmolarity.
MATERIALS AND METHODS
Bacterial strains and media.
The following rhizobial strains were used in this study: S. meliloti wild-type strains 102F34 (this laboratory), M5N1, and SU47 (both provided by J. N. Barbotin) and Bradyrhizobium japonicum USDA 110spc4, Rhizobium leguminosarum bv. phaseoli H132, R. leguminosarum bv. viciae ATCC 10006, S. fredii USDA 205T, and Mesorhizobium huakuii CCBAU 2609T (which were provided by D. Le Rudulier). Cells were grown aerobically at 30°C in MSY medium (21) to mid-exponential phase (optical density at 570 nm [OD570] of 1), harvested by centrifugation (5,000 × g, 10 min), washed once with minimal salt medium S (26), concentrated at an OD570 of 10 in the same minimal medium, and inoculated, at a final OD570 of 0.1, in S medium supplied with carbon and nitrogen sources at a final concentration of 10 mM each. The standard minimal medium (LAS) contains dl-lactate and l-aspartate as carbon and nitrogen sources, respectively. The protein content of the culture was determined by the method of Lowry et al. (17), with serum albumin as the standard.
Cryoelectron microscopy.
Copper grids were overlaid with a carbon film freshly glow discharged; 5 μl of bacterial culture was allowed to adhere to the grid for 1 min and blotted with filter paper. The grid was quickly immersed into liquid ethane and transferred to a Gatan cryoholder in a Philips CM12 electron microscope operating at 100 kV. The sample was maintained below −174°C throughout the observation.
Cell volume measurements.
Cells were harvested in the exponential phase of growth. Cell volume measurements were performed on digitized electron images, using the MACS program (29). Briefly, in cryoelectron microscopy, cells are embedded into a thin film of ice and then oriented parallel to the grid plan. Thus, in the projected image, both length and width are available. For each cell, the area and volume of the total cell and the cytoplasm were calculated.
Cell volume was also determined as described by Stock et al. (31) by measuring the differential retention of 3H2O (400 MBq/ml; Amersham, Les Ulis, France) and [carboxyl-14C]inulin (308 MBq/mmol; Amersham). Bacterial cells were collected by centrifugation and concentrated at an OD570 of 10 in their growth medium. [14C]inulin (2 × 105 dpm) and 3H2O (2 × 106 dpm) were added to 500 μl of this suspension. Cells were allowed to equilibrate for 30 min at 30°C prior to centrifugation (12,000 × g, 5 min). [14C] and [3H] from the supernatant and the pellet were simultaneously determined by liquid scintillation counting by using the dual dpm mode of a Packard Tri-Carb 1600-TR spectrometer.
Extraction of cellular solutes.
The pellet of freshly harvested cells was washed with S isotonic medium and extracted at least twice with 80% (vol/vol) ethanol–water under vigorous magnetic stirring at room temperature for 30 min. After centrifugation (8,000 × g, 15 min), the supernatants constituting the ethanol-soluble fraction (ESF) were pooled and evaporated to dryness at 40°C, and the dry residue was dissolved in deionized water. The pellet, called the ethanol-insoluble fraction (EIF), contained the intracellular macromolecules and cell envelopes.
NMR spectroscopy.
To identify the major intracellular compounds, the ESF extracted from about 2 × 1012 cells (350 mg of protein) was evaporated to dryness and dissolved in 1 ml of D2O. The natural-abundance 13C NMR spectra were recorded in the pulsed Fourier transform mode at an operational frequency of 75.4 MHz as previously described (34).
Chromatographic and quantitative analysis of endogenous osmolytes.
The N-acetylglutaminylglutamine amide (NAGGN) content was determined after passage of the ESF through a cation-exchange column (Bio-Rad AG 50 × 8, H+ form). NAGGN was converted into glutamate by chemical hydrolysis with 9 M KOH (110°C, 14 h). K+ ions were removed by titrating the hydrolyzed solution with 13 M perchloric acid to pH 9. Glutamate and trehalose were then quantified as already described (11, 33).
Uptake assays.
Cells grown in LAS medium with or without 0.5 M NaCl and with or without 0.5 mM sucrose were harvested by centrifugation, washed twice with isotonic S medium deprived of sucrose, and then concentrated to an OD570 of 1 in isotonic growth medium. Uptake experiments were performed as previously reported (33). [U-14C]sucrose (23.3 GBq/mmol; Amersham) was used at a final concentration of 500 μM in 400 μl of bacterial suspension.
Radiolabelling assays.
Cells were grown in LAS without or with 0.5 M NaCl or 0.5 mM sucrose or both. [14C]sucrose was introduced in the growth medium at a specific radioactivity of 0.2 MBq/mmol. At predetermined intervals, the OD570 of the culture was measured and 1 to 2 ml of cell suspension was harvested by centrifugation (12,000 × g, 2 min). The pellets were immediately extracted with 80% ethanol as described above. CO2 was trapped on a strip of filter paper (0.5 by 3 cm) moistened with 30 μl of 5 M KOH. This filter was changed at each sampling time. The radioactivity of subsamples of the ESF, the EIF, and CO2 was measured by scintillation counting. The remainder of the ESF and EIF were analyzed for composition. The ESF was analyzed by paper chromatography and high-voltage paper electrophoresis (2). To determine the nature and the amount of the labelled compounds, the chromatograms and electrophoregrams were analyzed by autoradiography. Identified spots were cut out, and their radioactivity was quantified by scintillation counting. The EIF was treated with 9 M KOH at 110°C for 20 h, neutralized with perchloric acid, and centrifuged (12,000 × g, 2 min). The supernatant was then analyzed by the same methods as used for the ESF.
RESULTS
Sucrose improves the growth of S. meliloti 102F34 in media of high osmolarity.
Sucrose is usually used as a nonionic osmotic agent to increase the osmotic strength of bacterial growth media (6). A solution of 0.84 M sucrose develops the same osmotic pressure as a solution with 0.65 M NaCl. Nevertheless, growth inhibition of S. meliloti 102F34 by iso-osmotic concentrations of these two solutes differed remarkably (Table 1). NaCl strongly reduced the growth rate and growth yield (9- and 6-fold, respectively), whereas sucrose reduced the growth rate only 2.4-fold; 1 mM GB, which is a powerful osmoprotectant for S. meliloti (2), partially reversed growth inhibition by NaCl but was unable to improve the growth of sucrose-stressed S. meliloti cells (Table 1). In addition, the growth parameters observed in LAS medium containing 0.65 M NaCl plus 1 mM GB were similar to those obtained for cultures grown in LAS medium containing 0.84 M sucrose, with or without 1 mM GB. Hence, the apparently weak osmotic effect of sucrose, compared to that of NaCl, could result from a concomitant osmoprotective effect displayed by this sugar. This might explain the observation that addition of a known osmoprotective solute, such as GB, to a sucrose-stressed culture did not improve sinorhizobial growth. To confirm this hypothesis, GB was used as an osmotic agent at a concentration of 0.9 M, which is iso-osmotic with 0.65 M NaCl. As expected, the growth parameters of S. meliloti 102F34 in LAS with 0.9 M GB were similar to those observed with 0.84 M sucrose. In contrast, when 0.9 M mannitol was used to increase the osmotic strength of the medium, the growth rate was inhibited by a factor of 5, and the addition of 1 mM GB significantly reduced growth inhibition caused by mannitol (Table 1). All of these data suggest that sucrose may act as an osmoprotectant for S. meliloti.
TABLE 1.
Comparative effects of various osmotic agents on the growth of S. meliloti 102F34
| Osmotic agent addeda | Growth parameter in LAS medium containingb:
|
|||
|---|---|---|---|---|
| No osmoprotectant
|
1 mM GB
|
|||
| μ | ODmax | μ | ODmax | |
| None | 0.200 | 1.9 | 0.200 | 2.0 |
| 0.65 M NaCl | 0.022 | 0.3 | 0.076 | 1.9 |
| 0.84 M sucrose | 0.083 | 2.1 | 0.083 | 2.1 |
| 0.9 M GB | 0.083 | 1.7 | NAc | NA |
| 0.9 M mannitol | 0.040 | 0.9 | 0.083 | 2.1 |
Cultures were grown in LAS medium containing the indicated osmoticum agents, all added at concentrations which developed an osmotic pressure of 3.14 MPa.
Growth parameters are expressed as the growth rate (μ, in generations/hour) and the maximal OD570 (ODmax) at the stationary phase.
NA, not applicable.
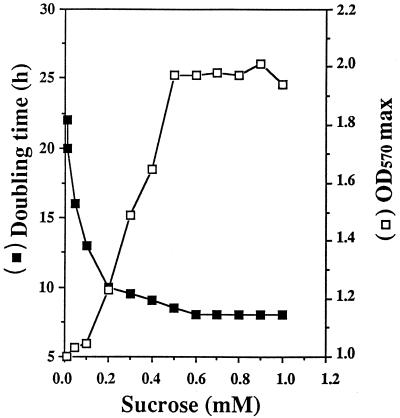
The putative osmoprotective activity of sucrose was assayed in LAS medium, with or without 0.5 M NaCl. Low concentrations of sucrose (1 μM to 1 mM) were used to minimize the utilization of this sugar as a carbon source (32). Sucrose added at these concentrations did not modify the growth parameters of unstressed cultures. In contrast, in media containing 0.5 M NaCl, the generation time and growth yield were improved for sucrose concentrations as low as 50 μM (Fig. 1). The greatest beneficial effect was reached with 0.5 mM sucrose (Fig. 1). Hence, sucrose was used at 0.5 mM for further experiments.
FIG. 1.
Effect of increasing concentrations of exogenous sucrose on the doubling time (in hours/generation) and the maximal cell yield (OD570 max) of stressed cultures of S. meliloti 102F34. Cells were inoculated in LAS minimal medium containing 0.5 M NaCl plus the indicated concentrations of sucrose.
KCl, K2SO4, and mannitol were added to LAS medium at the appropriate concentrations to obtain an osmolarity equivalent to that developed by 0.5 M NaCl. These compounds, like NaCl, impaired the proliferation of S. meliloti 102F34 (Table 2). The incorporation of 0.5 mM sucrose to these media also led to increases in both growth rates and growth yields (two- to threefold increases for KCl-, K2SO4-, and mannitol-stressed cultures [Table 2]). In contrast, sinorhizobial growth was not inhibited by the addition of glycerol, used as a control because it reduces water activity but does not cause osmotic stress; this compound diffuses freely across biological membranes (5). Sucrose had no effects when it was added to LAS with 0.9 M glycerol. Thus, sucrose specifically relieved osmotic stress rather than ionic stress.
TABLE 2.
Alleviation of osmotic stress by exogenous sucrose in S. meliloti 102F34
| Osmotic agent addeda | Growth parameter in LAS medium containingb:
|
|||
|---|---|---|---|---|
| No addition
|
0.5 mM sucrose
|
|||
| μ | ODmax | μ | ODmax | |
| None | 0.200 | 1.9 | 0.200 | 1.9 |
| 0.5 M NaCl | 0.051 | 0.9 | 0.120 | 1.9 |
| 0.5 M KCl | 0.042 | 0.8 | 0.105 | 1.7 |
| 0.45 M K2SO4 | 0.020 | 0.4 | 0.060 | 1.2 |
| 0.8 M mannitol | 0.060 | 1.1 | 0.100 | 1.8 |
| 0.8 M glycerol | 0.250 | 2.1 | 0.250 | 2.1 |
Each was added to LAS medium at a concentration which developed the same osmotic pressure as 0.5 M NaCl.
See Table 1, footnote b.
The improvement of cell growth by sucrose under high-osmolality conditions was also observed when S. meliloti was grown with 0.5 M NaCl in S minimal medium containing urea, ammonium, or glutamate as the nitrogen source and containing glucose, fructose, mannitol, succinate, malate, fumarate, or glycerol as the carbon source. Thus, sucrose acted in the same way, whatever the nitrogen and carbon sources used in the medium. Moreover, its osmoprotective effect did not result from its extracellular cleavage since its two constitutive hexoses, glucose and fructose, supplied separately or in combination at concentrations ranging from 1 μM to 10 mM, did not allow for any growth improvement (data not shown).
Because the beneficial effect of sucrose was observed at low concentrations of this disaccharide only when cells were subjected to an hyperosmotic stress, and independently of the medium composition, sucrose could be considered an osmoprotectant for S. meliloti 102F34.
Sucrose allows stressed cells to recover a normal cytoplasmic volume.
The cell volume of S. meliloti 102F34 grown in stressed or unstressed LAS minimal medium, with or without 0.5 mM sucrose, was calculated by the radioisotopic technique of Stock et al. (31). A total mean cell volume of 0.55 ± 0.07 10−9 μl/cell was obtained whatever the osmotic strength of the growth medium, in the presence or absence of sucrose. However, the size of the periplasmic space of these cells could not be determined because a suitable radioactive compound allowing the size of this compartment of S. meliloti to be determined was not available.
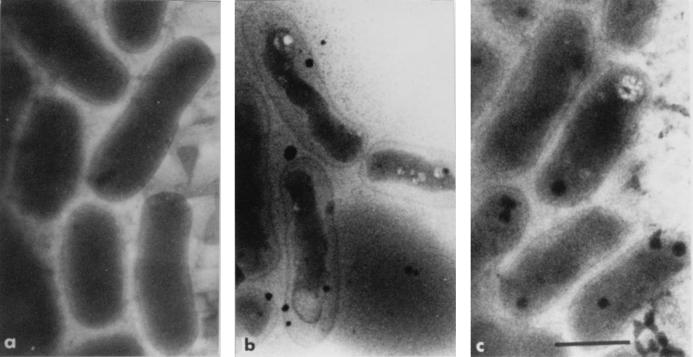
Cryoelectron microscopy offers the unique possibility of observing bacteria in their native state without the use of any fixative agent, dehydration, or embedding procedures which would alter the osmotic equilibrium of the cells (29). Therefore, this technique was used to measure the dimensions and calculate the volumes of the periplasmic space and the cytoplasm of S. meliloti 102F34 cells. Figure 2a shows that the cytoplasmic compartment of unstressed cells was pressed against the cell wall. The total volume of these cells was 0.57 ± 0.02 10−9 μl/cell (n = 90), and the volume of the periplasmic space was 0.12 ± 0.05 10−9 μl/cell. The mean cell volumes of stressed (0.5 M NaCl) bacteria grown with and without 0.5 mM sucrose were quite similar, 0.58 ± 0.02 10−9 (n = 75) and 0.56 ± 0.02 10−9 (n = 57) μl/cell, respectively. However, the cytoplasmic volume of stressed cells grown without added sucrose was drastically reduced; therefore, the periplasmic space of these cells increased to 0.35 ± 0.01 10−9 μl/cell (Fig. 2b). In contrast, the periplasmic space of stressed cells grown in the presence of 0.5 mM sucrose was quite similar to that observed for unstressed cells (Fig. 2a and c).
FIG. 2.
Restoration of cell turgor in S. meliloti 102F34 grown under osmotic stress in the presence of exogenous sucrose. Cultures were grown for 16 h in LAS minimal medium (a), in LAS plus 0.5 M NaCl (b), and in LAS supplemented with 0.5 M NaCl and 0.5 mM sucrose (c). Samples were processed for cryoelectron microscopy as described in Materials and Methods. Scale bar correspond to 1 μm.
External sucrose is not accumulated in S. meliloti.
Most cells attempt to increase their cytoplasmic volume by regulating the concentrations of intracellular osmolytes. Previous studies (30, 33, 34) showed that S. meliloti, in response to an osmotic upshift, synthesizes de novo three major osmolytes, glutamate, NAGGN, and trehalose, which is significant only during the stationary phase of growth.
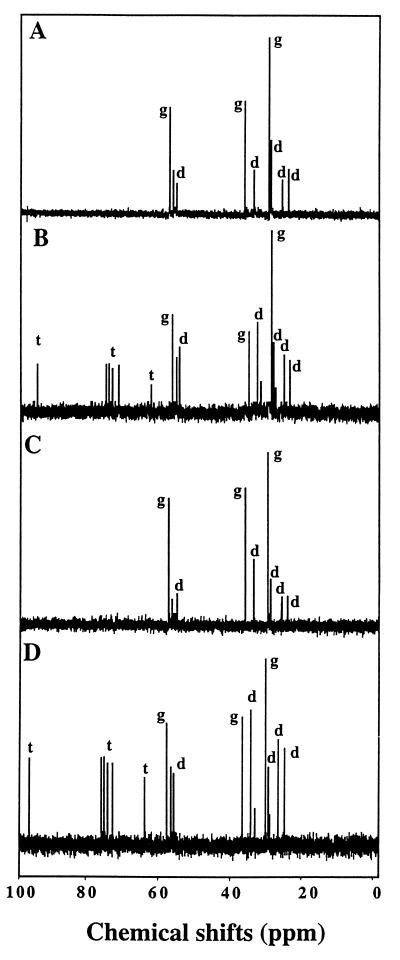
Osmolytes accumulated by S. meliloti grown in low- or high-osmolarity medium, in the presence or absence of 0.5 mM sucrose, were characterized by natural-abundance 13C NMR spectroscopy. No major solute was detected in cells cultivated in LAS medium deprived of NaCl. The presence of 0.5 M NaCl in the medium induced the accumulation of glutamate and NAGGN during exponential growth, whereas trehalose content started to increase in the late exponential growth phase (Fig. 3A and B). An identical spectrum was obtained from cells cultivated in LAS medium with 0.5 M NaCl and 0.5 mM sucrose (Fig. 3C and D). None of the peaks attributable to sucrose was observed. Thus, sucrose appears as a solute that is not accumulated in osmotically significant amounts by stressed cells of S. meliloti 102F34.
FIG. 3.
Natural-abundance 13C NMR of extracts of salt-stressed cultures of S. meliloti 102F34. Cells were grown in LAS medium containing 0.5 M NaCl without (A and B) or with (C and D) 0.5 mM sucrose. Samples were harvested in the early (A and C) and late (B and D) log phases of growth and processed as described in the text. All spectra (1,024 scans) were obtained from 2 × 1012 cells (2,000 OD570 units). Resonance (peaks) from l-glutamate (g), the dipeptide NAGGN (d), and trehalose (t) are indicated when these osmolytes were detected in the cytosolic extracts.
However, 13C NMR spectroscopy does not allow for the detection of solutes that are present in low cellular concentrations. To follow the behavior of sucrose, the 14C-labelled molecule was supplied (0.5 mM) to stressed or unstressed cultures of S. meliloti (Fig. 4). Extracellular sucrose was readily taken up at a constant velocity of 33 nmol/min/mg of protein in the low-osmolarity medium. About 80% of the supplied [14C]sucrose was transported in 10 h. At this time, 75% of the imported radiocarbon was incorporated into insoluble materials (the EIF), whereas 15% was released as [14C]CO2 and only 10% remained in the soluble pool (the ESF). Interestingly, no radiolabelled sucrose was detected in the ESF fraction, and the radiocarbon was distributed over several primary metabolites. The radioactivity of the EIF and the ESF started to decrease as soon as the external sucrose disappeared from the medium (Fig. 4A).
FIG. 4.
Fate of [14C]sucrose in S. meliloti 102F34. [U-14C]sucrose (0.5 mM, 0.2 MBq/mmol) was supplied to unstressed cells grown in LAS medium (A) and stressed cells grown in LAS plus 0.5 M NaCl (B). The distribution of the radioactivity in the growth medium and the cellular fractions was monitored as described in Materials and Methods. Symbols: □, growth expressed as OD570; ■, radioactivity in the medium; ⧫, radioactivity in 14CO2; •, radioactivity in the EIF; ▴, radioactivity in the ESF.
The stressed cultures grown in the presence of 0.5 M NaCl, supplied with 0.5 mM [14C]sucrose, incorporated the labelled disaccharide at an initial velocity of only 3.7 nmol/min/mg of protein (which is ninefold lower than that observed in cells grown at low osmolarity) during the first 6 h following the osmotic upshift. Then the rate of the radiocarbon influx increased steadily, reaching 5.4, 16, and 34 nmol/min/mg of protein 10, 15, and 20 h, respectively, after inoculation. Thus, the rate of sucrose uptake, which was severely inhibited during the lag phase, was progressively restored to the level observed in unstressed cells (Fig. 4B). The imported radiocarbon was again found primarily in the insoluble materials. Indeed, 50% of the radioactivity that was incorporated during the first 10 h was recovered in the EIF and 70% was recovered in the mid-exponential growth phase, whereas the level decreased as the external [14C]sucrose became limiting. The soluble pool never exceeded a maximum of 20% of the total imported radiocarbon. Interestingly, chromatographic analysis of this fraction enabled us to detect radiolabelled sucrose, which represented about 45% of the ESF radiocarbon during the lag phase; then, the sucrose ratio of the ESF decreased at the beginning of the exponential phase. Only traces of [14C]glutamate, [14C]NAGGN, and [14C]trehalose were detected. The incorporation of radioactivity in CO2 was reduced, suggesting that sucrose was not used as a preferential energetic substrate.
In summary, the osmotic upshift occurring at inoculation of S. meliloti in high-osmolarity LAS led to a lag phase of growth during which sucrose was accumulated temporarily at low levels. The major part of sucrose-derived radiocarbon was detected in insoluble materials, whereas only a low amount of radioactivity was found in the dominant osmolytes (i.e., glutamate, NAGGN, and trehalose) that were revealed by NMR analysis. Moreover, the stimulation of sucrose uptake and catabolism corresponded with the resumption of growth of the stressed cells.
Kinetics of endogenous osmolyte pool.
The amounts of intracellular osmolytes were determined during the growth cycle of S. meliloti cultivated in LAS medium deprived of NaCl. As previously described (33, 34), the levels of intracellular glutamate, NAGGN, and trehalose remained very low (below 0.05 μmol/mg of protein).
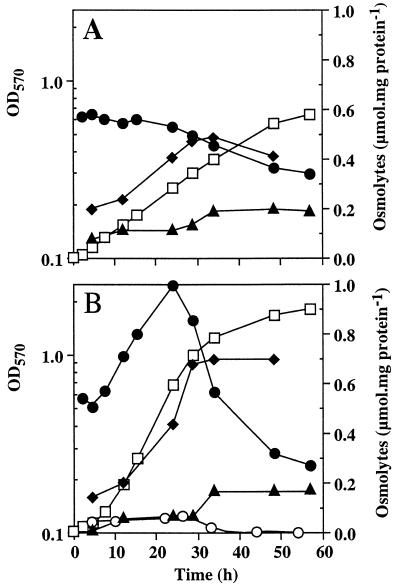
Two hours after the application of the osmotic upshift (0.5 M NaCl), the intracellular glutamate level had reached 0.55 μmol/mg of protein, a value about 10 times higher than the value measured in unstressed cells. This level remained constant during 24 h and then decreased progressively (Fig. 5A). In contrast, the level of NAGGN increased progressively throughout the growth period, reaching a maximum of 0.5 μmol/mg of protein after 30 h of growth. Trehalose remained at low levels (about 0.1 μmol/mg of protein) during the exponential growth and began to increase as glutamate and NAGGN decreased.
FIG. 5.
Effect of exogenous sucrose on the internal osmolyte composition of salt-stressed S. meliloti 102F34. Cells were grown in LAS medium containing 0.5 M NaCl without (A) or with (B) 0.5 mM sucrose supplied as an osmoprotectant. Endogenous osmolytes were quantified as described in the text. Symbols: □, growth expressed as OD570; •, glutamate; ⧫, NAGGN; ▴, trehalose; ○, sucrose.
The osmolyte levels in a stressed culture grown in the presence of 0.5 mM sucrose were also determined (Fig. 5B). The profile of osmolytes accumulation of these cells was remarkably different from that observed in a stressed culture grown without sucrose (Fig. 5A). Indeed, glutamate levels (which were similar in the two cultures for 8 h after inoculation) increased sharply during the exponential growth in the sucrose-supplemented culture (Fig. 5B). Consequently, the maximal level of this amino acid was about twofold higher in this culture (about 1 μmol/mg of protein in the late exponential phase) than in the stressed culture grown without sucrose. Furthermore, during the late exponential growth phase, cytoplasmic glutamate decreased faster in the sucrose-supplemented culture than in the culture grown without sucrose (Fig. 5). Interestingly, the level of cytosolic NAGGN also increased sharply (about sixfold) during the exponential phase of the culture grown with 0.5 mM sucrose, but NAGGN, in contrast to glutamate, did not decrease thereafter (Fig. 5B). The trehalose content was very similar in the stressed cultures grown with or without sucrose. It reached a steady-state level of about 0.2 μmol/mg of protein during the stationary phase (Fig. 5). The intracellular sucrose level was very similar to that of trehalose for about 24 h in stressed cells grown in the presence of 0.5 mM sucrose. Later, cytosolic sucrose (which never exceeded 75 nmol/mg of protein) decreased and became negligible (Fig. 5B).
To determine whether the cytosolic solutes described above played a part in osmotic adjustment, it was necessary to measure their specific cytoplasmic concentrations. Taking into account the different cytoplasmic volumes of the stressed cells grown with or without 0.5 mM sucrose (Fig. 2), the concentrations of the two major endogenous osmolytes were 187 mM for glutamate and 120 mM for NAGGN in stressed cells grown without sucrose and 247 mM for glutamate and 107 mM for NAGGN when 0.5 mM sucrose was added. The cytoplasmic concentration of sucrose was always much lower than that of glutamate and NAGGN and reached a maximal concentration of 21 mM, which explains why sucrose signals were never observed in the NMR spectra.
In conclusion, sucrose is not accumulated to osmotically significant levels in salt-stressed cells of S. meliloti. Consequently, this highly beneficial osmoprotectant cannot be considered a true compatible solute in this species. Moreover, this disaccharide contributes significantly to enhance glutamate and NAGGN levels in stressed cells and subsequently to promote cytoplasmic expansion and turgor recovery.
Effect of sucrose on other rhizobial strains.
To determine whether osmoprotection by sucrose was specific to S. meliloti 102F34, the osmoprotective activity of this compound was evaluated in several other strains of rhizobia. Growth rates and growth yields were also improved in NaCl-containing media in the presence of 0.5 mM sucrose for S. meliloti wild-type strains SU47 and M5N1 and for R. leguminosarum bv. phaseoli H132 (Table 3). In contrast, no beneficial effect of sucrose was observed for R. leguminosarum bv. viciae, S. fredii USDA 205T, M. huakuii CCBAU 2609T, and B. japonicum USDA 110spc4 (data not shown). The uptake and the fate of [14C]sucrose in S. meliloti strains SU47 and M5N1 grown in NaCl-containing medium were the same as those observed above for strain 102F34; i.e., sucrose was never accumulated in significant amounts and sucrose-derived radiocarbon was recovered essentially in the EIF fraction (Table 4). Sucrose uptake in R. leguminosarum bv. phaseoli was stimulated by NaCl, and most of the radiocarbon from [14C]sucrose was incorporated into CO2 but not into the EIF as observed in S. meliloti (Table 4). In all of these strains, the ESF represented the minor labelled fraction, which demonstrates that sucrose was not accumulated to significant levels.
TABLE 3.
Beneficial effect of exogenous sucrose on the growth of salt-stressed cultures of various rhizobial strainsa
| Strain | Growth parameter in minimal mediumb
|
|||||
|---|---|---|---|---|---|---|
| Without NaClc
|
With NaCld
|
|||||
| Without sucrose
|
With sucrose
|
|||||
| μ | ODmax | μ | ODmax | μ | ODmax | |
| S. meliloti 102F34 | 0.200 | 1.9 | 0.052 | 0.9 | 0.125 | 1.9 |
| S. meliloti SU47 | 0.400 | 3.0 | 0.125 | 1.5 | 0.333 | 3.0 |
| S. meliloti M5N1 | 0.100 | 2.7 | 0.059 | 0.7 | 0.143 | 2.9 |
| R. leguminosarum bv. phaseoli H132 | 0.111 | 1.0 | 0.050 | 0.5 | 0.091 | 1.0 |
Bacteria were grown in LAS minimal medium with the indicated additions.
See Table 1, footnote b.
Similar growth rates and yields were observed in unstressed cultures supplied with 0.5 mM sucrose.
The NaCl concentrations used were 0.5 and 0.3 M for S. meliloti and R. leguminosarum bv. phaseoli H132, respectively.
TABLE 4.
Fate of [U-14C]sucrose in various rhizobial speciesa
| Fraction | Distribution of 14C from [14C]sucrose in indicated fraction from control and salt-stressed cultures (% of total activity recovered)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
S. meliloti SU47
|
S. meliloti M5N1
|
R. leguminosarum bv. phaseoli H132
|
R. leguminosarum bv. viciae ATCC 10006
|
S. fredii USDA 205T
|
M. huakuii CCBAU 2609T
|
B. japonicum USDA 110 spc4
|
||||||||
| −NaCl | +NaCl | −NaCl | +NaCl | −NaCl | +NaCl | −NaCl | +NaCl | −NaCl | +NaCl | −NaCl | +NaCl | −NaCl | +NaCl | |
| Medium | 9 | 5 | 5 | 8 | 31 | 9 | 35 | 81 | 43 | 96 | 83 | 98 | 97 | 97 |
| 14CO2 | 25 | 22 | 15 | 18 | 32 | 45 | 39 | 12 | 36 | 3 | 7 | 2 | 2 | 2 |
| EIF | 60 | 56 | 75 | 71 | 30 | 37 | 23 | 5 | 18 | 0 | 9 | 0 | 0 | 0 |
| ESF | 6 | 17 | 5 | 2 | 6 | 9 | 4 | 2 | 3 | 1 | 1 | 0 | 1 | 1 |
Bacteria were grown for 24 h in S medium supplied with mannitol and aspartate. The fate of [U-14C]sucrose (0.5 mM, 23.3 GBq/mmol) was followed in the absence of NaCl (−NaCl) or in the presence of NaCl (+NaCl). Salt concentration was adjusted to allow 80% inhibition of growth of S. meliloti (0.5 M NaCl), R. leguminosarum bv. phaseoli, R. leguminosarum bv. viciae, S. fredii, and M. huakuii (0.3 M NaCl), and B. japonicum (0.15 M NaCl).
In sharp contrast, sucrose uptake and its fate appeared to be clearly different in strains that were not osmoprotected by sucrose. Salt-stressed cultures of M. huakuii CCBAU 2609T were unable to transport sucrose. Moreover, B. japonicum USDA 110spc4 did not take up [14C]sucrose in either low- or high-osmolarity medium (Table 4). This result is consistent with the nonutilization of sucrose as a carbon source by this strain (32). Last, the uptake and catabolism of sucrose were abolished or strongly reduced by an increase in medium osmolarity for S. fredii USDA 205T and R. leguminosarum bv. viciae ATCC 10006, respectively (Table 4).
DISCUSSION
In this study, we provide evidence that exogenously supplied sucrose acts as an osmoprotectant in stressed cultures of S. meliloti, the root symbiont of the plant crop alfalfa. First, we showed that a submolar concentration of sucrose (0.84 M) only weakly inhibits the growth rate of sinorhizobial cultures in comparison to iso-osmotic concentrations of inorganic salts and mannitol, which were highly inhibitory. Second, micromolar levels of exogenous sucrose (50 to 500 μM) strongly stimulate the growth of salt-stressed and mannitol-stressed cultures of S. meliloti 102F34. Moreover, sucrose appears to be as effective as GB in promoting the growth of S. meliloti under an hyperosmotic constraint. To the best of our knowledge, this is the first report demonstrating the pivotal role of sucrose in the alleviation of osmotic inhibition of growth in a nonphotosynthetic bacterium.
A few examples of sucrose accumulation in response to water and salt stresses have been reported for desiccation-tolerant (3) and salt-stressed (24) plants and for photosynthetic eubacteria (12, 19, 28, 35) in which sucrose is accumulated by de novo biosynthesis. In contrast, exogenously supplied sucrose is not accumulated as a compatible solute in osmotically stressed cells of S. meliloti but is actively catabolized. Moreover, its intracellular concentration did not reach levels compatible with its direct participation in the recovery of cell turgor and in the protection of intracellular macromolecular structures against the deleterious effects of high osmolarities. In contrast to the situation observed with other sinorhizobial and bacterial osmoprotectants (i.e., stimulated uptake in stressed cells [2, 6, 33]), the uptake of sucrose was strongly reduced by an osmotic upshift and started to increase only when cultures entered the exponential phase of growth. All of these features clearly indicate that sucrose, while allowing for growth improvement in adverse conditions, cannot be considered a classical osmoprotectant. Indeed, sucrose is neither, like choline (5), a precursor of an accumulated osmoprotectant nor preferentially used to synthesize any endogenous osmolyte, as observed with proline in Brevibacterium linens (14). Moreover, the behavior of sucrose is very closely related to that previously reported for ectoine in S. meliloti (33), because (i) both sucrose and ectoine induce osmoprotection without being accumulated at significant levels, (ii) no metabolites derived from these osmoprotectants were accumulated, and (iii) sucrose and ectoine (unlike GB and dimethylsulfoniopropionate [DMSP] [23, 34]) do not suppress the accumulation of the major endogenous compatible osmolytes (glutamate, NAGGN, and trehalose). Another interesting observation is the spectacular increase in the level of cytosolic glutamate during the initial steps preceding the resumption of growth. This increase seems to be the initial event that triggers the recovery of a maximal cytoplasmic volume. We do not know how sucrose and ectoine stimulate the accumulation of glutamate in stressed cells of S. meliloti. However, these osmoprotectants did not act as close precursors to glutamate. A significant correlation was observed among rhizobia between osmoprotection by sucrose and its metabolization under stressing conditions, suggesting that these two properties are linked. The same observation has been previously described for ectoine (33).
Until now, this kind of cell strategy, permitting the achievement of osmotic equilibrium without preferential accumulation of the supplied osmoprotectant, has not been reported for any other organism. Among the rhizobial strains analyzed, the beneficial effect of sucrose was observed only for S. meliloti strains and R. leguminosarum bv. phaseoli. This finding raises the question of the acquisition of this specific pathway of osmoprotection during evolution. One of the obvious differences between S. meliloti and the enteric bacteria is its ability to catabolize all of the compounds that it uses as exogenous osmoprotectants except DMSP (23). This catabolic ability is shared with various other bacteria such as the pseudomonads (15, 20), but no one has yet demonstrated a protective effect without preferential accumulation of an osmoprotectant in these organisms.
Osmoprotection without accumulation of the osmoprotectant is not exclusive of the classical phenomenon; in S. meliloti, both may coexist. Accumulated osmoprotectants, like GB and DMSP, inhibit the de novo synthesis of endogenous solutes (7, 23, 34), ensuring a metabolic economy for the cell. In contrast, nonaccumulated osmoprotectants (sucrose and ectoine) stimulate the adaptation capacities of the cell. In the most highly developed bacterial model, E. coli, the concentration of potassium glutamate is considered the intracellular signal of osmotic stress; it acts on the transcriptional regulation of genes involved in the osmoadaptative response (6, 20). Osmoadaptation is achieved in S. meliloti by accumulated and nonaccumulated osmoprotectants, which act in opposite manners on glutamate cellular content. Therefore, the conclusions derived from the E. coli model are not applicable to S. meliloti. Potassium glutamate cannot be considered the only intracellular osmotic signal for this bacterium; a novel mechanism of osmoregulation involving a different intracellular signal could occur in S. meliloti.
Sucrose is widespread in nature; it is produced mainly in plants and photosynthetic microorganisms. In the symbiotic association Medicago sativa-S. meliloti, sucrose is the most prominent carbohydrate in roots and nodules; its levels have been shown to increase in the latter under salt stress (8). Although the peribacteroid membrane is a very selective barrier through which no sugar is actively transported (32), sucrose could enter the bacteroid by slow and passive diffusion. These data suggest two consequences. First, sucrose could be released into the environment via plant exudates and wilting plants (20) and could be absorbed by free-living rhizosphere bacteria. Second, sucrose is available in low amounts but is not limiting for bacteroids. Thus, sucrose could participate in their osmoprotection rather than in their energy supply, which is mainly ensured through active dicarboxylic acid import systems and catabolism (32). These points must be analyzed since they are crucial for improvement of the resistance of this association to drought and, in general, to any osmotic stress.
ACKNOWLEDGMENTS
We thank M. Uguet for technical assistance, J. Hamelin for NMR experiments, J. N. Barbotin and D. Le Rudulier for providing rhizobial strains, J.-A. Pocard for helpful discussion, and V. James for language improvement.
This work was supported by the Direction de la Recherche et des Etudes Doctorales and by the Centre National de la Recherche Scientifique.
REFERENCES
- 1.Bernard T, Jebbar M, Rassouli Y, Himdi-Kabbab S, Hamelin J, Blanco C. Ectoine accumulation and osmotic regulation in Brevibacterium linens. J Gen Microbiol. 1993;139:129–138. [Google Scholar]
- 2.Bernard T, Pocard J-A, Perroud B, Le Rudulier D. Variations in the response of salt-stressed Rhizobium strains to betaines. Arch Microbiol. 1986;143:359–364. [Google Scholar]
- 3.Bianchi G, Gamba A, Limiroli R, Pozzi N, Elster R, Salamini F, Bartels D. The unusual sugar composition in leaves of the resurrection plant Myrothamnus flabellifolia. Physiol Plant. 1993;87:223–226. [Google Scholar]
- 4.Brown A D. Microbial water stress. Bacteriol Rev. 1976;40:803–846. doi: 10.1128/br.40.4.803-846.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Csonka L N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989;53:121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Csonka L N, Hanson A D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- 7.Dinnbier U, Limpinsel E, Schmid R, Bakker E P. Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K-12 to elevated sodium chloride concentrations. Arch Microbiol. 1988;150:348–357. doi: 10.1007/BF00408306. [DOI] [PubMed] [Google Scholar]
- 8.Fougère F, Le Rudulier D, Streeter J G. Effects of salt stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sativa L.) Plant Physiol. 1991;96:1228–1236. doi: 10.1104/pp.96.4.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galinski E A, Trüper H G. Microbial behaviour in salt-stressed ecosystems. FEMS Microbiol Rev. 1994;15:95–108. [Google Scholar]
- 10.Garcia-Perez A, Burg M B. Renal medullary organic osmolytes. Physiol Rev. 1991;71:1081–1115. doi: 10.1152/physrev.1991.71.4.1081. [DOI] [PubMed] [Google Scholar]
- 11.Guillouet S, Engasser J M. Sodium and proline accumulation in Corynebacterium glutamicum as a response to an osmotic saline upshock. Appl Microbiol Biotechnol. 1995;43:315–320. [Google Scholar]
- 12.Hellebust J A. Osmoregulation. Annu Rev Plant Physiol. 1976;27:485–505. [Google Scholar]
- 13.Imhoff J F. Osmoregulation and compatible solutes in eubacteria. FEMS Microbiol Rev. 1986;39:57–66. [Google Scholar]
- 14.Jebbar M, Gouesbet G, Himdi-Kabbab S, Blanco C, Bernard T. Osmotic adaptation in Brevibacterium linens: differential effect of proline and glycine betaine on cytoplasmic osmolyte pool. Arch Microbiol. 1995;163:380–386. [Google Scholar]
- 15.Kortstee G J J. The aerobic decomposition of choline by microorganisms. I. The ability of aerobic organisms, particularly coryneform bacteria, to utilize the choline as the sole carbon and nitrogen source. Arch Mikrobiol. 1970;71:235–244. [PubMed] [Google Scholar]
- 16.Kushner D J. Life in high salt and solute concentrations: halophilic bacteria. In: Kushner D J, editor. Microbial life in extreme environments. New York, N.Y: Academic Press; 1978. pp. 317–368. [Google Scholar]
- 17.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Lucht J M, Bremer E. Adaptation of Escherichia coli to high osmolarity environments: osmoregulation of the high-affinity glycine betaine transport system ProU. FEMS Microbiol Rev. 1994;14:3–20. doi: 10.1111/j.1574-6976.1994.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 19.Mackay M A, Norton R S, Borowitzka L J. Organic osmoregulatory solutes in cyanobacteria. J Gen Microbiol. 1984;130:2177–2191. [Google Scholar]
- 20.Miller K J, Wood J M. Osmoadaptation by rhizosphere bacteria. Annu Rev Microbiol. 1996;50:101–136. doi: 10.1146/annurev.micro.50.1.101. [DOI] [PubMed] [Google Scholar]
- 21.O’Gara F, Shanmugam K T. Regulation of nitrogen fixation by Rhizobia: export of fixed N2 as NH2+ Biochim Biophys Acta. 1976;437:313–321. doi: 10.1016/0304-4165(76)90001-5. [DOI] [PubMed] [Google Scholar]
- 22.Pichereau V, Cosquer A, Gaumont A C, Bernard T. Synthesis of trimethylated phosphonium and arsonium analogues of the osmoprotectant glycine betaine; contrasted biological activities in two bacterial species. Bioorg Med Chem Lett. 1997;7:2893–2896. [Google Scholar]
- 23.Pichereau V, Pocard J-A, Hamelin J, Blanco C, Bernard T. Differential effects of dimethylsulfoniopropionate, dimethylsulfonioacetate, and other S-methylated compounds on the growth of Sinorhizobium meliloti at low and high osmolarities. Appl Environ Microbiol. 1998;64:1420–1429. doi: 10.1128/aem.64.4.1420-1429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilon-Smits E A H, Ebskamp M J M, Paul M J, Jeuken M J W, Weisbeek P J, Smeekens S C M. Improved performance of transgenic fructan-accumulating tobacco under drought stress. Plant Physiol. 1995;107:125–130. doi: 10.1104/pp.107.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pocard J-A. Ph.D. thesis. Rennes, France: Université de Rennes 1; 1987. [Google Scholar]
- 26.Rains D W, Csonka L N, Le Rudulier D, Croughan T P, Yang S S, Stavarek S J, Valentine R C. Osmoregulation by organisms exposed to saline stress: physiological mechanisms and genetic manipulation. In: San Pietro A, editor. Biosaline research: a look to the future. New York, N.Y: Plenum Publishing Corp.; 1982. pp. 283–302. [Google Scholar]
- 27.Randall K, Lever M, Peddie B A, Chambers S T. Natural and synthetic betaines counter the effects of high NaCl and urea concentrations. Biochim Biophys Acta. 1996;1291:189–194. doi: 10.1016/s0304-4165(96)00057-8. [DOI] [PubMed] [Google Scholar]
- 28.Reed R H, Stewart W D P. Osmotic adjustment and organic solute accumulation in unicellular cyanobacteria from freshwater and marine habitats. Mar Biol. 1985;88:1–9. [Google Scholar]
- 29.Rolland J P, Bron P, Thomas D. MACS, automatic counting of objects based on shape recognition. Comput Appl Biosci. 1997;5:563–564. doi: 10.1093/bioinformatics/13.5.563. [DOI] [PubMed] [Google Scholar]
- 30.Smith L T, Smith G M. An osmoregulated dipeptide in stressed Rhizobium meliloti. J Bacteriol. 1989;171:4714–4717. doi: 10.1128/jb.171.9.4714-4717.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stock J B, Rauch B, Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977;252:7850–7861. [PubMed] [Google Scholar]
- 32.Stowers M D. Carbon metabolism in Rhizobium species. Annu Rev Microbiol. 1985;39:89–108. doi: 10.1146/annurev.mi.39.100185.000513. [DOI] [PubMed] [Google Scholar]
- 33.Talibart R, Jebbar M, Gouesbet G, Himdi-Kabbab S, Wroblewski H, Blanco C, Bernard T. Osmoadaptation in rhizobia: ectoine-induced salt tolerance. J Bacteriol. 1994;176:5210–5217. doi: 10.1128/jb.176.17.5210-5217.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talibart R, Jebbar M, Gouffi K, Pichereau V, Gouesbet G, Blanco C, Bernard T, Pocard J-A. Transient accumulation of glycine betaine and dynamics of endogenous osmolytes in salt-stressed cultures of Sinorhizobium meliloti. Appl Environ Microbiol. 1997;63:4657–4663. doi: 10.1128/aem.63.12.4657-4663.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welsh D T, Herbert R A. Identification of organic solutes accumulated by purple and green sulphur bacteria during osmotic stress using natural abundance 13C nuclear magnetic resonance spectroscopy. FEMS Microbiol Ecol. 1993;13:145–149. [Google Scholar]
- 36.Wyn Jones R G, Gorham J. Phytochemical aspects of osmotic adaptation. Recent Adv Phytochem. 1984;18:55–78. [Google Scholar]
- 37.Yancey P H, Clark M E, Hand S C, Bowlus R D, Somero G N. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]