Abstract
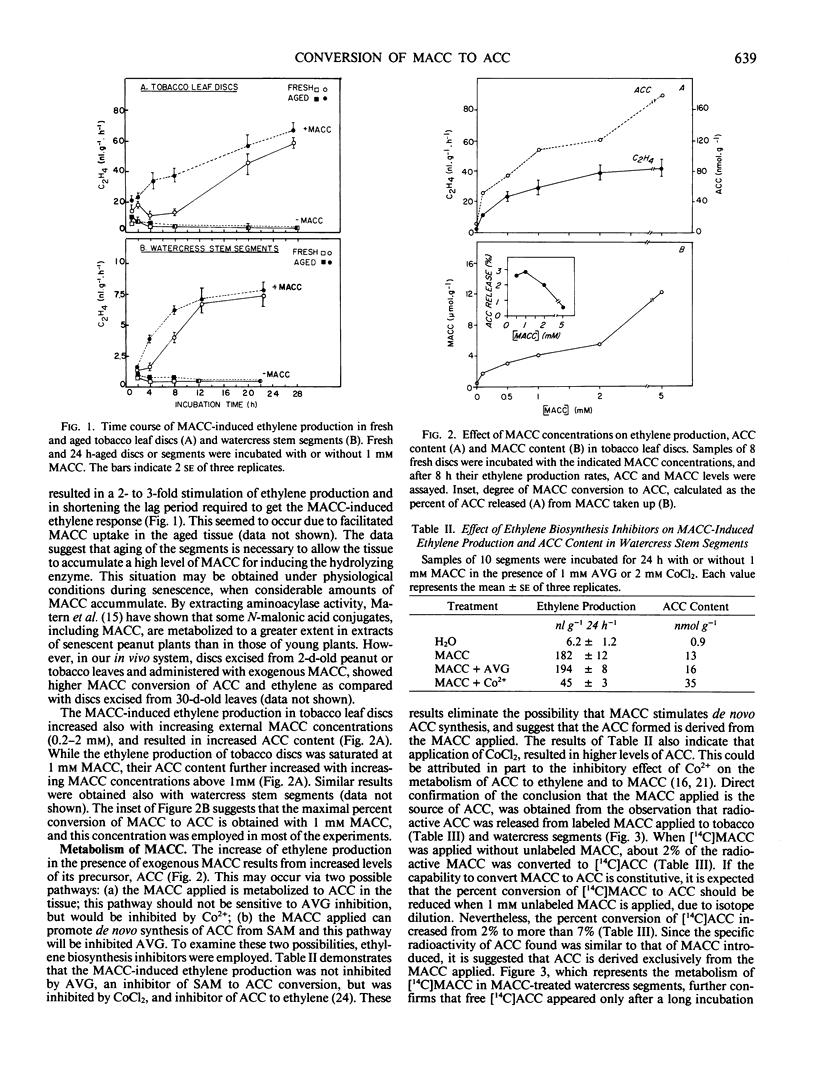
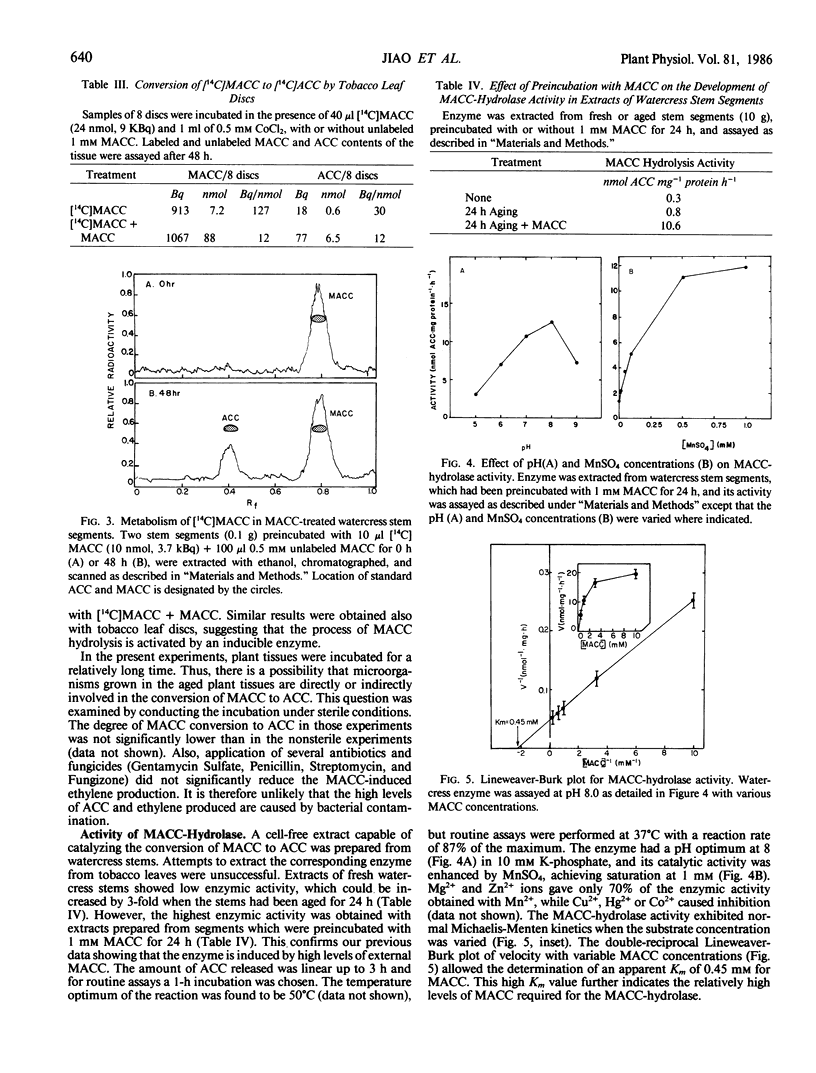
Since 1-(malonylamino)cyclopropane-1-carboxylic acid (MACC), the major conjugate of 1-aminocyclopropane-1-carboxylic acid (ACC) in plant tissues, is a poor ethylene producer, it is generally thought that MACC is a biologically inactive end product of ACC. In the present study we have shown that the capability of watercress (Nasturtium officinale R. Br) stem sections and tobacco (Nicotiana tabacum L.) leaf discs to convert exogenously applied MACC to ACC increased with increasing MACC concentrations (0.2-5 millimolar) and duration (4-48 hours) of the treatment. The MACC-induced ethylene production was inhibited by CoCl2 but not by aminoethoxyvinylglycin, suggesting that the ACC formed is derived from the MACC applied, and not from the methionine pathway. This was further confirmed by the observation that radioactive MACC released radioactive ACC and ethylene. A cell-free extract, which catalyzes the conversion of MACC to ACC, was prepared from watercress stems which were preincubated with 1 millimolar MACC for 24 hours. Neither fresh tissues nor aged tissues incubated without external MACC exhibited enzymic activity, confirming the view that the enzyme is induced by MACC. The enzyme had a Km of 0.45 millimolar for MACC and showed maximal activity at pH 8.0 in the presence of 1 millimolar MnSO4. The present study indicates that high MACC levels in the plant tissue can induce to some extent the capability to convert MACC to ACC.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni N., Lieberman M. Patterns of ehtylene production in senescing leaves. Plant Physiol. 1979 Nov;64(5):796–800. doi: 10.1104/pp.64.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryce G. F., Rabin B. R. The function of the metal ion in leucine aminopeptidase and the mechanism of action of the enzyme. Biochem J. 1964 Mar;90(3):513–518. doi: 10.1042/bj0900513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman N. E., Fu J. R., Yang S. F. Identification and Metabolism of 1-(Malonylamino)cyclopropane-1-carboxylic Acid in Germinating Peanut Seeds. Plant Physiol. 1983 Jan;71(1):197–199. doi: 10.1104/pp.71.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman N. E., Yang S. F., McKeon T. Identification of 1-(malonylamino) cyclopropane-1-carboxylic acid as a major conjugate of 1-aminocyclopropane-1-carboxylic acid, an ethylene precursor in higher plants. Biochem Biophys Res Commun. 1982 Jan 29;104(2):765–770. doi: 10.1016/0006-291x(82)90703-3. [DOI] [PubMed] [Google Scholar]
- Liu Y., Su L. Y., Yang S. F. Ethylene Promotes the Capability To Malonylate 1-Aminocyclopropane-1-carboxylic Acid and d-Amino Acids in Preclimacteric Tomato Fruits. Plant Physiol. 1985 Apr;77(4):891–895. doi: 10.1104/pp.77.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Matern U., Feser C., Heller W. N-malonyltransferases from peanut. Arch Biochem Biophys. 1984 Nov 15;235(1):218–227. doi: 10.1016/0003-9861(84)90271-6. [DOI] [PubMed] [Google Scholar]
- Rosa N., Neish A. C. Formation and occurrence of N-malonylphenylalanine and related compounds in plants. Can J Biochem. 1968 Aug;46(8):799–806. [PubMed] [Google Scholar]


