Abstract
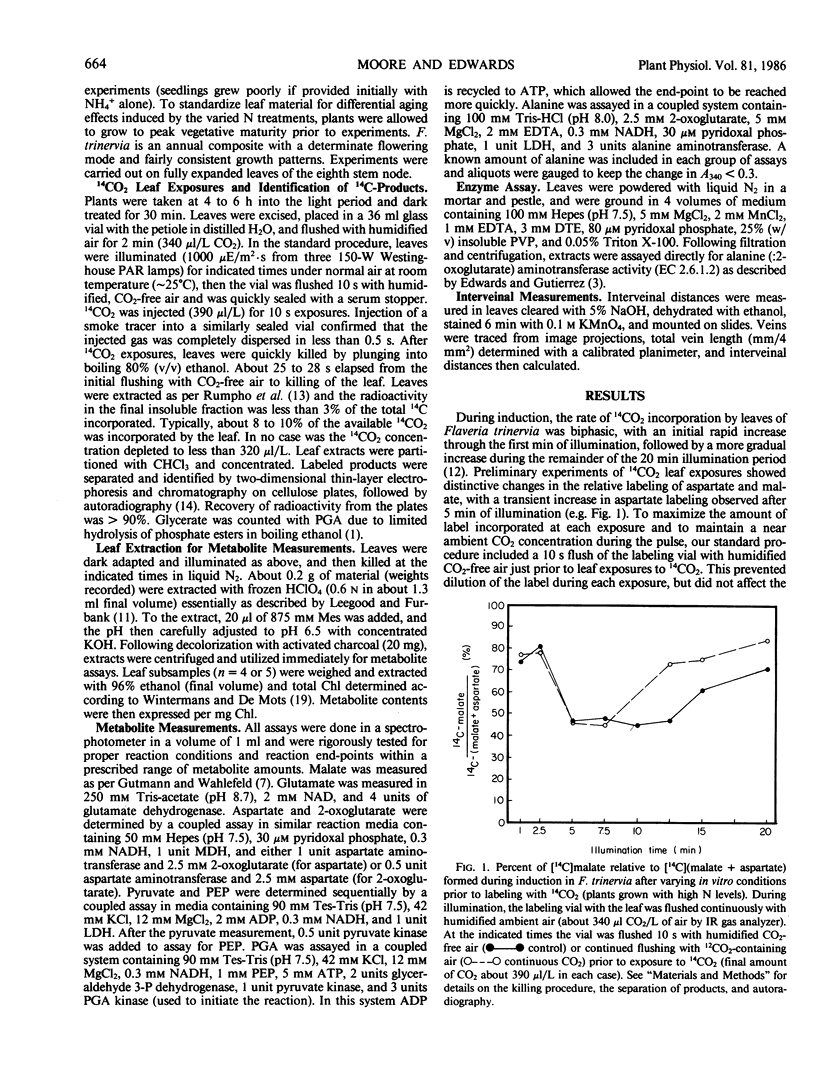
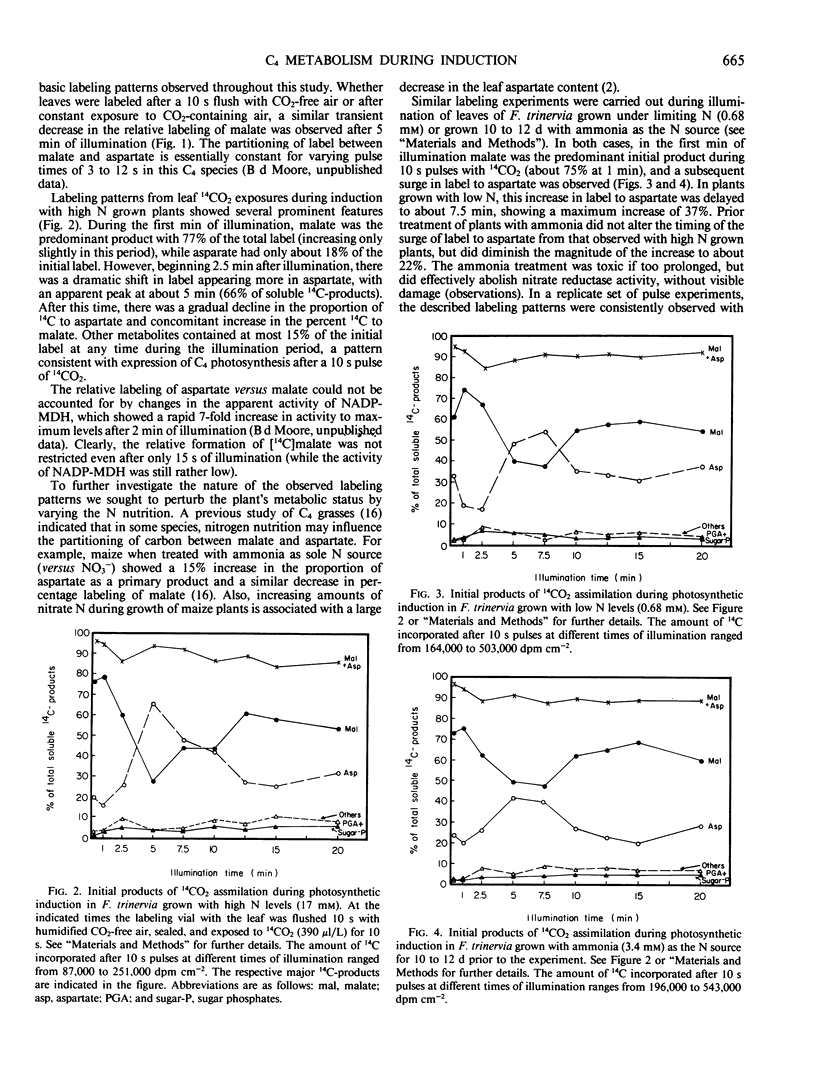
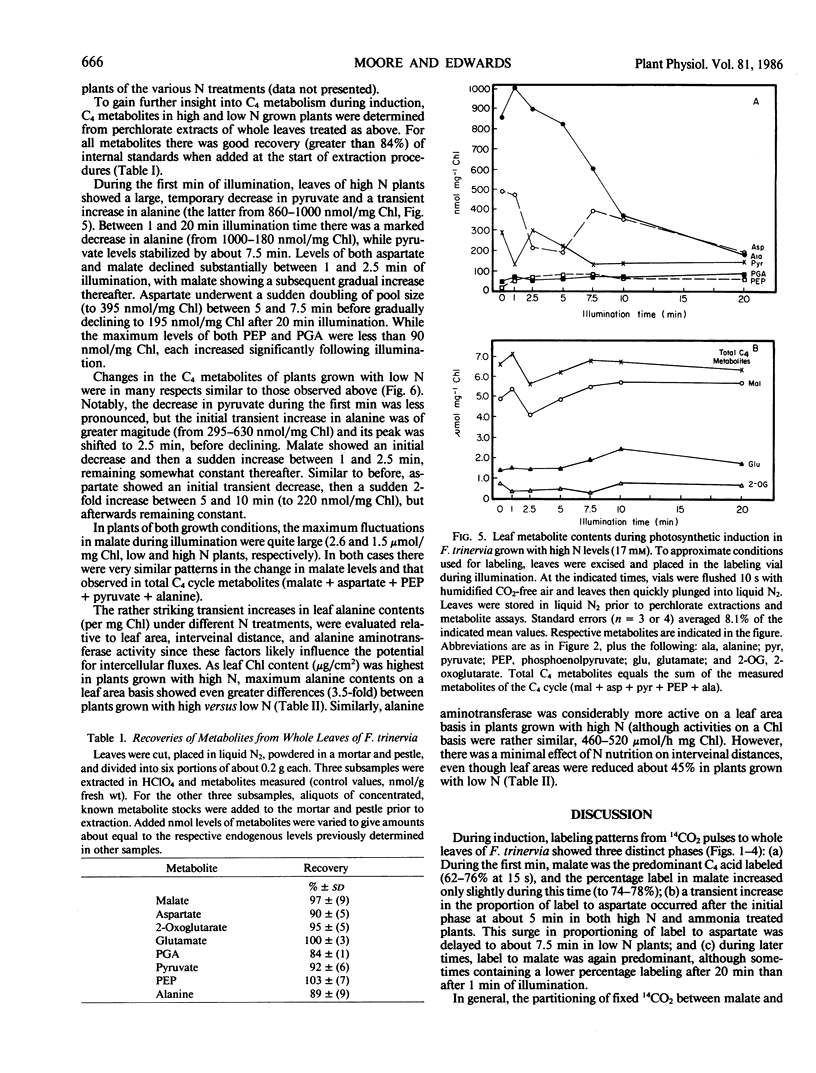
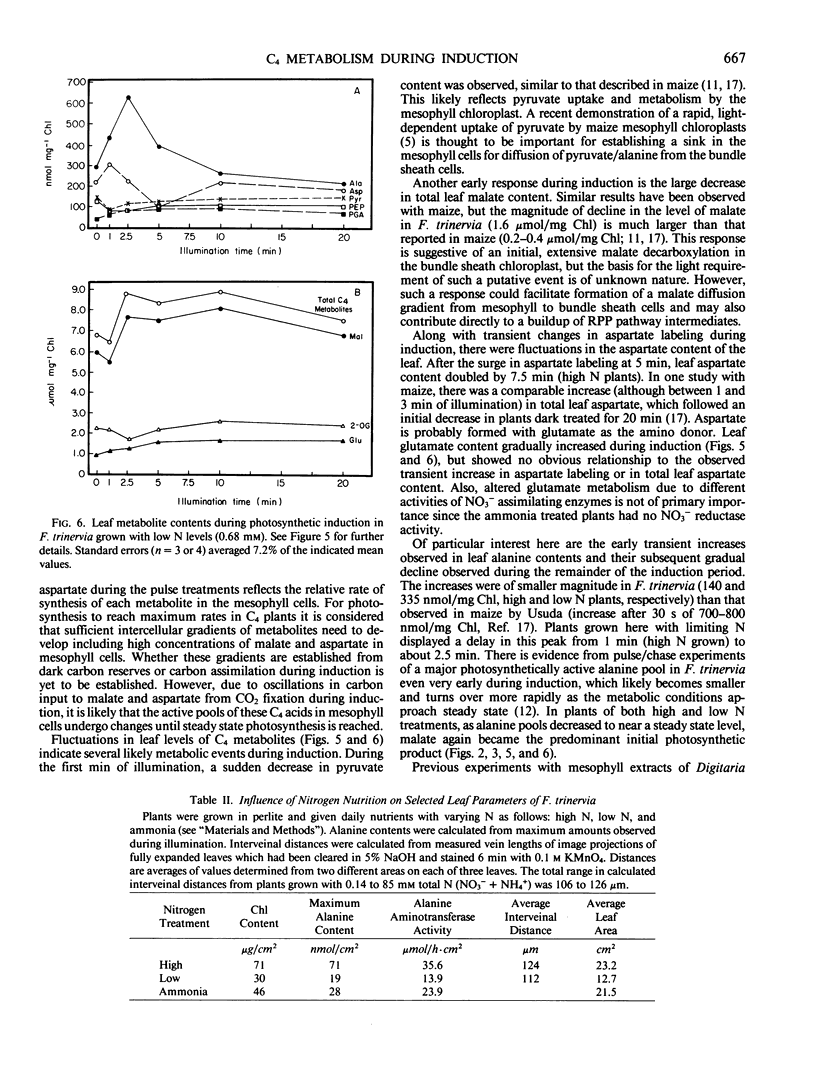
Labeling patterns from 14CO2 pulses to leaves and whole leaf metabolite contents were examined during photosynthetic induction in Flaveria trinervia, a C4 dicot of the NADP-malic enzyme subgroup. During the first one to two minutes of illumination, malate was the primary initial product of 14CO2 assimiltion (about 77% of total 14C incorporated). After about 5 minutes of illumination, the proportion of initial label to aspartate increased from 16 to 66%, and then gradually declined during the following 7 to 10 minutes of illumination. Nutrition experiments showed that the increase in 14CO2 partitioning to aspartate was delayed about 2.5 minutes in plants grown with limiting N, and was highly dampened in plants previously treated 10 to 12 days with ammonia as the sole N source. Measurements of C4 leaf metabolites revealed several transients in metabolite pools during the first few minutes of illumination, and subsequently, more gradual adjustments in pool sizes. These include a large initial decrease in malate (about 1.6 micromoles per milligram chlorophyll) and a small initial decrease in pyruvate. There was a transient increase in alanine levels after 1 minute of illumination, which was followed by a gradual, prolonged decrease during the remainder of the induction period. Total leaf aspartate decreased initially, but temporarily doubled in amount between 5 and 10 minutes of illumination (after its surge as a primary product). These results are discussed in terms of a plausible sequence of metabolic events which lead to the formation of the intercellular metabolite gradients required in C4 photosynthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edwards G. E., Gutierrez M. Metabolic Activities in Extracts of Mesophyll and Bundle Sheath Cells of Panicum miliaceum (L.) in Relation to the C(4) Dicarboxylic Acid Pathway of Photosynthesis. Plant Physiol. 1972 Dec;50(6):728–732. doi: 10.1104/pp.50.6.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D. Mechanism of C4 photosynthesis in Chloris gayana: pool sizes and kinetics of 14CO2 incorporation into 4-carbon and 3-carbon intermediates. Arch Biochem Biophys. 1979 Apr 15;194(1):117–127. doi: 10.1016/0003-9861(79)90601-5. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Edwards G. E. Regulation of oxaloacetate, aspartate, and malate formation in mesophyll protoplast extracts of three types of c(4) plants. Plant Physiol. 1975 Aug;56(2):324–331. doi: 10.1104/pp.56.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B. D., Edwards G. E. Photosynthetic Induction in a C(4) Dicot, Flaveria trinervia: II. Metabolism of Products of CO(2) Fixation after Different Illumination Times. Plant Physiol. 1986 Jun;81(2):669–673. doi: 10.1104/pp.81.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpho M. E., Ku M. S., Cheng S. H., Edwards G. E. Photosynthetic Characteristics of C(3)-C(4) Intermediate Flaveria Species : III. Reduction of Photorespiration by a Limited C(4) Pathway of Photosynthesis in Flaveria ramosissima. Plant Physiol. 1984 Aug;75(4):993–996. doi: 10.1104/pp.75.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann P. Separation of phosphate esters and algal extracts by thin-layer electrophoresis and chromatography. J Chromatogr. 1969 Feb 25;39(4):507–509. [PubMed] [Google Scholar]
- Usuda H. Changes in Levels of Intermediates of the C(4) Cycle and Reductive Pentose Phosphate Pathway during Induction of Photosynthesis in Maize Leaves. Plant Physiol. 1985 Aug;78(4):859–864. doi: 10.1104/pp.78.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda H., Ku M. S., Edwards G. E. Activation of NADP-Malate Dehydrogenase, Pyruvate,Pi Dikinase, and Fructose 1,6-Bisphosphatase in Relation to Photosynthetic Rate in Maize. Plant Physiol. 1984 Sep;76(1):238–243. doi: 10.1104/pp.76.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]


