Abstract
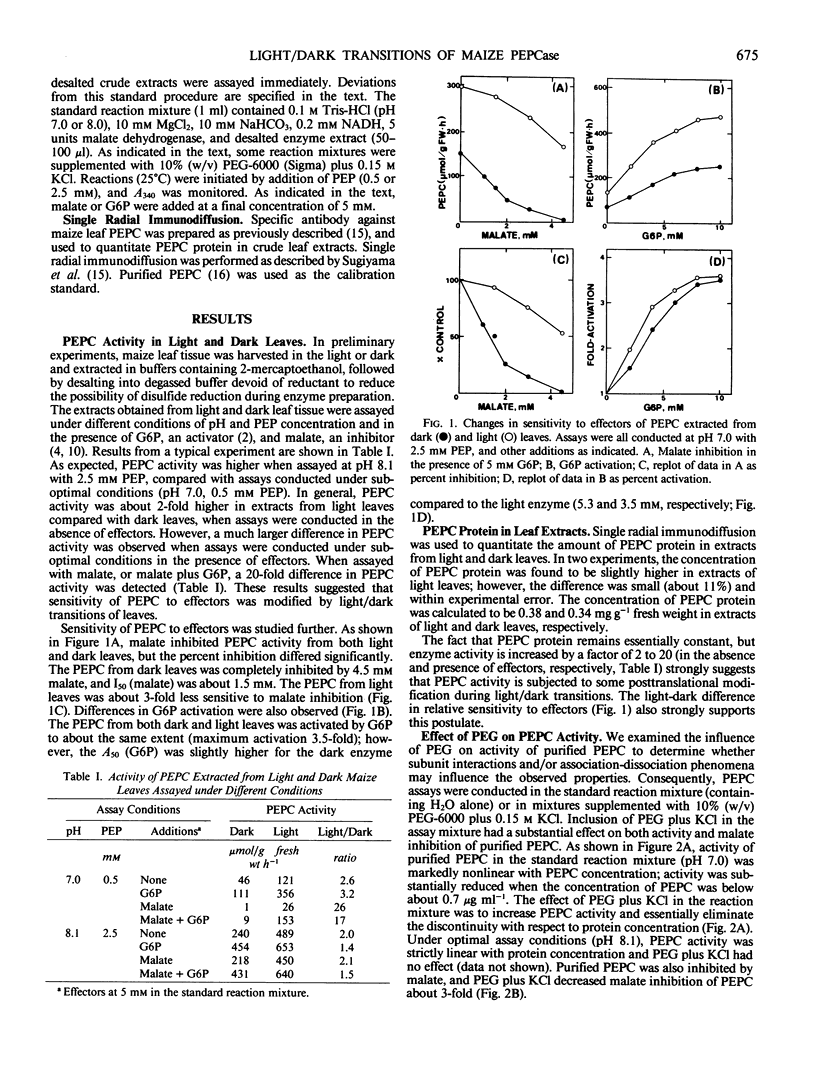
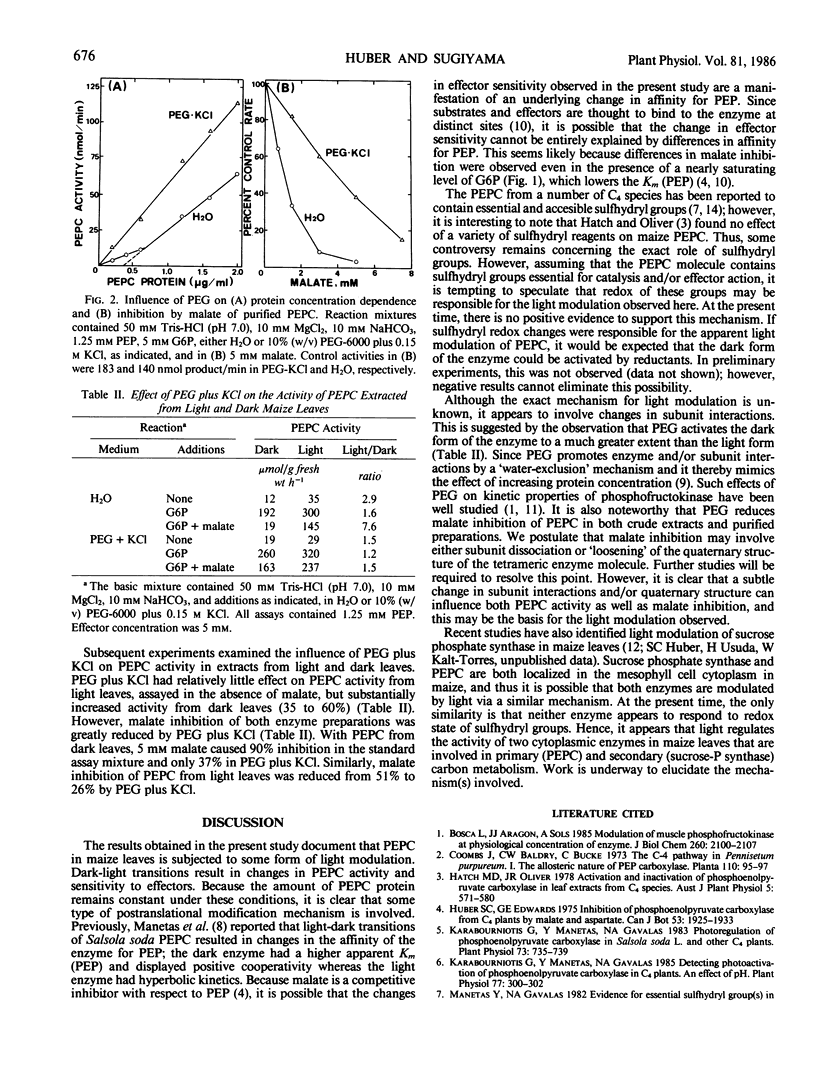
Illumination of previously darkened maize (Zea mays L. cv Golden Cross Bantam T51) leaves had no effect on the concentration of phosphoenolpyruvate (PEP) carboxylase protein, but increased enzyme activity about 2-fold when assayed under suboptimal conditions (pH 7.0 and limiting PEP). In addition, sensitivity to effectors of PEP carboxylase activity was significantly altered; e.g. malate inhibition was reduced and glucose-6-phosphate activation was increased. Consequently, 10- to 20-fold differences in PEP carboxylase activity were observed during dark to light transitions when assayed in the presence of effectors. At pH 7.0 activity of purified PEP carboxylase was not proportional to enzyme concentrations. Below 0.7 microgram PEP carboxylase protein per milliliter, enzyme activity was disproportionately reduced. Including polyethylene glycol plus potassium chloride in the reaction mixture eliminated this discontinuity and substantially increased PEP carboxylase activity and reduced malate inhibition dramatically. Inclusion of polyethylene glycol in the assay mixture specifically increased the activity of PEP carboxylase extracted from dark leaves, and reduced malate inhibition of the enzyme from both light and dark leaves. Collectively, the results suggest that PEP carboxylase in maize leaves is subjected to some type of protein modification that affects both activity and effector sensitivity. We postulate that changes in quaternary structure (dissociation or altered subunit interactions) may be involved.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boscá L., Aragón J. J., Sols A. Modulation of muscle phosphofructokinase at physiological concentration of enzyme. J Biol Chem. 1985 Feb 25;260(4):2100–2107. [PubMed] [Google Scholar]
- Karabourniotis G., Manetas Y., Gavalas N. A. Detecting Photoactivation of Phosphoenolpyruvate Carboxylase in C(4) Plants : An Effect of pH. Plant Physiol. 1985 Feb;77(2):300–302. doi: 10.1104/pp.77.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabourniotis G., Manetas Y., Gavalas N. A. Photoregulation of Phosphoenolpyruvate Carboxylase in Salsola soda L. and Other C(4) Plants. Plant Physiol. 1983 Nov;73(3):735–739. doi: 10.1104/pp.73.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miekka S. I., Ingham K. C. Influence of self-association of proteins on their precipitation by poly(ethylene glycol). Arch Biochem Biophys. 1978 Dec;191(2):525–536. doi: 10.1016/0003-9861(78)90391-0. [DOI] [PubMed] [Google Scholar]
- Reinhart G. D. Influence of polyethylene glycols on the kinetics of rat liver phosphofructokinase. J Biol Chem. 1980 Nov 25;255(22):10576–10578. [PubMed] [Google Scholar]
- Sicher R. C., Kremer D. F. Possible control of maize leaf sucrose-phosphate synthase activity by light modulation. Plant Physiol. 1985 Nov;79(3):695–698. doi: 10.1104/pp.79.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R. The photoactivation of a phosphopyruvate synthase in leaves of Amaranthus palmeri. Biochem Biophys Res Commun. 1968 Mar 12;30(5):483–488. doi: 10.1016/0006-291x(68)90077-6. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Mizuno M., Hayashi M. Partitioning of Nitrogen among Ribulose-1,5-bisphosphate Carboxylase/Oxygenase, Phosphoenolpyruvate Carboxylase, and Pyruvate Orthophosphate Dikinase as Related to Biomass Productivity in Maize Seedlings. Plant Physiol. 1984 Jul;75(3):665–669. doi: 10.1104/pp.75.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976 Jun;57(6):906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]


